Abstract
The maintenance of the immune system can be costly, and a lack of dietary protein can increase the susceptibility of organisms to disease. However, few studies have investigated the relationship between protein nutrition and immunity in insects. Here, we tested in honeybees (Apis mellifera) whether dietary protein quantity (monofloral pollen) and diet diversity (polyfloral pollen) can shape baseline immunocompetence (IC) by measuring parameters of individual immunity (haemocyte concentration, fat body content and phenoloxidase activity) and glucose oxidase (GOX) activity, which enables bees to sterilize colony and brood food, as a parameter of social immunity. Protein feeding modified both individual and social IC but increases in dietary protein quantity did not enhance IC. However, diet diversity increased IC levels. In particular, polyfloral diets induced higher GOX activity compared with monofloral diets, including protein-richer diets. These results suggest a link between protein nutrition and immunity in honeybees and underscore the critical role of resource availability on pollinator health.
Keywords: immunocompetence, diet, pollen, honeybee, social immunity
1. Introduction
The maintenance of the immune system is one of the most costly physiological systems in animals (Lochmiller & Deerenberg 2000; Schmid-Hempel 2005) and deficient nutrition can impair immune function and increase the susceptibility of individuals to disease. In humans, dietary protein deficiency reduces the concentrations of most amino acids in plasma and compromises the immune system (Li et al. 2007). Therefore, an adequate provision of proteins is required to sustain normal immunocompetence (IC), defined as the capacity of an organism to mount an immune response (Wilson-Rich et al. 2008). However, besides a few studies showing that the dietary protein quantity and quality can enhance immune functions (Lee et al. 2006, 2008), this relationship has been poorly investigated in insects. Also, to our knowledge, it is unknown whether diet diversity also affects IC in insects.
To fill this gap, we tested the effect of both dietary protein quantity and diet diversity on honeybee (Apis mellifera) IC by feeding bees with mono- and polyfloral pollen diets. The honeybee is a valuable model for such studies, because they usually pollinate a variety of plants but sometimes are forced to feed on single crops (monocultures). In addition, pollen, which is the main source of dietary protein and contains essential amino acids for their development (de Groot 1953), can influence longevity, the development of hypopharyngeal glands (HPG) and ovaries (Pernal & Currie 2000) and the susceptibility to pathogens (Rinderer et al. 1974; Rinderer & Elliott 1977); but to what extent pollen can affect IC is not known. Finally, honeybee populations have been declining over the last years and a current idea suggests that honeybee colonies may suffer from a compromised immune system (van Engelsdorp et al. 2008), which could be related to poor nutrition commonly associated with colony losses (van Engelsdorp et al. 2008). Such a study will provide a framework to better understand how the abundance and diversity of environmental resources can shape an organism's immunity.
The effects of pollen diet on the capacity for disease resistance were empirically tested by measuring haemocyte concentration and fat body content as indirect assessments of cellular and humoral IC, respectively, and phenoloxidase (PO) activity, which is involved in both. Haemocytes are involved in the phagocytosis and encapsulation of parasites, the latter process requiring PO activity, and fat body is the main site of antimicrobial peptide synthesis. As social organisms, honeybees depend not only on individual immunity, but also on the overall functioning of the hive. So, we also analysed glucose oxidase (GOX) activity as a parameter of social immunity. Mainly expressed in the HPGs (Ohashi et al. 1999), GOX catalyses the oxidation of β-d-glucose to gluconic acid and hydrogen peroxide, the latter having antiseptic properties. The antiseptic products are secreted into larval food and honey, which contributes to colony-food sterilization (White et al. 1963), and hence, to the prevention of disease contamination at the group level.
2. Material and methods
Experiments were performed in Avignon (France) with local hybrid colonies (A. m. ligustica/A. m. mellifera). For controlling the pollen intake, 1-day-old bees were reared in cages in the dark at 32°C and 70 per cent RH. They were obtained from honeycombs containing late-stage pupae removed from source colonies. Bees were fed ad libitum with candy (30% honey from the source colonies, 70% powdered sugar), water and one of the pollen diets prepared with 1/10 water. Pollen diets were replaced every day for 10 days and bees were collected at days 5 and 10 for IC sampling. To simulate as much as possible the colony rearing conditions, cages were exposed to a Beeboost (Pherotech), releasing one queen-equivalent of queen mandibular pheromone per day.
(a). Pollen diets
Seven blends of fresh pollen commonly found in France and with a respective predominance of Acer, Castanea, Cistus, Erica, Quercus, Salix and Taraxacum pollen were obtained from Pollenergie (France) and frozen at −20°C. To produce monofloral pollen diets, we sorted, by colour, pellets of the predominant pollen from each of the commercial blend. Nitrogen and then protein contents of the different pollens were determined by microkjeldahl analysis using a Vapodest 45 (Gerhardt) and according to the procedures described in ISO 5983 (1997). In order to test the effect of protein quantity and pollen diversity, groups of 80 bees were, respectively, fed with monofloral diets that differed in the quantity of protein or polyfloral diets that had the same amount of protein as monofloral diets (table 1). Control groups had no pollen. The experiment was repeated on five different colonies.
Table 1.
Mono- and polyfloral diets of pollen. The percentage of the different pollens used for each polyfloral blend is given. The protein content of Acer, Erica and Salix pollen was 25.9, 17.1 and 25.8%, respectively.
| diet | pollen type | % protein | blend composition |
|---|---|---|---|
| monofloral | Cistus | 15.5 | — |
| Taraxacum | 19.8 | — | |
| Castanea | 23.6 | — | |
| Quercus | 29.6 | — | |
| polyfloral | first pollen blend | 19.8 | 32% Erica, 28% Cistus, 16% Castanea, 12% Salix, 12% Acer |
| second pollen blend | 23.6 | 24% Quercus, 20% Salix, 20% Taraxacum, 18% Acer, 18% Cistus |
(b). Immunocompetence
IC was assessed indirectly in the absence of an actual pathogen challenge. To determine the haemocyte concentration, haemolymph was extracted with micro capillaries (10 µl) from the second abdominal tergite and diluted 1 : 5 in ice-cold phosphate-buffered saline (pH 7.4). The number of haemocytes per microlitre of haemolymph was counted using a phase contrast microscope (200×) with haemocytometer. Fat body mass was estimated using an ether extraction method according to Wilson-Rich et al. (2008), then the relative mass of fat body was given as the per cent change in abdominal weight after the ether wash. There are no data yet showing how the fat body size correlates with immune response, but this tissue is the main site of immunoproteins synthesis, energy and protein storage used for brood food and vitellogenin synthesis involved in longevity (Amdam & Omholt 2002). PO and GOX activity were, respectively, measured on whole abdomen and head (see electronic supplementary material). Diet and age effects on IC were determined using two-way ANOVA followed by Newman–Keuls post hoc tests.
3. Results
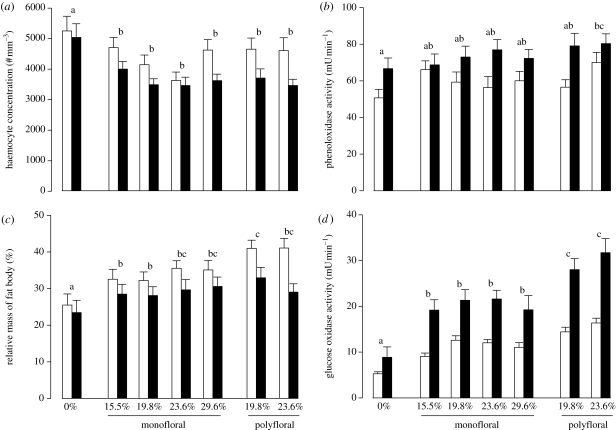
The amount of pollen consumed per bee and per day did not differ between the diets (one-way ANOVA: F5,204 = 0.863, p = 0.51, 5.6 ± 2.1 mg per bee per day) but we found a significant effect of pollen diet on the different immune parameters. Control bees had a higher haemocyte concentration compared with bees fed with pollen (F6,489 = 5.27, p < 0.001; figure 1a). Regarding PO activity, a slight pollen–diet effect was observed (F6,428 = 2.54, p = 0.02), which was due to a higher PO activity in bees fed with the protein-richest polyfloral blend compared with control bees (figure 1b). The fat body contents increased with pollen diets (F6,489 = 6.74, p < 0.001). In addition, the first polyfloral blend induced a higher fat body content compared with its monofloral counterpart (same protein content) and the protein-lowest monofloral diets (figure 1c). Pollen consumption also greatly increased GOX activity, and furthermore, the type of diet had a pronounced effect on its activity (F6,446 = 19.9, p < 0.001). Both, polyfloral diets induced a higher GOX activity compared with the same protein-level monofloral diets and the protein-richest diet (figure 1d). Finally, for each immune parameter, no difference was observed between the different monofloral diets (figure 1a–d).
Figure 1.
Effect of pollen diet on IC in 5 (open bars) and 10 days old bees (filled bars). (a) Haemocyte concentration, (b) phenoloxidase activity, (c) fat body mass and (d) GOX activity. Eight bees per cage for each experimental group were analysed for each immune parameter. The protein percentage of each pollen diet is indicated on the x-axis. Each letter indicates significant differences between diets (p < 0.05, Newman–Keuls post hoc tests). No significant interaction between the diet and age factors was found (p > 0.05 for each immune parameter). Data show mean ± s.e.
A significant age effect was also observed on the different immune parameters. Haemocyte concentration and fat body contents decreased with age (F1,489 = 19.1, p < 0.001 and F1,489 = 25.2, p < 0.001, respectively) contrary to PO and GOX activity, which increased with age (F1,428 = 24.9, p < 0.001 and F1,446 = 123.6, p < 0.001, respectively). In addition, the effect of diet on IC was consistent between 5 and 10-day-old bees, as no significant interaction between the diet and age factors was found (p > 0.05 for each immune parameter).
4. Discussion
In this study, we provided experimental evidence for a link between pollen nutrition and baseline IC in honeybees. These results suggest that the abundance and diversity of environmental resources can have a direct impact on pollinator's health.
Haemocyte concentration was augmented in bees fed a diet with no protein. Higher haemocyte concentrations are expected to be associated with protein supply and higher resistance to disease. However, bees fed with protein might invest more resources in certain types of haemocytes (e.g. granulocyte or plasmatocyte) at the expense of others types. An investment in the type of haemocytes that are more costly to produce would ultimately lead to an overall decrease in haemocyte numbers. Or, since the metabolic activity of haemocytes increases with a pollen diet (Sżymaś & Jędruszuk 2003), haemocyte concentration might increase in bees fed without protein to compensate for the lower metabolic activity. This age decrease in concentration was also found by Schmid et al. (2007). However, Wilson-Rich et al. (2008) observed a lower haemocyte concentration in 1-day-old bees compared to foragers, perhaps because collected bees were very young and in the process of reaching the maximum haemocyte concentration. Pollen feeding did not clearly affect the PO activity. Similar results were found in bumble-bees, where limited nutrition did not affect the encapsulation response mediated by PO (Schmid-Hempel & Schmid-Hempel 1998). Perhaps, the costs induced by pathogens would reveal a critical role of protein diet on PO activity. On the contrary, GOX activity, which does not provide an immune protection to the bee itself but allows prevention of infectious disease in the colony, was greatly affected by the protein diets, suggesting that bees would invest more resources in social rather than individual immunity. Indeed, honeybees possess only one-third the numbers of immune response genes known for solitary insects (Evans et al. 2006), which indicates that other types of defence against pathogens might be important (e.g. GOX).
Regarding the diet quality, the different monofloral diets did not induce changes in the IC levels. This could be explained by a nutritive compensation of bees fed with protein-poor pollen; however, the amount of pollen consumed per bee did not differ between the diets. Interestingly, a comparable range of protein content induces differences in ovary and HPG development (Pernal & Currie 2000), suggesting that IC in bees is not sensitive to the amount of protein. However, we cannot exclude the possibility that other pollen with poorer or richer protein content or challenges with pathogens would induce differences in IC.
Interestingly, polyfloral diets enhanced some immune functions compared with monofloral diets, in particular GOX activity, meaning that the diversity in floral resources confers bees with better in-hive antiseptic protection. This demonstrates that diet diversity is important and that a minimal nutrient diversity may not meet all honeybee needs. Because nitrogen content was equal between the mono- and polyfloral diets, additional properties might be present in the pollen mix. For example, essential amino acids from protein digested are required in specific proportions to complete their normal growth and development (de Groot 1953). Low pollen diversity might represent a major limiting factor for honeybee's development, but a polyfloral diet might increase the diversity and/or the proportion of specifics proteins and amino acids required for tissue development (fat body and HPGs). This assumption is supported by the study of Tasei & Aupinel (2008) showing that bumble-bee larvae fed with a polyfloral blend were heavier than larvae fed with monofloral diets of higher protein content.
If nutrition is a critical factor in immune response, then malnutrition is probably one of the causes of immunodeficiency in honeybee colonies. This work also emphasizes the importance of diet diversity and underscores the need for further studies to test different blends of proteins and identify protein ‘cocktails’ essential for developing normal immune function.
Acknowledgements
We thank Pollenergie for pollen supply, J. L. Brunet, D. Feuillet, F. Mondet, M. R. Schmid and B. Vaissière for help and advice and A. Brockmann, C. M. McDonnell and S. F. Pernal and three anonymous referees for comments that improved the manuscript. Funding was provided by HFSP (RGP0042/2007). C.A. was supported by an INRA young researcher position (INRA SPE department).
References
- Amdam G. V., Omholt S. W.2002The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 216, 209–228 (doi:10.1006/jtbi.2002.2545) [DOI] [PubMed] [Google Scholar]
- de Groot A. P.1953Protein and amino acid requirements of the honey bee (Apis mellifica L.). Physiol. Comp. Oecol. 3, 197–285 [Google Scholar]
- Evans J. D., et al. 2006Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect. Mol. Biol. 15, 645–656 (doi:10.1111/j.1365-2583.2006.00682.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 5983 1997Animal feeding stuffs. Determination of nitrogen content and calculation of crude protein content—Kjeldahl method. Geneva, Switzerland: International Organization for Standardization [Google Scholar]
- Lee K. P., Cory J. S., Wilson K., Raubenheimer D., Simpson S. J.2006Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. R. Soc. B 273, 823–829 (doi:10.1098/rspb.2005.3385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. P., Simpson S. J., Wilson K.2008Dietary protein-quality influences melanization and immune function in an insect. Funct. Ecol. 22, 1052–1061 (doi:10.1111/j.1365-2435.2008.01459.x) [Google Scholar]
- Li P., Yin Y. L., Li D., Kim S. W., Wu G.2007Amino acids and immune function. Br. J. Nutr. 98, 237–252 (doi:10.1017/S000711450769936X) [DOI] [PubMed] [Google Scholar]
- Lochmiller R. L., Deerenberg C.2000Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98 (doi:10.1034/j.1600-0706.2000.880110.x) [Google Scholar]
- Ohashi K., Natori S., Kubo T.1999Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Eur. J. Biochem. 265, 127–133 (doi:10.1046/j.1432-1327.1999.00696.x) [DOI] [PubMed] [Google Scholar]
- Pernal S. F., Currie R. W.2000Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31, 387–409 (doi:10.1051/apido:2000130) [Google Scholar]
- Rinderer T. E., Elliott K. D.1977Worker honey bee response to infection with Nosema apis. J. Econ. Entomol. 70, 431–433 [Google Scholar]
- Rinderer T. E., Rothenbuhler W. C., Gochnauer T. A.1974The influence of pollen on the susceptibility of honey-bee larvae to Bacillus larvae. J. Invertebr. Pathol. 23, 347–350 (doi:10.1016/0022-2011(74)90100-1) [DOI] [PubMed] [Google Scholar]
- Schmid M. R., Brockmann A., Perk C. W., Stanley D. W., Tautz J.2007Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect. Physiol. 54, 215–221 [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P.2005Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551 (doi:10.1146/annurev.ento.50.071803.130420) [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel R., Schmid-Hempel P.1998Colony performance and IC of a social insect, Bombus terrestris, in poor and variable environments. Funct. Ecol. 12, 22–30 (doi:10.1046/j.1365-2435.1998.00153.x) [Google Scholar]
- Sżymaś B., Jędruszuk A.2003The influence of different diets on haemocytes of adult worker honey bees, Apis mellifera. Apidologie 34, 97–102 (doi:10.1051/apido:2003012) [Google Scholar]
- Tasei J. N., Aupinel P.2008Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39, 397–409 (doi:10.1051/apido:2008017) [Google Scholar]
- van Engelsdorp D., Hayes J., Jr, Underwood R. M., Pettis J.2008A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 3, e4071 (doi:10.1371/journal.pone.0004071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. W. J., Subers M. H., Schepartz A. I.1963The identification of inhibine, antibacterial factor in honey, as hydrogen peroxide, and its origin in a honey glucose oxidase system. Biochem. Biophys. Acta 73, 57–70 (doi:10.1016/0006-3002(63)90359-7) [DOI] [PubMed] [Google Scholar]
- Wilson-Rich N., Dres S. T., Starks P. T.2008The ontogeny of immunity: development of innate immune strength in the honey bee (Apis mellifera). J. Insect Physiol. 54, 1392–1399 (doi:10.1016/j.jinsphys.2008.07.016) [DOI] [PubMed] [Google Scholar]