Abstract
During jumping or falling in humans and various other mammals, limb muscles are activated before landing, and the intensity and timing of this pre-landing activity are scaled to the expected impact. In this study, we test whether similarly tuned anticipatory muscle activity is present in hopping cane toads. Toads use their forelimbs for landing, and we analysed pre-landing electromyographic (EMG) timing and intensity in relation to hop distance for the m. coracoradialis and m. anconeus, which act antagonistically at the elbow, and are presumably important in stabilizing the forelimb during landing. In most cases, a significant, positive relationship between hop distance and pre-landing EMG intensity was found. Moreover, pre-landing activation timing of m. anconeus was tightly linked to when the forelimbs touched down at landing. Thus, like mammals, toads appear to gauge the timing and magnitude of their impending impact and activate elbow muscles accordingly. To our knowledge these data represent the first demonstration of tuned pre-landing muscle recruitment in anurans and raise questions about how important the visual, vestibular and/or proprioceptive systems are in mediating this response.
Keywords: forelimbs, toads, muscle, EMG, locomotion, jumping
1. Introduction
Nearly 40 years ago, electromyographic (EMG) recordings were used to show that humans activate ankle muscles well before their feet touch the ground when falling, jumping or stepping down (Melvill Jones & Watt 1971a,b). Such anticipatory activation is critical, because limb muscles need to be tensed prior to impact or the limb could collapse. Closer examination of these anticipatory activation patterns revealed that their intensities are scaled to the expected magnitude of the impending impact. For example, jumping down from a height of 1 m elicits more intense pre-landing EMG activity in ankle muscles than does jumping down from 20 cm (Santello & McDonagh 1998). Moreover, the initiation of pre-landing EMG activity is timed so as to maintain a fixed interval between muscle activation and landing, regardless of jump height (Santello 2005). Such alterations in the magnitude and timing of pre-landing limb muscle activity are also present in cats (Prochazka et al. 1977) and monkeys (Dyhre-Poulsen & Laursen 1984). While such modulation seems intuitively reasonable, its underlying neural control is probably complex (Santello 2005). Whether similarly tuned anticipation is present in limb muscles of animals other than mammals is not known, but anurans (frogs and toads), because of their proclivity for jumping, provide a good system for addressing this question.
In anurans the forelimbs are important during landing, as they are usually the first structures to touch down (Peters et al. 1996; Gillis & Biewener 2000; Nauwelaerts & Aerts 2006). Toads, in particular, exhibit coordinated landings in which the forelimbs decelerate and stabilize the body (electronic supplementary material, video). To test whether toads can modulate pre-landing forelimb muscle recruitment patterns during hopping, we quantify relationships between hop distance and pre-landing EMG intensity and timing in two elbow antagonists: m. coracoradialis and the lateral head of m. anconeus.
2. Material and methods
(a). Animals
Seven cane toads, Bufo marinus (mean mass = 139 ± 11 g, mean snout-vent length = 10.7 ± 0.2 cm), were obtained from a commercial supplier and housed in plastic containers at approximately 24°C with a 12-h light : 12-h dark cycle. They were fed mealworms and provided with fresh water several times per week.
(b). Electromyography experiments
Two muscles that act antagonistically at the elbow were studied, m. coracoradialis and the lateral head of m. anconeus. The m. coracoradialis originates from the sternum and its fibres converge laterally onto a long tendon that attaches to the proximal end of the radio-ulna; it is a major elbow flexor (Duellman & Trueb 1986). The m. anconeus has three heads. Its lateral head originates from the scapula and humerus with fibres converging on a large tendon that spans the elbow to insert proximally onto the radio-ulna. It is a major elbow extensor (Duellman & Trueb 1986). Given their antagonistic nature and co-activation during landing, these muscles are probably important in stabilizing the elbow.
To implant EMG electrodes, animals were anaesthetized by immersion in 1 l of a tricaine methanesulphonate solution (MS-222, 1.4 g l−1) for approximately 45 min. Following anaesthesia, skin incisions were made by scalpel over the chest and upper-arm to reveal the muscles. Fine-wire bipolar electrodes were made by twisting together two silver wires (0.1 mm diameter) whose tips were stripped of insulation and offset by approximately 0.5 mm. Wires were implanted into muscles using a 23-gauge needle, and were sutured to the surface of the muscle near the site of entry using 6.0 silk.
After implantation, skin incisions were sutured shut using 4.0 silk and the exiting electrodes were bonded together with model cement and sutured onto the animal's back. Opposite the animal, electrodes were soldered into connectors, and wires from those connectors carried signals to Grass P511 pre-amplifiers. Signals were amplified 1000× and filtered to eliminate 60 Hz noise and reduce frequency components lower than 100 Hz and higher than 3000 Hz. Signals were digitized at 5000 Hz using Axon Instruments' Digidata 1322A 16-bit A/D converter, and saved onto a computer.
After recovering from anaesthesia, toads were placed into a terrarium (89 × 43 × 43 cm) with a plywood base covered in felt. The terrarium was lit using 500 W lights and a Photron Fast-Cam was positioned to obtain a lateral view of the animal. Toads were placed at one end of the terrarium and encouraged to jump, using claps, voices or light taps. Jumps were recorded at 250 or 500 fps and a resolution of 1280 × 512 pixels. Distance scales were created using images of a meter stick at different positions in the tank varying in their distance from the camera. Video data were synchronized with EMG signals, using a 5-V trigger that stopped video recording and was saved to its own channel along with the muscle activity patterns. After recording 10–12 hops over a range of distances, toads were euthanized by overnight submersion in MS-222 (1.4 g l−1). Dissection was then used to confirm electrode placement.
(c). Data analysis
All videos were analysed to identify the onset of animal movement and the time of take-off (when the feet left the ground) and forelimb touch-down. In addition, hop distance was determined by measuring the horizontal distance travelled by a mark on the animal's trunk (either natural or made using Wite-Out) between the onset of movement and hindlimb touch-down. For every jump, the 50 ms prior to forelimb touch-down was identified on the EMG traces (from here on called pre-landing EMG activity), and the average amplitude of the rectified signal during this interval was calculated. To control for differences in signal intensities between individuals, these amplitudes were scaled to the maximum observed for a given muscle in a given individual. Linear regression (SPSS) was then used to examine relationships between hop distance and scaled pre-landing EMG intensity. In addition, the onset timing of EMG activity was identified for each hop and evaluated with respect to the start of the hop and forelimb touch-down.
3. Results
We analysed 78 hops ranging between 10 and 50 cm in seven animals. We collected data from m. coracoradialis in four animals (43 hops) and m. anconeus in four animals (46 hops); in one animal data from both muscles were collected simultaneously. Toads underwent a stereotyped set of movements during hopping (electronic supplementary material, video), and always landed on their forelimbs, which were protracted and extended prior to impact.
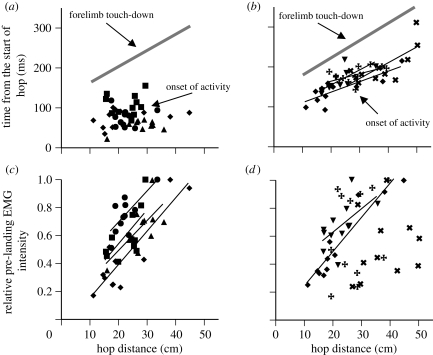
The m. coracoradialis was always activated before take-off, roughly 50–150 ms after the start of the hop (figure 1). Its onset timing relative to the start of the hop was not affected by the hop distance in any animal (figure 2a). Onset of m. anconeus was later than in m. coracoradialis, typically between 100 and 250 ms after the start of the hop. Its onset timing was affected by hop distance in all animals and paralleled that of forelimb touch-down (figure 2b).
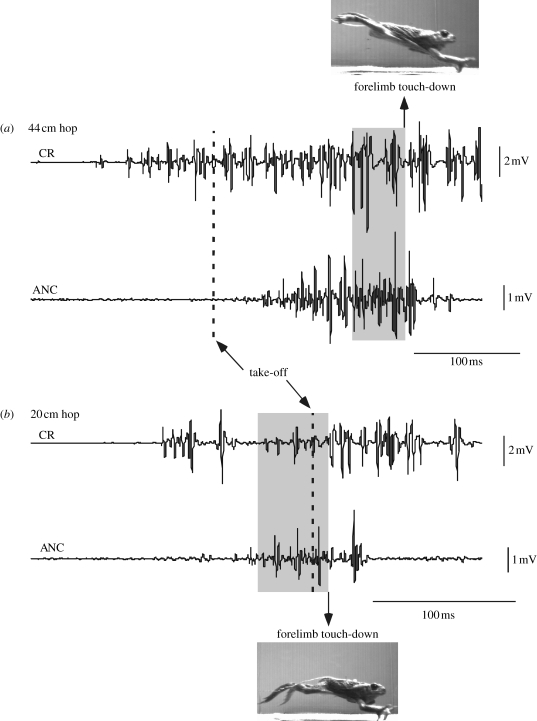
Figure 1.
Representative EMG traces for m. coracoradialis (CR) and m. anconeus (ANC) during a long, 44 cm hop (a) and short, 20 cm hop (b) in the same animal. The vertical dashed lines represent takeoff (when the hindlimbs leave the ground). The grey boxes highlight the 50 ms intervals used for assessing pre-landing EMG intensity (i.e. the right edge of the boxes represents forelimb touch-down). Note the higher average pre-landing signal intensities during the long hop.
Figure 2.
Plots of onset timing and relative EMG intensity in m. coracoradialis (a,c) and m. anconeus (b,d) as a function of hop distance. In (a,b) black symbols represent the onset timing of muscle activity and grey lines represent the best-fit line for the time of forelimb impact in these hops for comparison. In (c,d) intensities are scaled within each animal relative to the largest value observed. Different symbols represent different individuals and lines are drawn for any significant relationships (p < 0.05). Note that the onset of m. coracoradialis activity is not related to hop distance, but its pre-landing intensity increases significantly with hop distance. The onset of m. anconeus activity gets delayed with increasing hop distance paralleling the time of forelimb impact; its pre-landing intensity increases significantly with hop distance in two of four animals.
Both muscles were always active in the 50 ms prior to forelimb touch-down (figure 1). The intensity of this pre-landing activity was highly variable but often showed a striking relationship with hop distance. In m. coracoradialis a significant positive relationship was found between distance and pre-landing EMG intensity in all animals (figure 2c). In m. anconeus, significant relationships between distance and pre-landing intensity were found in two animals (figure 2d). In the other two animals, despite non-significant relationships, pre-landing m. anconeus intensities were much greater during the longest hop than the shortest hop.
4. Discussion
Our work demonstrates tuned, pre-landing EMG activity in antagonistic elbow muscles in hopping toads. In long hops, these muscles exhibit more intense activity just before landing than during short hops, suggesting that toads prepare for impact in a distance-dependent manner. Longer hops lead to greater impact forces in jumping anurans (Nauwelaerts & Aerts 2006), and different levels of forelimb muscle recruitment are probably important for managing different degrees of impact. In addition, onset timing in m. anconeus changes with hop distance in a manner that parallels the timing of forelimb touch-down (figure 2b). This strongly suggests that the onset of m. anconeus is modulated so as to create a fixed interval (approx. 90 ms in these animals) between muscle activation and landing, as in various limb muscles in humans and other mammals (Santello 2005). The onset of m. anconeus activity occurs before take-off in hops shorter than approximately 30 cm, indicating that this modulation or tuning can take place even before the animal's feet leave the ground.
Unlike in m. anconeus, the onset of m. coracoradialis activity always occurs before take-off and at a roughly fixed interval from the start of the hop (figure 2a). Its onset closely corresponds to the time when the forelimbs leave the ground, shortly after which they are pulled forward to prepare for landing (electronic supplementary material, video). The onset timing of m. coracoradialis suggests its importance in these early forelimb movements, while its intensity modulation just before landing suggests that it is also important in forelimb stabilization on impact.
Previous work on pre-landing activation patterns in various mammals (e.g. humans, monkeys and cats) has shown prescient modulation of recruitment timing and intensity in limb muscles important for resisting impact during jumping or falling (Prochazka et al. 1977; Dyhre-Poulsen & Laursen 1984; Santello & McDonagh 1998). However, to our knowledge our work represents the first direct evidence of this phenomenon in an anuran, which, like jumping mammals, must modulate limb muscle stiffness to absorb the impact of hops covering a range of distances, or suffer the consequences (e.g. poorly coordinated transitions between hops or even injury). Here we show that pre-landing recruitment intensity (in m. coracoradialis and to a lesser extent in m. anconeus) and onset timing (in m. anconeus but not m. coracoradialis) are tuned to hop distance in cane toads. The extent to which visual, vestibular and/or proprioceptive feedback are important for these anticipatory responses remains to be tested (electronic supplementary material), but all three mechanisms have been implicated in mammals (McKinley & Smith 1983; Avela et al. 1996; Santello et al. 2001). How widespread this ability is among other anurans (e.g. those that more commonly jump into water than on land) is currently unknown, but cane toads in some sense, seem to ‘know’ how far they are hopping, even before they take off, and prepare for landing accordingly.
Acknowledgements
This work was approved by Mount Holyoke College's IACUC Committee. Funding for this work came from HHMI and Mount Holyoke's Department of Biological Sciences.
We are grateful to Andrea Laizer, Jo Niepoort, Toshikah Wheatley and Rebekah Wieland for assistance with experiments and to Thomas Liimatainen and Len McEachern for constructing aspects of the hopping arena. Len also helped come up with the title. Two anonymous reviewers helped improve the paper substantially.
References
- Avela J., Santos P. M., Komi P. V.1996Effects of differently induced stretch loads on neuromuscular control in drop jump exercise. Eur. J. Appl. Physiol. 72, 553–562 (doi:10.1007/BF00242290) [DOI] [PubMed] [Google Scholar]
- Duellman W. E., Trueb L.1986Biology of amphibians. New York, NY: McGraw-Hill [Google Scholar]
- Dyhre-Poulsen P., Laursen A. M.1984Programmed electromyographic activity and negative incremental muscle stiffness in monkeys jumping downward. J. Physiol. 350, 121–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis G. B., Biewener A. A.2000Hindlimb extensor muscle function during jumping and swimming in the toad (Bufo marinus). J. Exp. Biol. 203, 3547–3563 [DOI] [PubMed] [Google Scholar]
- McKinley P., Smith J. L.1983Visual and vestibular contributions to prelanding EMG during jump-down in cats. Exp. Brain Res. 52, 439–448 (doi:10.1007/BF00238037) [DOI] [PubMed] [Google Scholar]
- Melvill Jones G., Watt D. G. D.1971aMuscular control of landing from unexpected falls in man. J. Physiol. 219, 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvill Jones G., Watt D. G. D.1971bObservations on the control of stepping and hopping movements in man. J. Physiol. 219, 709–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauwelaerts S., Aerts P.2006Take-off and landing forces in jumping frogs. J. Exp. Biol. 209, 66–77 (doi:10.1242/jeb.01969) [DOI] [PubMed] [Google Scholar]
- Peters S. E., Kamel L. T., Bashor D. P.1996Hopping and swimming in the leopard frog, Rana pipiens: I. Step cycles and kinematics. J. Morph. 230, 1–16 (doi:10.1002/(SICI)1097-4687(199610)230:1<1::AID-JMOR1>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- Prochazka A., Schofield P., Westerman R. A., Ziccone S. P.1977Reflexes in cat ankle muscles after landing from falls. J. Physiol. 272, 705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M.2005Review of motor control mechanisms underlying impact absorption from falls. Gait Posture 21, 85–94 [DOI] [PubMed] [Google Scholar]
- Santello M., McDonagh M. J. N.1998The control of timing and amplitude of EMG activity in landing movements in humans. Exp. Physiol. 83, 857–874 [DOI] [PubMed] [Google Scholar]
- Santello M., McDonagh M. J. N., Challis J. H.2001Visual and non-visual control of landing movements in humans. J. Physiol. 537, 313–327 (doi:10.1111/j.1469-7793.2001.0313k.x) [DOI] [PMC free article] [PubMed] [Google Scholar]