Abstract
Double cones (DCs) are the most common cone types in fish, reptiles and birds. It has been suggested that DCs are used for achromatic tasks such as luminance, motion and polarization vision. Here we show that a reef fish Rhinecanthus aculeatus can discriminate colours on the basis of the difference between the signals of individual members of DCs. This is the first direct evidence that individual members of DCs are used in colour vision as independent spectral channels.
Keywords: colour vision, double cones, fish
1. Introduction
Double cones (DC) are two cone photoreceptor cells that are fused together and may be optically and/or electrically coupled (Marchiafava 1985; Smith et al. 1985). They are present in the eyes of most vertebrate animals, but are conspicuously absent from retina of placental mammals, elasmobranches and catfish (Walls 1942; Ali & Anctil 1976; Ebrey & Koutalos 2001). Although DC are the most common cone photoreceptors in fish, reptiles and birds, the function of DC is largely unknown. Here, we use a behavioural method to reveal the role of DC in colour vision in a reef fish Rhinecanthus aculeatus (Linnaeus 1758). Rhinecanthus aculeatus belongs to the family of triggerfish (Balistidae, Order Tetraodontiformes) and known as Blackbar triggerfish. Recent behavioural observations have demonstrated that this fish is capable of colour vision, but the role of cone types in colour vision has not been determined (Marshall et al. 2004). Blackbar triggerfish possesses one type of single cone (SC), with visual pigment peaking at 413 nm (S for short-wavelength), and a DC with different visual pigments in each member, one peaking at 480 nm (M for middle-wavelength) and the second peaking at 530 nm (L for long-wavelength) (Marshall et al. 2004).
For a number of animal groups including fish, it has been suggested that, while SCs certainly contribute to colour vision, DCs are likely to be involved in achromatic tasks, such as luminance, motion and polarization vision (Boehlert 1978; Lythgoe 1979; Cameron & Pugh 1991; McFarland 1991; Hawryshyn et al. 2003). Indeed, analysis of colour thresholds in some birds suggests that DC do not participate in colour vision (Maier & Bowmaker 1993; Vorobyev & Osorio 1998; Goldsmith & Butler 2003), and it has been demonstrated that motion vision in goldfish and chickens is mediated by the long wavelength-sensitive visual pigment housed in DCs (Schaerer & Neumeyer 1996; Campenhausen & Kirschfeld 1998). Summation of the signals of individual members of DC would be beneficial for luminance vision, as this would broaden the spectral sensitivity and improve the ability of fish to detect targets contrasting to background in different parts of the spectrum (Lythgoe 1979; Lythgoe & Partridge 1989; Marshall & Vorobyev 2003; Marshall et al. 2003). Numerous gap junctions between members of fish DCs form an anatomical basis for such summation (Marchiafava 1985). Therefore, it has been hypothesized that the signals of the two members of DC are summed in the retina and the signals of separate members of DC are not conveyed to the brain (Marshall & Vorobyev 2003; Marshall et al. 2003). Hence, a fish with three types of visual pigments, one housed in single cones and two pigments housed in two members of DC may be predicted to be dichromatic (Lythgoe 1979; Marshall & Vorobyev 2003; Marshall et al. 2003). Several modelling papers on colour perception by reef fishes were based on the assumption that reef fishes are effectively dichromatic (Chiao et al. 2000; Marshall & Vorobyev 2003; Marshall et al. 2006).
A behavioural outcome of the DC summation hypothesis is that a fish should not be able to discriminate between colour stimuli that differ in signals from individual DC members, given that such stimuli provide similar summed DC signal and SC signals. Here we test this prediction by training a fish to discriminate stimuli with adjusted spectral properties.
2. Material and methods
(a). Stimuli and their spectral properties
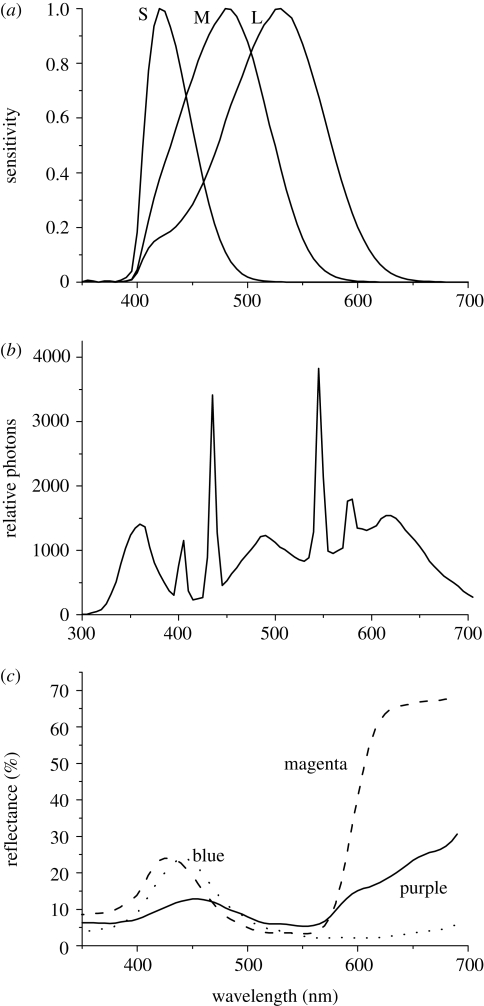
Coloured circular targets (5 cm in diameter) were printed on white paper using an Epson Stylus Photo 1290 colour printer and, in order to water-proof them, were laminated. Reflectance spectra of the laminated stimuli and the aquarium illumination were measured using an Ocean Optics USB 2000 spectrometer (figure 1b,c). The spectral properties of stimuli were adjusted by changing the red, green and blue (RGB) values of colours in Photoshop (Giurfa & Vorobyev 1997; Giurfa et al. 1997). Spectral sensitivities of S, M and L cones were modelled by combining the visual pigment absorption curve with the ocular media spectrum (Siebeck & Marshall 2001; Marshall et al. 2004; figure 1a). Cone signals, qi, were calculated as normalized quantum catches:
| 2.1 |
where i denotes the spectral type cone (i = S, M, L), λ the wavelength, Ri(λ) the spectral sensitivity of a cone i, S(λ) the reflectance spectrum of a stimulus, I(λ) the illumination spectrum, ki = 1/∫I(λ)Ri(λ)dλ is the scaling factor whose value is chosen so that the quantum catch corresponding to an ideal reflector is equal to 1 (Kelber et al. 2003). Such scaling describes adaptation of cones to ambient illumination. Because members of DCs in R. aculeatus are of equal size (Marshall et al. 2004), the combined DC signal can be modelled as:
| 2.2 |
Figure 1.
(a) Spectral sensitivity of the S, M and L cones of a triggerfish, Rhinecanthus aculeatus; (b) aquarium illumination spectrum in relative photons; (c) reflectance spectra of ‘magenta’, ‘blue’ and ‘purple’ stimuli.
Three spectral types of stimuli have been chosen for experiments, which we label according to their colour appearance to our eyes as ‘magenta’, ‘purple’ and ‘blue’. The reflectance spectra of the stimuli are given in figure 1, and the quantum catches are given in table 1.
Table 1.
Cone signals corresponding to magenta, blue and purple stimuli. Cone signals of S, M and L cones were calculated as normalized quantum catches using equation (2.1) and the signal of DC was calculated as the mean signal of L and M cones (equation 2.2).
| S | M | L | DC | |
|---|---|---|---|---|
| magenta | 0.203 | 0.105 | 0.091 | 0.098 |
| blue | 0.200 | 0.122 | 0.062 | 0.092 |
| purple | 0.112 | 0.094 | 0.080 | 0.087 |
(b). Training and testing
The method is modified from Siebeck et al. (2009). Rhinecanthus aculeatus were kept and tested in identical individual sea water aquaria (permit 55 605, obtained under the Fisheries Act 1994). Training was identical to testing. Two stimuli, one having a ‘rewarded’ colour and one having an alternative colour, were attached to grey plastic plates (10 cm width, 24 cm depth) and inserted side by side at the front end of aquarium. To avoid the influence of cues not related to the colour of stimuli, a reward (fish flake HBH Marine Flake Frenzy and/or crustacean paste) was given at the rear end of aquarium after fish pecked beneath a ‘rewarded’ stimulus at the front end of the aquarium. We started to record choices after fish learned to peck on stimuli at the front end of aquarium and collect the reward to the rear end of the aquarium. The position of stimuli was changed in a pseudorandom manner, but the stimuli never appeared on the same side more than two times in a row (Giurfa et al. 1997). Between tests, stimuli were removed from aquaria. Choices were collected in sets of 30 or less per day, and the tests were interrupted if fish did not swim towards the stimuli.
3. Results and discussion
Three spectral types of stimuli—‘magenta’, ‘blue’ and ‘purple’—were presented to fish in pairs. The first pair of stimuli—‘magenta’ and ‘blue’—differed in the signals to individual DC members, but provided similar signals to SC and to the combined DC (mean signal of the DC members; table 1). Therefore, according to the DC summation hypothesis, a fish would not be able to discriminate between ‘magenta’ and ‘blue’ stimuli. The second pair—‘magenta’ and ‘purple’—provided similar signals to both members of DC, but different signals to SC, and hence they could be discriminated on the basis of the difference between signals of SC alone.
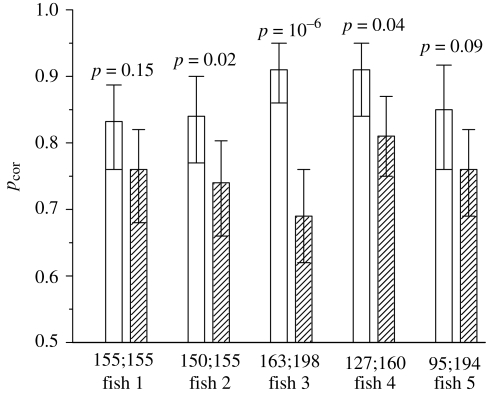
Fish was rewarded after pecking beneath ‘magenta’ stimulus. The alternative was ‘blue’ stimulus in the first series of experiments, and ‘purple’ stimulus in the second series. The probability of correct choice, pcor, was calculated as the ratio of the number of trials where ‘magenta’ stimulus was chosen to the total number of trials. Each individual fish could easily discriminate ‘magenta’ from ‘blue’ in the first series of experiments, and ‘magenta’ from ‘purple’ in the second series of experiments, with pcor varying from 0.68 to 0.91 (figure 2). All five fish performed better in discriminating ‘magenta’ from ‘blue’ than ‘magenta’ from ‘purple’ indicating that individual members of DCs provided a more salient signal than the SCs (p = 0.03, n = 5; binomial test). Therefore, we can exclude the possibility that ‘magenta’ was discriminated from ‘blue’ on the basis of the SC signals, because the difference in the SC signals between ‘magenta’ and ‘blue’ stimuli was lower than the difference in the SC signals between ‘magenta’ and ‘purple’ stimuli (table 1). We can also exclude the possibility that ‘magenta’ was discriminated from ‘blue’ on the basis of the combined DC signal, because the difference in the combined DC signals between ‘magenta’ and ‘blue’ stimuli was lower than the difference in the combined DC signals between ‘magenta’ and ‘purple’ stimuli (table 1). Therefore, we conclude that, in Blackbar triggerfish, the signals of individual members of DC (L and M cones) are used as independent spectral channels.
Figure 2.
Proportion of correct choices (pcor) in discriminating ‘magenta’ stimulus from ‘blue’ stimulus (open bars) and ‘magenta’ stimulus from ‘purple’ stimulus (dashed bars). Error bars indicate 95% confidence intervals (binomial test). Numbers above the bars indicate the probability of ‘magenta’–‘blue’ pair and ‘magenta’–‘purple’ pair being discriminated equally easily by each individual fish (Fisher's exact test). Numbers below the bars indicate the number of trials in each experiment.
While the DC involvement in fish colour vision has been implied previously (Neumeyer 1986; McFarland 1991; Hughes et al. 1998), this is the first direct evidence that individual members of DCs are used in colour vision as independent spectral channels. Our results indicate that at least some fishes with one type of visual pigment housed in single cones and two pigments housed in two members of DC are trichromatic, rather then dichromatic.
Acknowledgements
We are grateful to Alan Goldizen and Kylie Jennings for the help in maintaining aquaria. The work was supported by the Australian Research Council.
References
- Ali M. A., Anctil M.1976Retinas of fishes: an atlas. New York, NY: Springer [Google Scholar]
- Boehlert G. W.1978Intraspecific evidence for function of single and double cones in teleost retina. Science 202, 309–311 (doi:10.1126/science.694534) [DOI] [PubMed] [Google Scholar]
- Cameron D. A., Pugh E. N.1991Double cones as a basis for a new type of polarization vision in vertebrates. Nature 353, 161–164 (doi:10.1038/353161a0) [DOI] [PubMed] [Google Scholar]
- Campenhausen M. V., Kirschfeld K.1998Spectral sensitivity of the accessory optic system of the pigeon. J. Comp. Physiol. A 183, 1–6 (doi:10.1007/s003590050229) [Google Scholar]
- Chiao C., Vorobyev M., Cronin T. W., Osorio D.2000Spectral tuning of dichromats to natural scenes. Vision Res. 40, 3257–3271 (doi:10.1016/S0042-6989(00)00156-5) [DOI] [PubMed] [Google Scholar]
- Ebrey T., Koutalos Y.2001Vertebrate photoreceptors. Prog. Retinal Eye Res. 20, 49–94 (doi:10.1016/S1350-9462(00)00014-8) [DOI] [PubMed] [Google Scholar]
- Giurfa M., Vorobyev M.1997The detection and recognition of color stimuli by honeybees: performance and mechanisms. Isr. J. Plant Sci. 45, 129–140 [Google Scholar]
- Giurfa M., Vorobyev M., Brandt R., Posner B., Menzel R.1997Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J. Comp. Physiol. A 180, 235–243 (doi:10.1007/s003590050044) [Google Scholar]
- Goldsmith T. H., Butler B. K.2003The roles of receptor noise and cone oil droplets in the photopic spectral sensitivity of the budgerigar, Melopsittacus undulatus. J. Comp. Physiol. A 189, 135–142 [DOI] [PubMed] [Google Scholar]
- Hawryshyn C. W., Moyer H. D., Allison W. T., Haimberger T. J., McFarland W. N.2003Multidimensional polarization sensitivity in damselfishes. J. Comp. Physiol. A 189, 213–220 [DOI] [PubMed] [Google Scholar]
- Hughes A., Saszik S., Bilotta J., DeMarco P. J., Patterson W. F.1998Cone contributions to the photopic spectral sensitivity of the zebrafish ERG. Vis. Neurosci. 15, 1029–1037 (doi:10.1017/S095252389815602X) [DOI] [PubMed] [Google Scholar]
- Kelber A., Vorobyev M., Osorio D.2003Animal colour vision: behavioural tests and physiological concepts. Biol. Rev. 78, 81–118 (doi:10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- Lythgoe J. N.1979The ecology of vision. Oxford, UK: Clarendon Press [Google Scholar]
- Lythgoe J. N., Partridge J. C.1989Visual pigments and the acquisition of visual information. J. Exp. Biol. 146, 1–20 [DOI] [PubMed] [Google Scholar]
- Maier E. J., Bowmaker J. K.1993Color-vision in the passeriform bird, Leiothrix lutea: correlation of visual pigment absorbency and oil droplet transmission with spectral sensitivity. J. Comp. Physiol. A 172, 295–301 (doi:10.1007/BF00216611) [Google Scholar]
- Marchiafava P. L.1985Cell coupling in double cones of the fish retina. Proc. R. Soc. Lond. B 226, 211–215 (doi:10.1098/rspb.1985.0091) [Google Scholar]
- Marshall N. J., Vorobyev M.2003The design of color signals and color vision in fishes. In Sensory processing in aquatic environments, pp. 194–222 New York, NY: Springer [Google Scholar]
- Marshall N. J., Jennings K., McFarland W. N., Loew E. R., Losey G. S.2003Visual biology of Hawaiian coral reef fishes. II. Colors of Hawaiian coral reef fish. Copeia 2003, 455–466 (doi:10.1643/01-055) [Google Scholar]
- Marshall J., Jennings K., Goldizen A., Vorobyev M.2004Colour vision in reef fish. Vision down under. Brisbane, Australia: Fraser Island [Google Scholar]
- Marshall N. J., Vorobyev M., Siebeck U. E.2006What does a reef fish see when it sees a reef fish? Eating ‘Nemo’©. In Communication in fishes (eds Laddich F., Collin S. P., Moller P., Kapoor B. G.), pp. 393–422 Plymouth, UK: Science Publishers Inc. [Google Scholar]
- McFarland W. N.1991The visual world of coral reef fishes. In The ecology of fishes on coral reefs (ed. Sale P. F.), pp. 16–38 San Diego, CA: Academic Press, Inc [Google Scholar]
- Neumeyer C.1986Wavelength discrimination in the goldfish. J. Comp. Physiol. A 158, 203–213 (doi:10.1007/BF01338563) [Google Scholar]
- Schaerer S., Neumeyer C.1996Motion detection in goldfish investigated with the optomotor response is ‘color blind’. Vision Res. 36, 4025–4034 (doi:10.1016/S0042-6989(96)00149-6) [DOI] [PubMed] [Google Scholar]
- Siebeck U. E., Marshall N. J.2001Ocular media transmission of coral reef fish: can coral reef fish see ultraviolet light? Vision Res. 41, 133–149 (doi:10.1016/S0042-6989(00)00240-6) [DOI] [PubMed] [Google Scholar]
- Siebeck U. E., Litherland L., Wallis G. M.2009Shape learning and discrimination in reef fish. J. Exp. Biol. 212, 2112–2118 [DOI] [PubMed] [Google Scholar]
- Smith R. L., Nishimura Y., Raviola G.1985Interreceptor junction in the double cone of the chicken retina. J. Submicrosc. Cytol. Pathol. 17, 183–186 [PubMed] [Google Scholar]
- Vorobyev M., Osorio D.1998Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls G. L.1942The vertebrate eye and its adaptive radiation. Cranbrook Inst. Sc. Bull. 19, 785 pp [Google Scholar]