Abstract
The functional characteristics and cellular localization of the γaminobutyric acid (GABA) ρ1 receptor and its nonfunctional isoform ρ1Δ450 were investigated by expressing them as gene fusions with the enhanced version of the green fluorescent protein (GFP). Oocytes injected with ρ1-GFP had receptors that gated chloride channels when activated by GABA. The functional characteristics of these receptors were the same as for those of wild-type ρ1 receptors. Fluorescence, because of the chimeric receptors expressed, was over the whole oocyte but was more intense near the cell surface and more abundant in the animal hemisphere. Similar to the wild type, ρ1Δ450-GFP did not lead to the expression of functional GABA receptors, and injected oocytes failed to generate currents even after exposure to high concentrations of GABA. Nonetheless, the fluorescence displayed by oocytes expressing ρ1Δ450-GFP was distributed similarly to that of ρ1-GFP. Mammalian cells transfected with the ρ1-GFP or ρ1Δ450-GFP constructs showed mostly intracellularly distributed fluorescence in confocal microscope images. A sparse localization of fluorescence was observed in the plasma membrane regardless of the cell line used. We conclude that ρ1Δ450 is expressed and transported close to, and perhaps incorporated into, the plasma membrane. Thus, ρ1- and ρ1Δ450-GFP fusions provide a powerful tool to visualize the traffic of GABA type C receptors.
Keywords: GABAC, Xenopus oocyte
Fast inhibitory synaptic transmission in the vertebrate brain occurs mainly by the action of the amino acid γ-aminobutyrate (GABA) on at least three different classes of membrane receptors: GABA type A (GABAA), GABA type B (GABAB), and GABA type C (GABAC). The GABAC receptors were identified functionally after expressing mammalian retina mRNA in Xenopus oocytes (1). Those oocytes responded to GABA with a current made up of two components caused by activation of GABAA and GABAC receptors, both of which gated chloride channels. The latter component was distinguished clearly by, among other things, a very slow rate of desensitization, resistance to the GABAA receptor antagonist bicuculline, and insensitivity to the GABAB agonist baclofen (1–5). Subsequently, the highly selective GABAC antagonist (1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic (TPMPA) was designed, synthesized, and proven to be effective for distinguishing among the three classes of GABA receptors (6–7). Thus, the new class of GABAC receptors was defined by their functional and pharmacological properties and their high abundance in the vertebrate retina.
So far, three cDNAs encoding GABAC subunits have been cloned: ρ1–ρ3. The human ρ1 and ρ2 genes are located in tandem in chromosome 6, whereas the ρ3 gene is located in chromosome 3 (8–9). When those subunits are expressed heterologously in frog oocytes or mammalian cultured cells, they form functional homomeric receptors that exhibit the typical electrophysiological and pharmacological profile of retinal GABAC receptors (8, 10–12), suggesting that those subunits and/or their combination form the native GABAC receptors of retinal cells.
It is known that the human ρ1 gene produces at least three isoforms by alternative splicing: ρ1, ρ1Δ51, and ρ1Δ450 (13). The ρ1 and ρ1Δ51 isoforms express functional homomeric receptors in oocytes whereas ρ1Δ450 does not (13–14). ρ1Δ450 lacks 150 residues in the extracellular N-terminal domain, and no evidence is known of either its translational efficacy or its intracellular distribution. Although ρ1Δ450 conserves domains necessary for the proper function of the receptor, such as the signal peptide and the channel-forming second transmembrane region, the deletion removes critical regions for proper cell sorting, agonist binding, and assembly of the receptor (15–16). Production of nonfunctional subunits is not an event exclusive to GABAC receptors. For example, the α6 gene of the GABAA receptor produces two isoforms: one that assembles into fully functional heteromeric receptors and one that inhibits formation of the receptor (17). The detailed mechanisms and implications of this interaction are still unknown. Therefore, it was of interest to determine whether the gene carrying the Δ450 deletion is not functional because: (i) its RNA is unstable or fails to be translated, (ii) the receptor is expressed but fails to be incorporated into the plasma membrane, or (iii) the receptor is nonfunctional even if incorporated into the membrane.
To begin to address these questions, we constructed gene fusions between the enhanced version of the green fluorescent protein (GFP) and either ρ1 or ρ1Δ450, and the properties of the receptors expressed by these chimeras were studied in both frog oocytes and cultured cells.
Materials and Methods
Plasmids and DNA Manipulations.
A ScaI site was introduced at the end of the coding sequence of ρ1 by using site-directed mutagenesis (mutagenic primer 5′-TACAAGCATAGTACTGAAAATAGACCAGTA-3′), thus eliminating the translational stop codon and changing the last serine codon (TCC 254 TCA). This method allowed subcloning of the ρ1 cDNA (BamHI–ScaI) into the BglII and SmaI sites of plasmid pEGFP-N2 (CLONTECH) to produce the plasmid pρ1-GFP. ρ1Δ450 was introduced by replacing the SmaI–HindIII fragment of pρ1-GFP by the EcoRV–HindIII fragment from the plasmid pAV113. The resulting plasmids (1 μg/μl) were injected (13–15 nl) into the nucleus of Xenopus oocytes. After 2–7 days, expression was assessed by electrophysiological methods and laser confocal microscopy.
To produce cRNAs, the fragments encoding the gene fusions had to be subcloned into a different vector. Therefore, ρ1-GFP and ρ1Δ450-GFP were inserted as SmaI–NotI fragments between the EcoRV and NotI sites of the expression plasmid pcDNA3 (Invitrogen). The resulting recombinants were linearized with NotI and capped cRNAs were synthesized by using the T7 Riboprobe kit (Promega). For expression, 50 nl of cRNA (1 μg/μl) was injected into each oocyte.
Confocal Imaging.
Images were obtained by using a confocal microscope, built by I. Parker and N. Callamaras (University of California, Irvine) using an Olympus inverted microscope. The system has been modified to produce axial scanning (18–19).
Electrophysiological Recordings in Xenopus Oocytes.
Procedures for expressing ρ1 receptors in Xenopus oocytes and recording were as described (14, 20–21).
Expression in HEK293 and 3T3 Cells.
The mouse fibroblast cell line 3T3 and human HEK293 cells were grown and transfected according to the lipofectamine (GIBCO/BRL) method.
Results
ρ1-GFP Expresses Functional GABA Receptors.
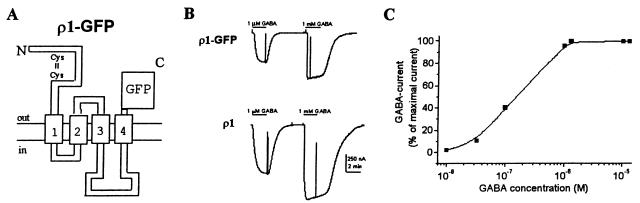
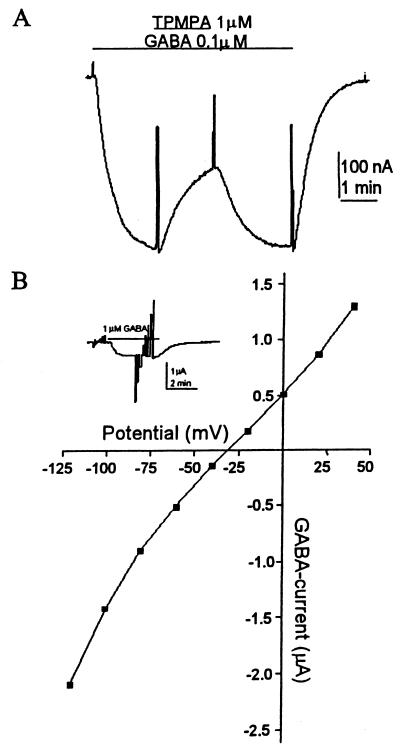
From its structure, ρ1-GFP would be predicted to form a chimeric receptor in which the extracellular C terminus is tagged with the fluorescent protein. Therefore, the N terminus and the four transmembrane segments including the second one, which forms the ion channel, would be physically distant from the GFP (Fig. 1A). Fig. 1B shows GABA currents elicited by oocytes injected with either the wild-type (wt) ρ1 or ρ1-GFP. The GABA-dose-response relation for ρ1-GFP receptors yielded an EC50 of 0.7 μM (Fig. 1C), which is more like that of the ρ1Δ51 isoform (EC50 = 0.57 μM) than that of the ρ1 receptors (EC50 = 1.2 μM). Essentially, the electrophysiological and pharmacological properties of the chimeric receptors were the same as those of the wt ρ1. For example, the receptors desensitized very little even after long exposures to high concentrations of GABA, and they were not blocked by bicuculline (up to 1 mM). Moreover, the GABAC-antagonist TPMPA reduced the GABA current with an IC50 of 1.6 μM (Fig. 2A), which is the same as that of the wt receptor (6–7). In addition, the reversal potential of GABA currents generated by ρ1-GFP receptors was the same as those for the wt ρ1, both of which were close to −30 mV (Fig. 2B) which corresponds to the chloride equilibrium potential (22).
Figure 1.
Functional expression of ρ1-GFP. (A) Diagram of the receptor fusion ρ1-GFP. (B) Comparison of GABA currents elicited by oocytes injected with either ρ1-GFP or ρ1. For this and other figures, the membrane was held at −60 mV, and GABA was applied during the time indicated by the bars above the records as well as by 20-mV depolarizing pulses to monitor membrane conductance. (C) GABA-dose-response curve of an oocyte injected with ρ1-GFP (EC50 = 0.7 μM).
Figure 2.
Characterization of ρ1-GFP. (A) The chimeric receptor is blocked efficiently by TPMPA. GABA (0–3 μm) and TPMPA were applied while holding the oocyte at −60 mV. (B) The current elicited by GABA (1 μM) and voltage steps from −120 to +40 mV (20-mV steps) were applied to an oocyte expressing ρ1-GFP (Inset). The current–voltage relation gave an equilibrium potential close to −30 mV, similar to the chloride equilibrium potential in oocytes.
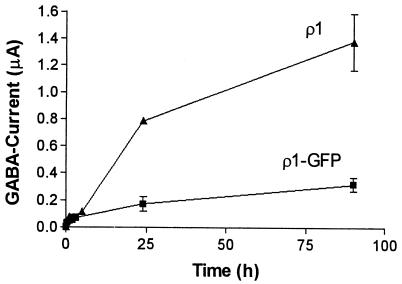
Although the functional characteristics of the ρ1-GFP fusion receptors were essentially the same as those for the wt-ρ1 receptor, some clear differences were observed. First, it took longer for ρ1-GFP than for ρ1 to express functional receptors (Fig. 3). Second, after cDNA injections, the amplitude of the GABA currents as well as the proportion of oocytes expressing functional ρ1-GFP receptors (10–30%) were also smaller than those of oocytes injected with the wt ρ1 (90–100%). In contrast, in oocytes injected with cRNAs the ρ1-GFP GABA currents were again smaller than the ρ1 GABA currents; but the number of oocytes expressing functional receptors was the same for both (90–100%). In some experiments, we compared the time course of appearance of GABA currents in oocytes from the same donor injected with cRNAs synthesized from either construct. As soon as 1 h after injection, the GABA currents averaged 47 nA for ρ1-GFP and 77.6 nA for ρ1 oocytes (1 μM GABA, n = 3). However, although ρ1-GFP-injected oocytes reached a plateau of expression 24–48 h after injection, in ρ1-injected oocytes the magnitude of GABA currents continued to increase (Fig. 3). It is worth noting that fluorescence was not clearly evident above background fluorescence in oocytes producing GABA currents smaller than ≈300 nA (1 μM GABA); and note also that only a small proportion of oocytes injected with ρ1-GFP cRNA reached sufficiently high levels of expression for detailed fluorescent imaging.
Figure 3.
Time course of appearance of GABA currents. Each point shows the mean + SEM of 2–5 oocytes.
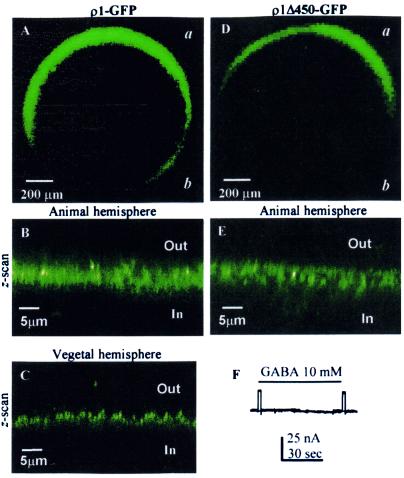
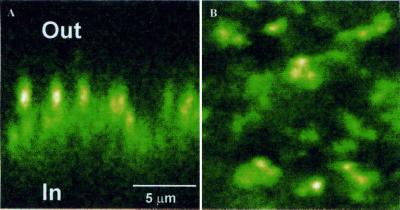
Oocytes injected with ρ1-GFP, which elicited large GABA currents of 700 nA or more (1 μM GABA), showed strong fluorescence, and only low background fluorescence was observed in wt-ρ1 or noninjected oocytes. Fig. 4A illustrates the distribution of fluorescence in the oocyte that generated the GABA currents shown in Fig. 1B. Although the fluorescence was distributed around the entire oocyte, more often the fluorescence was clear only in the animal hemisphere and was faint or undetectable near the vegetal hemisphere, suggesting a clear polarization of receptor distribution. A z-scan near the animal or vegetal poles of the same oocyte showed that the fluorescence was concentrated at and just below (3–5 μm) the oocyte's surface (Fig. 4 B and C).
Figure 4.
Confocal images of frog oocytes expressing ρ1-GFP and ρ1Δ450-GFP. (A) Overall image of an oocyte expressing ρ1-GFP. (B) Oocyte surface near the animal pole. (C) Part of the vegetal hemisphere. (D) Overall image of an oocyte expressing ρ1Δ450. (D) An axial image near the animal pole. (E) Lack of response to GABA. D and E were taken from the same oocyte. “a” and “b” indicate the animal and vegetal hemispheres, respectively.
ρ1Δ450-GFP Produces Fluorescent Proteins but Not Functional Receptors.
As already stated, the wt ρ1Δ450 fails to express GABA-gated currents (13). Oocytes injected with the ρ1Δ450-GFP construct again were unresponsive to GABA (Fig. 4F); but it was clear that the RNA was translated efficiently because the oocytes displayed fluorescence (Fig. 4 D–E). The distribution of this fluorescence was very similar to that observed in oocytes expressing functional ρ1-GFP receptors (compare to Fig. 4 A–C); that is, the animal hemisphere was more fluorescent than the vegetal hemisphere, and the fluorescence was concentrated near the oocyte surface (Fig. 4E). Thus, the translation and distribution of ρ1Δ450-GFP is clear, and the absence of functional receptors therefore is not the result of untranslatable cRNA.
ρ1-GFP and ρ1Δ450-Fluorescence Clusters.
The fluorescence distribution in xy-scans near the animal poles of oocytes expressing either ρ1-GFP or ρ1Δ450-GFP evidenced that both constructs lead to a “patchy” distribution of fluorescence. Higher magnification images of an oocyte injected with ρ1-GFP, which elicited a GABA current of 756 nA (1 μM GABA), are shown in Fig. 5. The strong fluorescence patches in the z- and xy-scans show clearly that the distribution of the receptors is clustered over the oocyte surface.
Figure 5.
Detail within the animal hemisphere of an oocyte expressing ρ1-GFP. Fluorescence is concentrated in the oocyte's filopodia and probably in endoplasmic reticulum compartments. (A) Z-scan. (B) Surface view.
Expression of ρ1-GFP and ρ1Δ450-GFP in Cultured Cells.
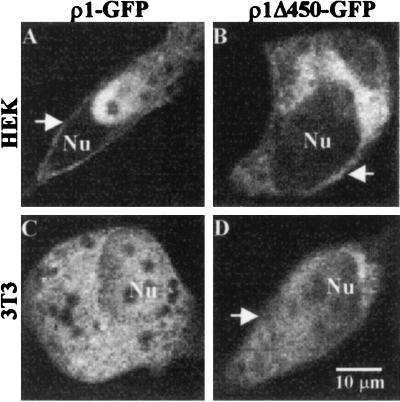
After transfection (1–3 days), mammalian cells efficiently produced ρ1-GFP or ρ1Δ450-GFP proteins. These proteins were located mostly in intracellular compartments and contrasted clearly with a fairly homogeneous distribution of fluorescence in cells transfected with GFP alone. Cells positively transfected (i.e., with strong fluorescence) showed an altered morphology with shrunken bodies and shorter filopodia. Despite these changes, fluorescent proteins were located clearly near the surface in some cells, suggesting that the receptor probably was anchored properly in the surface membrane (Fig. 6). The distribution of fluorescence was similar in HEK293 and 3T3 cells, but electrophysiological experiments will be necessary to determine whether the GFP constructs are really assembling into functional GABA receptors in the plasma membranes of both types of cells. Furthermore, expression of the chimeric receptors led to the premature death of the overproducing cells 4–5 days after transfection. This was not the case for cells expressing wt ρ1 alone.
Figure 6.
Expression of ρ1-GFP and ρ1Δ450-GFP in mammalian cells. Arrows indicate the fluorescence located at or near the plasma membrane. Nu, nucleus. Cells were observed 72 h after transfection.
Discussion
To compare the cellular distribution of functional ρ1 receptors with the nonfunctional isoform ρ1Δ450, we fused GFP to their corresponding C termini. This strategy was chosen to reduce the possibility of alterations in the function of the receptor, because the GFP would be physically far from the ion channel and the agonist-binding sites.
The ρ1-GFP receptors expressed were functional, and the traits typical of the wt-ρ1 receptor were conserved. However, two differences were observed: a delay in the expression of functional receptors and a decreased amplitude of the GABA currents generated, possibly caused by a decrease in the number of receptors assembled in the membrane. Somewhat similar results have been reported for GABAA receptors fused to GFP. For example, Bueno et al. (23) fused GFP to the C terminus of the α1 subunit, and coexpression of this chimera with the β2 and γ2 subunits produced functional receptors with properties similar to those of wt receptors. However, slight but nonsignificant differences in the time of appearance and in the magnitude of the GABA currents were observed. Similar variations in GABA currents of heteromeric GABAA receptors (α1, β2-GFP, and γ2) were observed by Flippova et al. (24).
Oocytes injected with either ρ1-GFP or ρ1Δ450-GFP displayed similar fluorescence patterns. However, consistent with the wild-type ρ1Δ450 receptors, oocytes injected with ρ1Δ450-GFP cDNA or cRNA failed to generate a GABA current, even after exposure to 10 mM GABA. Therefore, although it still is possible that ρ1Δ450 receptors are not being assembled properly in the cell surface, the efficient translation of this subunit is now clear. Moreover, because the fluorescence near the plasma membrane was very similar in oocytes injected with ρ1-GFP, which did express functional GABA receptors, and in oocytes injected with ρ1Δ450-GFP, which did not respond to GABA, it seems likely that ρ1Δ450-GFP receptors actually were inserted in the membrane but were nonfunctional.
Mammalian cells expressing the gene fusions usually showed more fluorescence in intracellular compartments than in the cell surface, and only a few cells presented clear fluorescence close to or at the plasma membrane. This kind of fluorescence distribution was found also in BOSC23 and HEK293 cells expressing a homomeric subunit of the zebrafish glycine receptor tagged with GFP (25). On the other hand, ρ1 expressed in COS cells had an intracellular as well as a cell-membrane distribution, as determined by immunofluorescence, and coexpression of the microtubule associated protein MAP1b and ρ1 led to a redistribution of the receptor into punctate intracellular compartments (26). Nevertheless, aggregation of the receptor (presumptively triggered by MAP1b) is not necessary to produce functional GABA-ρ1 receptors in cultured cells, because it has been shown previously that ρ1 produces functional receptors in HEK293 and COS cells (24, 27–29). The intracellular distribution of both ρ1-GFP and ρ1Δ450-GFP in cultured cells correlates well with that found by immunolocalization of the wt-ρ1 receptor (26), and therefore the fluorescent tag may be a useful tool to study proteins associated with the receptors and their sorting to the cell membrane in mammalian cells in culture. However, it is still desirable to reduce the cell toxicity induced by overexpressing the receptors.
The prevailing presence of ρ1-GFP and ρ1Δ450-GFP in intracellular compartments could be due to the absence of adequate plasma-membrane-anchoring proteins such as MAP1b. However, this may be explained also by the requirement of a second GABAC subunit that facilitates or makes more efficient the sorting process. For example, it is known that intracellular coassembly of several subunits of nicotinic and GABAA receptors is necessary to produce functional receptors in the plasma membrane (30–31). Those experiments suggested that heteromeric acetylcholine receptors are assembled even if they lack 1–3 of the subunits that form the receptor complex. However, assembly of all the subunits was needed for efficient insertion of receptors into the plasma membrane (30). Thus, if this mechanism is preserved among the other members of the nicotinic acetylcholine- and GABAA-receptor families, it could be possible that ρ1 requires the presence of other proteins or GABAC subunits to have a more efficient sorting of the receptor to the cell surface.
Other interesting issues are the highly polarized and patchy distribution of the ρ1-GFP receptors expressed in the oocyte. For some time now it has been known that receptors such as the nicotinic and muscarinic acetylcholine receptors are distributed differentially in the Xenopus oocyte cell membrane—more abundance for either native muscarinic or heterologous nicotinic receptors in the animal hemisphere or specially early after mRNA's injection in the vegetal hemisphere, respectively (22, 32). GABAA receptors tagged with GFP were localized preferentially in the animal hemisphere (23). That distribution is very much like what we found for both GABA-ρ1 and ρ1Δ450-GFP receptors in oocytes injected into the nucleus or with cRNA. On the other hand, the patchy distribution of functional receptors within a small area of the oocyte membrane was found originally by ionophoretically mapping the acetylcholine sensitivity of oocytes injected with cat-muscle mRNA (32). Confocal images of ρ1-GFP confirm that some membrane patches have higher concentrations of receptors. This polarization and differential distribution could result from a preexisting arrangement of factors that anchor the receptors to the plasma membrane or from an asymmetrical distribution of materials required for protein translation and translocation to the plasma membrane. The combination of ρ1-GFP gene fusions with expression in Xenopus oocytes is an attractive model to dissect the molecular and cellular elements that lead to the differential incorporation of the receptors in the cell membrane.
Acknowledgments
We thank Dr. I. Parker and the University of California at Irvine image facility for use of confocal microscopes. We are indebted to Dr. J. S. Marchant (University of California, Irvine) for valuable help and suggestions in some experiments and Prof. Fabrizio Eusebi (Universitá di Roma, La Sapienza, Rome, Italy) for help with the manuscript. This work was supported by the National Science Foundation (Neural and Glial Mechanisms Program) Grant 9982856.
Abbreviations
- GABA
γ-aminobutyric acid
- GABAA
GABA type A
- GABAC
GABA type C
- TPMPA
(1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic
- GFP
green fluorescent protein
- wild type
wt
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031584898.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031584898
References
- 1.Polenzani L, Woodward R M, Miledi R. Proc Natl Acad Sci USA. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward R M, Polenzani L, Miledi R. Mol Pharmacol. 1992;42:165–173. [PubMed] [Google Scholar]
- 3.Woodward R M, Polenzani L, Miledi R. Mol Pharmacol. 1992;41:1107–1115. [PubMed] [Google Scholar]
- 4.Woodward R M, Polenzani L, Miledi R. Mol Pharmacol. 1992;41:89–103. [PubMed] [Google Scholar]
- 5.Woodward R M, Polenzani L, Miledi R. Mol Pharmacol. 1993;34:609–625. [PubMed] [Google Scholar]
- 6.Murata Y, Woodward R M, Miledi R, Overman L E. Bioorg Med Chem Lett. 1996;7:2073–2076. [Google Scholar]
- 7.Ragozzino D, Woodward R M, Murata Y, Eusebi F, Overman L E, Miledi R. Mol Pharmacol. 1996;50:1024–1030. [PubMed] [Google Scholar]
- 8.Cutting G R, Curristin S, Zoghbi H, O'Hara B, Seldin M F, Uhl G R. Genomics. 1992;12:801–806. doi: 10.1016/0888-7543(92)90312-g. [DOI] [PubMed] [Google Scholar]
- 9.Bailey M E, Albrecht B E, Johnson K J, Darlison M G. Biochim Biophys Acta. 1999;1447:307–312. doi: 10.1016/s0167-4781(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 10.Cutting G R, Lu L, O'Hara B F, Kasch L M, Montrose-Rafizadeh C, Donovan D M, Shimada S, Antonarakis S E, Guggino W B, Uhl G R. Proc Natl Acad Sci USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo D J, Vazquez A E, Miledi R. Proc Nat Acad Sci USA. 1994;91:12725–12729. doi: 10.1073/pnas.91.26.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogurusu T, Shingai R. Biochim Biophys Acta. 1996;1305:15–18. doi: 10.1016/0167-4781(95)00205-7. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Torres A, Vazquez A E, Panicker M M, Miledi R. Proc Natl Acad Sci USA. 1998;95:4019–4022. doi: 10.1073/pnas.95.7.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuro A, Martinez-Torres A, Miledi R. Neurosci Res (NY) 2000;36:141–146. doi: 10.1016/s0168-0102(99)00116-9. [DOI] [PubMed] [Google Scholar]
- 15.Enz R, Cutting G R. Brain Res. 1999;846:177–185. doi: 10.1016/s0006-8993(99)02008-9. [DOI] [PubMed] [Google Scholar]
- 16.Hackam A S, Wang T L, Guggino W B, Cutting G R. NeuroReport. 1997;8:1425–1430. doi: 10.1097/00001756-199704140-00020. [DOI] [PubMed] [Google Scholar]
- 17.Korpi E R, Kuner T, Kristo P, Kohler M, Herb A, Luddens H, Seeburg P H. J Neurochem. 1994;63:1167–1170. doi: 10.1046/j.1471-4159.1994.63031167.x. [DOI] [PubMed] [Google Scholar]
- 18.Callamaras N, Parker I. Cell Calcium. 1999;26:271–279. doi: 10.1054/ceca.1999.0085. [DOI] [PubMed] [Google Scholar]
- 19.Parker I, Callamaras N, Wier W G. Cell Calcium. 1997;21:441–452. doi: 10.1016/s0143-4160(97)90055-5. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Torres A, Demro A, Miledi R. Proc Natl Acad Sci USA. 2000;97:3562–3566. doi: 10.1073/pnas.050582397. . (First Published March 21, 2000; 10.1073/pnas.050582397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miledi R. Proc R Soc London Ser B. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 22.Kusano K, Miledi R, Stinnakre J. J Physiol. 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bueno O F, Robinson L C, Alvarez-Hernandez X, Leidenheimer N J. Brain Res Mol Brain Res. 1998;59:165–177. doi: 10.1016/s0169-328x(98)00129-6. [DOI] [PubMed] [Google Scholar]
- 24.Filippova N, Sedelnikova A, Zong Y, Fortinberry H, Weiss D S. Mol Pharmacol. 2000;57:847–856. [PubMed] [Google Scholar]
- 25.David-Watine B, Shorte S L, Fucile S, de Saint Jan D, Korn H, Bregestovski P. Neuropharmacology. 1999;38:785–792. doi: 10.1016/s0028-3908(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 26.Hanley J G, Koulen P, Bedford F, Gordon-Weeks P R, Moss S J. Nature (London) 1999;397:66–69. doi: 10.1038/16258. [DOI] [PubMed] [Google Scholar]
- 27.Filippova N, Dudley R, Weiss D S. J Physiol. 1999;518:385–399. doi: 10.1111/j.1469-7793.1999.0385p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusama T, Spivak C E, Whiting P, Dawson V L, Schaeffer J C, Uhl G R. Br J Pharmacol. 1993;109:200–206. doi: 10.1111/j.1476-5381.1993.tb13554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wotring V E, Chang Y, Weiss D S. J Physiol. 1999;521:327–336. doi: 10.1111/j.1469-7793.1999.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumikawa K, Miledi R. Brain Res Mol Brain Res. 1989;5:183–192. doi: 10.1016/0169-328x(89)90034-x. [DOI] [PubMed] [Google Scholar]
- 31.Gorrie G H, Vallis Y, Stephenson A, Whitfield J, Browning B, Smart T G, Moss S J. J Neurosci. 1997;17:6587–6596. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miledi R, Sumikawa K. Biomed Res. 1982;3:390–399. [Google Scholar]