Abstract
Theoretical analyses have reported that in most circumstances where natural selection favours reliance on social learning, conformity (positive frequency-dependent social learning) is also favoured. These findings suggest that much animal social learning should involve a copy-the-majority strategy, yet there is currently surprisingly little evidence for conformist learning among animals. Here, we investigate this possibility in the nine-spined stickleback (Pungitius pungitius) by manipulating the number of demonstrator fish at two feeders, one rich and one poor, during a demonstration phase and evaluating how this affects the likelihood that the focal fish copy the demonstrators' apparent choices. As predicted, we observed a significantly increased level of copying with increasing numbers of demonstrators at the richer of the two feeders, with copying increasing disproportionately, rather than linearly, with the proportion of demonstrators at the rich foraging patch. Control conditions with non-feeding demonstrators showed that this was not simply the result of a preference for shoaling with larger groups, implying that nine-spined sticklebacks copy in a conformist manner.
Keywords: social learning strategy, evolutionary game theory, cultural evolution, social learning, conformity
1. Introduction
Boyd & Richerson (1985; Richerson & Boyd 2005) and their collaborators (e.g. Henrich & Boyd 1998; McElreath et al. 2005; Efferson et al. 2008) have long argued that conformist learning is an important mechanism of human cultural evolution, stressing the evidence for conformity found in the human psychology literature (e.g. Asch 1952; Jacobs & Campbell 1961; Gerard et al. 1968; Tanford & Penrod 1984). Here, ‘conformity’ refers to a positive frequency-dependent social learning where the probability of acquiring a trait increases disproportionately with the proportion of other individuals performing it. Theoretical analyses (Boyd & Richerson 1985; Henrich & Boyd 1998) have found that in most circumstances where natural selection favours reliance on social learning, conformity is also favoured. In principle, these findings apply to non-human animals too, suggesting that much animal social learning should involve individuals adopting the behaviour of the majority (Laland 2004).
The literature on animal social learning provides only indirect evidence of conformity (Kendal et al. 2009). Studies of fish (Laland & Williams 1997; Lachlan et al. 1998; Day et al. 2001), birds (Lefebvre & Giraldeau 1994) and mammals (Beck & Galef 1989; Chou & Richerson 1992; Whiten et al. 2005; Galef & Whiskin 2008) report an increase in the rate of social learning with an increase in the proportion of demonstrators performing a target behaviour, but fail to demonstrate an accelerating increase in the likelihood of learning. Here, we describe an experiment that exploits a previously verified experimental design of public information use in nine-spined sticklebacks (Coolen et al. 2003; van Bergen et al. 2004) in order to investigate whether a species of freshwater fish, the nine-spined stickleback (Pungitius pungitius), deploys a conformist strategy when it engages in social learning.
2. Material and methods
(a). Subjects and apparatus
Nine-spined sticklebacks were caught using dip nets from Melton Brook, Leicester (52°39′ N, 01°06′ W), and housed in tanks at the Gatty Marine Laboratory, St Andrews. Water temperature was maintained at 8–12°C in order to suppress the onset of sexual maturation; any fish showing signs of sexual maturation were not included in the experiment. Fish were fed daily on frozen Chironomid larvae (bloodworm), except prior to training when test fish were deprived of food for 24 h. Experiments were conducted in an aquarium (30 cm × 90 cm, 18 cm water level) divided into three sections with two transparent partitions, with a feeder placed at each end of the tank (electronic supplementary material, figure S1). Feeders consisted of columns (5 cm × 5 cm × 35 cm high) with opaque sides and a transparent front (van Bergen et al. 2004). To facilitate learning, one feeder was coloured blue and the other yellow (Girvan & Braithwaite 1998), although which colour of feeder was designated ‘rich’ or ‘poor’ (see below) was balanced within experimental groups. A pilot experiment confirmed that fish showed no prior preference for either colour of feeder (binomial test: n = 20, p = 0.82). To ensure that objects or events outside the tank would not distract the fish, the outside of three sides of the tank were covered with opaque black plastic and the experimenter observed the tank from within a hide.
(b). Experimental procedure
Adult sticklebacks were allocated randomly to one of either three experimental conditions or four control conditions. For the experimental conditions (termed public-information conditions), we adopted a similar procedure to van Bergen et al. (2004) whereby fish were given (i) personal information which provided them with the opportunity to feed at two feeders, and to learn that one, the personal-rich feeder, provided more food than the other, the personal-poor feeder (training); (ii) a ‘pre-test’ to establish that this personal training was effective; (iii) conflicting public information, where they observed two shoals of conspecific demonstrators feeding at the two feeders, but with the rich and poor feeders reversed compared with training; and (iv) a test to determine their final choice of feeder, which is indicative of reliance on either personal or public information. Final feeder preference was quantified as the difference (personal-poor minus personal-rich) in the number of instances that the focal fish was present in the ‘goal zone’ around each feeder (electronic supplementary material, figure S1), from point sampling every 6 s during the 90 s preference test. These public-information conditions differed in the number of demonstrators at each feeder, with the number of demonstrators in the shoal at the public-poor (P) and public-rich (R) feeders being three and three (denoted ‘3P–3R’), two and four (‘2P–4R’) or one and five (‘1P–5R’). The relative pay-off to demonstrators at the rich and poor feeders (six and two feeding bouts per session, respectively) remained constant across the three conditions.
Fish in the first three control conditions (termed social-information only conditions) received (i) personal information, (ii) pre-test and (iv) test, exactly as described above, but (iii) a public demonstration with three and three (denoted ‘3–3’), two and four (‘2–4’) or one and five (‘1–5’) demonstrators at the personal-rich and personal-poor feeders, respectively, in which no food was delivered to the demonstrators. These controls allowed us to determine to what extent any variation in the experimental conditions was due to non-foraging factors, such as shoaling behaviour. A further control (termed the personal-information only condition) experienced (i) personal information, (ii) pre-test and (iv) test, without receiving (iii) a public demonstration, but instead experienced a time delay of equivalent duration. This allowed us to establish the impact of the public demonstration on feeder choice. Full details of each stage of the experimental procedure are given in the electronic supplementary material.
(c). Predictions
If the decision to copy is a foraging decision dependent on the number of demonstrators, consistent with conformity, then fish in the 1P–5R condition should copy more than 2P–4R, and still more than 3P–3R (with greater levels of conformity in the public-information conditions compared with the social-information only conditions and a nonlinear increase in the probability of copying with increasing demonstrator group sizes; Laland 2004). If fish use both public information and the number of conspecifics to guide feeder choice, in accordance with an ideal-free distribution, we would expect the reverse pattern. If the decision to copy is based solely on public information, then there should be no difference in the level of copying between public-information conditions since the relative pay-off to demonstrators at the rich and poor feeders remained constant.
(d). Data analysis
In order to test for the patterns predicted above, linear mixed-effects models were fitted to the data and orthogonal contrasts used to test for predicted differences between pairs of conditions. Full details of the statistical analyses are given in the electronic supplementary material.
3. Results
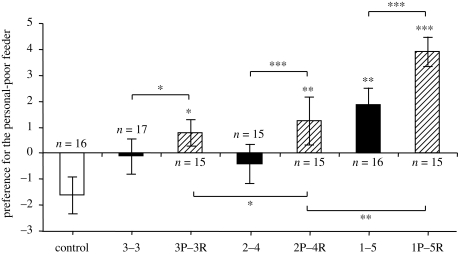
Fish in the personal-information only (control) condition, that received no public demonstration, showed a significant preference for the personal-rich feeder (one-sample t-test against a test mean of 0: t = 2.23, n = 16, p = 0.042). Fish in all six remaining conditions chose the personal-poor feeder more than this control group, indicative of copying, although this was not always significant (figure 1).
Figure 1.
Mean ± s.e preference for the personal-poor feeder (time spent near the personal-poor feeder minus time spent near the personal-rich feeder from instantaneous sampling every 6 s for 90 s) in the personal-information only condition (‘control’, white bar), the social-information only conditions (black bars, where 3–3 denotes three fish shoaling at one feeder and three fish at the other feeder) and the public-information conditions (hashed bars, where 3P–3R denotes three fish feeding at the public-poor feeder and three fish at the public-rich feeder, respectively). See text for full details. Asterisks above bars indicate a significant difference from the control group; horizontal lines indicate orthogonal pair-wise comparisons between groups. *p < 0.05, **p < 0.01 and ***p < 0.001.
In the public-information conditions, the number of fish copying the apparent foraging patch choice of the demonstrators at the rich feeder increased with the number of demonstrators at that feeder, consistent with conformist learning (figure 1). Fish in 2P–4R chose the public-rich (personal-poor) feeder significantly more than those in 3P–3R (t20 = 2.11, p = 0.047), and fish in 1P–5R chose the public-rich feeder significantly more than those in 2P–4R (t20 = 3.41, p = 0.003). The magnitude of the difference in mean preference between fish in 2P–4R and 1P–5R (2.66) was approximately 5.7 times greater than that between 3P–3R and 2P–4R (0.47) (permutation test, p = 0.008; figure 1).
There was more copying in all public-information conditions compared with their associated social-information only (control) conditions (figure 1). There were significant differences between 3–3 and 3P–3R (t63 = 2.19, p = 0.032), 2–4 and 2P–4R (t63 = 3.98, p < 0.001) and 1–5 and 1P–5R (t63 = 4.20, p < 0.001), with in each instance fish in the public-information condition choosing the personal-poor feeder more than those in the social-information only condition.
4. Discussion
We carried out an experimental investigation to explore the extent to which nine-spined sticklebacks' decision to copy conspecifics' foraging patch choice is dependent on the number of demonstrator fish at that patch. At test, fish in all three public-information conditions chose the feeder that was demonstrated to be rich in the public demonstration more than fish in the personal-information only control condition, indicating that the choices of these fish were influenced by the demonstrators' foraging. The fact that fish in all three public-information groups also chose the personal-poor feeder more than fish in the social-information only conditions, which had the same number of non-feeding demonstrators at each feeder, clearly shows that these decisions to copy relate, at least to some extent, to the rate at which the demonstrators fed. At test there were no demonstrators present at the feeders, so the experimental subjects' decisions cannot be dismissed as shoaling behaviour.
We find clear evidence that social learning increases with the number of demonstrators at the rich feeder in the public-information conditions. Fish in the 1P–5R condition copied significantly more than those in the 2P–4R condition, which in turn copied more than those in the 3P–3R condition. Moreover, and critical to any claim of conformity, social learning appears to increase disproportionately, rather than linearly, with the number of demonstrators (the magnitude of the difference between the public-rich preference in the 2P–4R and 1P–5R conditions was more than five times greater than the difference in preference between the 3P–3R and 2P–4R conditions). This pattern is exactly what a conformist strategy of social learning would predict (Boyd & Richerson 1985; Laland 2004). Our data are also consistent with other definitions of conformity, such as the discounting of personal experience in favour of majority opinion (Jacobs & Campbell 1961).
A capacity for conformist learning may well complement nine-spined sticklebacks' other social learning capabilities (van Bergen et al. 2004; Kendal et al. 2009) since conformity appears to be most beneficial in spatially variable environments (Boyd & Richerson 1985), an environmental context likely to apply broadly to stickleback habitat (Wootton 1976).
Acknowledgements
This work was supported by a BBSRC grant to K.N.L. (BB/C005430/1).
References
- Asch S. E.1952Social psychology. Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- Beck M., Galef B. G.1989Social influences on the selection of a protein-sufficient diet by Norway rats (Rattus norvegicus). J. Comp. Psychol. 103, 132–139 (doi:10.1037/0735-7036.103.2.132) [Google Scholar]
- Boyd R., Richerson P. J.1985Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- Chou L.-S., Richerson P. J.1992Multiple models in social transmission of food selection by Norway rats, Rattus norvegicus. Anim. Behav. 44, 337–343 (doi:10.1016/0003-3472(92)90039-C) [Google Scholar]
- Coolen I., Day R. L., Laland K. N.2003Species difference in adaptive use of public information in sticklebacks. Proc. R. Soc. Lond. B 270, 2413–2419 (doi:10.1098/rspb.2003.2525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. L., MacDonald T., Brown C., Laland K. N., Reader S. M.2001Interactions between shoal size and conformity in guppy social foraging. Anim. Behav. 62, 917–925 (doi:10.1006/anbe.2001.1820) [Google Scholar]
- Efferson C., Lalive R., Richerson P. J., McElreath R., Lubell M.2008Conformists and mavericks: the empirics of frequency-dependent cultural transmission. Evol. Hum. Behav. 29, 56–64 (doi:10.1016/j.evolhumbehav.2007.08.003) [Google Scholar]
- Galef B. G., Whiskin E. E.2008‘Conformity’ in Norway rats? Anim. Behav. 75, 2035–2039 (doi:10.1016/j.anbehav.2007.11.012) [Google Scholar]
- Gerard H. B., Whilhelmy R. A., Connolly R. S.1968Conformity and group size. J. Pers. Soc. Psychol. 8, 79–82 (doi:10.1037/h0025325) [DOI] [PubMed] [Google Scholar]
- Girvan J. R., Braithwaite V. A.1998Population differences in spatial learning and memory in threespined sticklebacks. Proc. R. Soc. B 265, 913–918 (doi:10.1098/rspb.1998.0378) [Google Scholar]
- Henrich J., Boyd R.1998The evolution of conformist transmission and the emergence of between-group differences. Evol. Hum. Behav. 19, 215–241 (doi:10.1016/S1090-5138(98)00018-X) [Google Scholar]
- Jacobs R. C., Campbell D. T.1961The perpetuation of an arbitrary tradition through several generations of a laboratory microculture. J. Abnorm. Soc. Psychol. 62, 649–658 (doi:10.1037/h0044182) [DOI] [PubMed] [Google Scholar]
- Kendal J. R., Giraldeau L. A., Laland K. N.2009The evolution of social learning rules: payoff-biased and frequency-dependent biased transmission. J. Theor. Biol. 260, 210–219 (doi:10.1016/j.jtbi.2009.05.029) [DOI] [PubMed] [Google Scholar]
- Lachlan R. F., Crooks L., Laland K. N.1998Who follows whom? Shoaling preferences and social learning of foraging information in guppies. Anim. Behav. 56, 181–190 (doi:10.1006/anbe.1998.0760) [DOI] [PubMed] [Google Scholar]
- Laland K. N.2004Social learning strategies. Learn. Behav. 32, 4–14 [DOI] [PubMed] [Google Scholar]
- Laland K. N., Williams K.1997Shoaling generates social learning of foraging information in guppies. Anim. Behav. 53, 1161–1169 (doi:10.1006/anbe.1996.0318) [DOI] [PubMed] [Google Scholar]
- Lefebvre L., Giraldeau L. A.1994Cultural transmission in pigeons is affected by the number of tutors and bystanders present. Anim. Behav. 47, 331–337 (doi:10.1006/anbe.1994.1046) [Google Scholar]
- McElreath R., Lubell M., Richerson P. J., Waring T., Baum W., Edsten E., Efferson C., Paciotti B.2005Applying evolutionary models to the laboratory study of social learning. Evol. Human Behav. 26, 483–508 [Google Scholar]
- Richerson P. J., Boyd R.2005Not by genes alone. Chicago, IL: Chicago University Press [Google Scholar]
- Tanford S., Penrod S.1984Social influence model: a formal integration of research on majority and minority influence processes. Psychol. Bull. 95, 189–225 (doi:10.1037/0033-2909.95.2.189) [Google Scholar]
- van Bergen Y., Coolen I., Laland K. N.2004Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. Lond. B 271, 957–962 (doi:10.1098/rspb.2004.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A., Horner V., de Waal F. B. M.2005Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737–740 (doi:10.1038/nature04047) [DOI] [PubMed] [Google Scholar]
- Wootton R. J.1976The biology of the sticklebacks. London, UK: Academic Press [Google Scholar]