Abstract
Tetrapod biodiversity today is great; over the past 400 Myr since vertebrates moved onto land, global tetrapod diversity has risen exponentially, punctuated by losses during major extinctions. There are links between the total global diversity of tetrapods and the diversity of their ecological roles, yet no one fully understands the interplay of these two aspects of biodiversity and a numerical analysis of this relationship has not so far been undertaken. Here we show that the global taxonomic and ecological diversity of tetrapods are closely linked. Throughout geological time, patterns of global diversity of tetrapod families show 97 per cent correlation with ecological modes. Global taxonomic and ecological diversity of this group correlates closely with the dominant classes of tetrapods (amphibians in the Palaeozoic, reptiles in the Mesozoic, birds and mammals in the Cenozoic). These groups have driven ecological diversity by expansion and contraction of occupied ecospace, rather than by direct competition within existing ecospace and each group has used ecospace at a greater rate than their predecessors.
Keywords: biodiversity, diversity, ecology, expansion, tetrapods, vertebrates
1. Introduction
Life on Earth today is diverse. Though tetrapods make up a small fraction of Earth's biomass, there are currently some 30 000 species in over 300 families, occupying 75 broad ecological modes of life. This current high diversity has presumably grown from a single species that crept onto land in the Mid Devonian, representing a single family and a single ecological mode. The pattern of diversity increase for tetrapods appears to have been essentially exponential, with many setbacks and evident damping (Benton 1995; Benton & Emerson 2007).
The exponential increase from one species to many tens of thousands occupying a great variety of ecological modes could have been driven by either (i) the expansion of habitats occupied as tetrapods moved from the waterside to exploit new sectors of Earth's surface, such as forests, plains and deserts, or (ii) competition within a more-or-less constant amount of habitat, leading to specialization within communities so that more species live together, with each exploiting an ever-narrower range of resources.
Diversity is most commonly assessed by tallying taxa on a global scale but can also be considered from an ecological, morphological or genetic perspective. Our ability to understand the complex relationships among these types of diversity is constantly improving, allowing us to draw correlations and better define the history of life on Earth. This is the first numerical study investigating the link between tetrapod taxonomic and ecological diversity on a global scale.
2. Material and methods
Tetrapod taxonomic and ecological diversity was tabulated at the family level over 66 stages of time. One thousand and thirty-four extinct and extant tetrapod families were initially tabulated, but families without a fossil record and monotypic families, those represented by only a single species, were discarded from the analysis, leaving 840 families (81%). Families were given broad geographical, chronological and ecological assignments. The time scale employed was that of Gradstein & Ogg (2004), stratigraphic ranges and ecomorphs (body size, diet and habitat) were taken from Benton (1993, 1996). Ecomorphs were combined to define 288 potential modes of life (3 sizes × 16 diets × 6 habitats), 81 of which are considered unlikely to be filled (for a variety of reasons such as incompatible habitat and dietary category), leaving 207 habitable modes of life. For further details see the electronic supplementary material.
3. Results
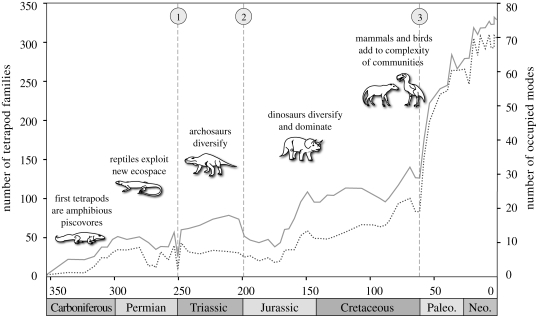
The global taxonomic diversity of tetrapod families correlates strongly with the number of ecological modes they occupied through time (Spearman's ρ = 0.9727; p < 0.001), represented by an overall exponential increase, and punctuated by losses at major extinction events in the late to end Permian, end of the Triassic and end of the Cretaceous (figure 1).
Figure 1.
Global taxonomic diversity of monotypic tetrapod families and ecological diversity of modes used by tetrapod families. These two measures of diversity correlate, with a Spearman's rank correlation coefficient of ρ = 0.9727, p < 0.001 and a linear regression of y = 0.2518x. Mass extinctions are  end-Permian extinction,
end-Permian extinction,  end-Triassic extinction and
end-Triassic extinction and  end-Cretaceous extinction (solid line, families; dashed line, modes).
end-Cretaceous extinction (solid line, families; dashed line, modes).
There are 207 habitable ecological modes for tetrapods to use, but only 75 have been populated (36%). The number of families occupying each realized mode has increased from an average of one to four families, and is well correlated with the taxonomic diversity of the different tetrapod classes; amphibians in the Palaeozoic, reptiles in the Mesozoic and birds and mammals in the Cenozoic (figure 2a). Increasing mode utilization is also associated with dominant faunas: amphibians maximally added 0.5 families to a mode per Myr until the end of the Palaeozoic; in the Mesozoic, reptiles added up to 1.0 families until the Cretaceous–Tertiary extinction; and in the Cenozoic, birds and mammals peaked at adding 3.0 families (figure 2b). Maximum decrease in mode utilization is consistent across all classes at 0.5 families per mode per Myr with four exceptions: stages that follow the end-Permian extinction (252 Ma), end-Triassic extinction (200 Ma), end-Cretaceous extinction (65 Ma), and the Grande Coupure (Eocene-Oligocene boundary; 34 Ma) show a significantly larger loss (figure 2b).
Figure 2.
Ecological role of tetrapods through time. (a) The average number of tetrapod families occupying a single mode and the global taxonomic diversity of the different tetrapod classes. (b) Rate of expansion/contraction of mode utilization and the general ‘normal’ range of this rate (shaded area) with the exception of troughs and peaks at major extinction events. Mass extinctions are  end-Permian extinction,
end-Permian extinction,  end-Triassic extinction,
end-Triassic extinction,  end-Cretaceous extinction and
end-Cretaceous extinction and  Grande Coupure (solid line, families per mode; long-dashed black line, mammals; short-dashed black line, birds; dashed grey line, reptiles; dotted grey line, amphibians).
Grande Coupure (solid line, families per mode; long-dashed black line, mammals; short-dashed black line, birds; dashed grey line, reptiles; dotted grey line, amphibians).
4. Discussion
The fossil record reveals that tetrapod taxonomic diversity has increased in an exponential fashion. While many investigators argue that biodiversity patterns are biased by poor-quality sampling of older parts of the record, and have found correlations between marine geological measures and biodiversity (Smith 2007), these correlations are not clear in the terrestrial fossil record (Fara 2002). There are three lines of evidence that indicate the tetrapod fossil record at family level is reasonably reliable. (i) Tetrapods have hard skeletons, and hence are more likely to be preserved than entirely soft-bodied organisms. It is unlikely that major groups have been missed, even those with small, delicate skeletons such as the first mammals and birds (Benton 1999; Foote et al. 1999). (ii) Intense collecting and description of tetrapod fossils over the past 150 years has not yielded any major surprises: fossil vertebrates more often fill gaps rather than create gaps (Benton & Storrs 1994). (iii) Molecular phylogenies show good congruence with the fossil record, suggesting that not many key fossils are missing and that the fossil record of tetrapods is of comparable quality to that of fishes or of echinoderms (Hitchin & Benton 1997; Benton et al. 2000).
Tetrapods have a great ability for adaptation and their taxonomic and ecological diversity have seemingly been shaped over the last 400 Myr primarily by expansion. Initially tetrapods moved into empty ecospace where no other large animal life existed. They filled empty modes of life further away from the water and began to burrow, climb, fly, take advantage of specialized feeding strategies and then continued to invade new habitats evolved by other organisms such as forests, canopies and grasslands. At the same time abiotic processes such as continental breakup, geographical barriers, latitudinal temperature differentiation and changing climate contributed to greater complexity of Earth's surface, creating endemism.
The data show multiple lines of evidence for the role of expansion as the main driver of tetrapod diversification: (i) tetrapods have only explored a third of habitable modes of life; (ii) tetrapods have occupied an exponentially increasing number of modes; (iii) ecological diversification has been driven at an increasing rate by the different tetrapod classes; (iv) successively dominant tetrapod classes have increased the maximum rate of mode utilization; and (v) Tetrapoda exhibit ecological incumbency, observed by a limit at which mode utilization decreased, except at times of mass extinction.
Tetrapods have filled 36 per cent of habitable modes. In contrast, Bambach et al. (2007) found that marine animals have explored 78 per cent of habitable modes, categorized by tiering position, motility level and feeding strategy. This may be because the ocean is in essence a giant Petri dish, whose diversity is saturated and can only diversify further by packing more species into already existing modes or subdividing niches. The terrestrial realm does not appear to have such restrictions or perhaps this limit has not yet been reached.
The records of taxonomic and ecological diversity of tetrapods are closely linked and are good reflections of the expansion of tetrapods. Tetrapod taxonomic and ecological diversity has increased dramatically through time (figure 1) and the high correlation between these two measures is in keeping with observations of the marine realm, where the ecological and taxonomic diversity histories of marine animals are broadly parallel (Bambach et al. 2007).
Tetrapod ecological diversity, like taxonomic diversity, is driven by the four tetrapod classes (figure 2a). Mode utilization by multiple families has risen from a single Devonian amphibious piscivore to four families filling each mode today. During each of the three eras the rate of mode utilization was contained within a range that increased with successively dominant tetrapod classes (figure 2b), since each had a greater ability to share a mode of life among multiple families, doubtless related to their key adaptive features. Palaeozoic amphibians were largely tied to waterside habitats because of breeding constraints, but amniotes exploited a wide variety of new, entirely terrestrial habitats, and the endothermy of mammals and birds allowed these groups to conquer cooler habitats and explore alternative behaviors such as nocturnality. The innovative adaptations of mammals and birds led to a dramatic increase in the rate of mode invasion, tripling the number of occupied modes in 30 Myr.
Although the rate at which tetrapods have expanded mode utilization has increased, the typical limit at which families decreased the use of ecospace is a loss of 0.5 families per mode per Myr with the exception of stages immediately following the end-Permian, end-Triassic, end-Cretaceous extinctions and the Grande Coupure; they show a rate of loss and then addition outside the ‘normal range’ (figure 2b) of the dominant fauna. These four mass extinctions removed incumbent families, and new invading tetrapod groups used their key adaptations to refill ecospace at a greater rate than their predecessors.
Given the unrestricted access tetrapods have to ecospace, perhaps there is little need for competitive interactions to shape diversification. Though traditional views cite inter-clade competition as a driver of evolution (Colbert 1955; Romer 1966) there is little evidence of competition guiding large-scale biotic replacements (Gould & Calloway 1980; Sepkoski 1996). Benton (1996) first indicated that competitive replacement played a minor role in the evolution of tetrapods. He estimated that seven-eighths of tetrapod familial diversification was unrestrained expansion into ecospace rather than the result of biological interaction; the remainder were candidate competitive replacers (CCRs), families that could have originated by competitively displacing another. This study considered ecological, geographical and stratigraphical information, but the resolution of the fossil record does not allow observation of behavioural characteristics (e.g. sleep/wake cycles or reproductive cycles) or spatial partitioning (e.g. animals living at different levels of a forest canopy). If these further partitions of ecospace could be added, the count of CCRs would probably decline further.
Though the tetrapod record does not provide evidence for direct competition, there is evidence of competition in the manner of incumbent replacement, in which established groups can exclude competitors, even if those competitors possess advantageous key adaptations, until the incumbents are removed from their foothold by a major environmental disruption such as a mass extinction, at which time the key adaptations of the invading clade allows them to colonize the area before the incumbents can reestablish themselves (Rosenzweig & McCord 1991). The data support the growing evidence that, except following mass extinctions, tetrapod diversity was primarily achieved by unrestricted expansion into empty ecospace, that is by the filling of unrealized modes of life, and multiplying into already realized modes. As taxonomic diversity has increased, there have been incentives for tetrapods to move into new modes of life, where initially resources may seem unlimited, there are few competitors and possible refuge from danger. And as ecological diversity increases, taxa diversify from their ancestors at a much greater rate among faunas with more superior, innovative or more flexible adaptations.
Tetrapods have not yet invaded 64 per cent of potentially habitable modes, and it could be that without human influence the ecological and taxonomic diversity of tetrapods would continue to increase in an exponential fashion until most or all of the available ecospace is filled.
Acknowledgements
We would like to thank colleagues in the Palaeobiology & Biodiversity Research Group at the University of Bristol and Graeme Lloyd for discussions. Also appreciated are useful comments from two anonymous reviewers and Mike Lee.
References
- Bambach R. K., Bush A. M., Erwin D. H.2007Autecology and the filling of ecospace: key metazoan radiations. Palaeontology 50, 1–22 (doi:10.1111/j.1475-4983.2006.00611.x) [Google Scholar]
- Benton M. J.1993The fossil record 2. London, UK: Chapman & Hall [Google Scholar]
- Benton M. J.1995Diversification and extinction in the history of life. Science 268, 52–58 (doi:10.1126/science.7701342) [DOI] [PubMed] [Google Scholar]
- Benton M. J.1996Testing the roles of competition and expansion in tetrapod evolution. Proc. R. Soc. Lond. B 263, 641–646 (doi:10.1098/rspb.1996.0096) [Google Scholar]
- Benton M. J.1999Early origins of modern birds and mammals: molecules vs. morphology. Bioessays 21, 1043–1051 (doi:10.1002/(SICI)1521-1878(199912)22:1<1043::AID-BIES8>3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- Benton M. J., Emerson B. C.2007How did life become so diverse? The dynamics of diversification according to the fossil record and molecular phylogenetics. Palaeontology 50, 23–40 (doi:10.1111/j.1475-4983.2006.00612.x) [Google Scholar]
- Benton M. J., Storrs G. W.1994Testing the quality of the fossil record—paleontological knowledge is improving. Geology 22, 111–114 (doi:10.1130/0091-7613(1994)022<0111:TTQOTF>2.3.CO;2) [Google Scholar]
- Benton M. J., Wills M. A., Hitchin R.2000Quality of the fossil record through time. Nature 403, 534–537 (doi:10.1038/35000558) [DOI] [PubMed] [Google Scholar]
- Colbert E. H.1955Evolution of the vertebrates. New York, NY: Wiley [Google Scholar]
- Fara E.2002Sea-level variations and the quality of the continental fossil record. J. Geol. Soc. 159, 489–491 [Google Scholar]
- Foote M., Hunter J. P., Janis C. M., Sepkoski J. J.1999Evolutionary and preservational constraints on origins of biologic groups: divergence times of eutherian mammals. Science 283, 1310–1314 (doi:10.1126/science.283.5406.1310) [DOI] [PubMed] [Google Scholar]
- Gould S. J., Calloway C. B.1980Clams and brachiopods—ships that pass in the night. Paleobiology 6, 383–396 [Google Scholar]
- Gradstein F. M., Ogg J. G.2004Geologic time scale 2004—why, how, and where next!. Lethaia 37, 175–181 (doi:10.1080/00241160410006483) [Google Scholar]
- Hitchin R., Benton M. J.1997Congruence between parsimony and stratigraphy: comparisons of three indices. Paleobiology 23, 20–32 [Google Scholar]
- Romer A. S.1966Vertebrate paleontology. Chicago, IL: University of Chicago Press [Google Scholar]
- Rosenzweig M. L., McCord R. D.1991Incumbent replacement—evidence for long-term evolutionary progress. Paleobiology 17, 202–213 [Google Scholar]
- Sepkoski J. J.1996Competition in macroevolution: the double wedge revisited. In Evolutionary paleobiology (eds Jablonski D., Erwin D. H., Lipps J. H.), pp. 211–255 Chicago, IL: Chicago University Press [Google Scholar]
- Smith A. B.2007Marine diversity through the Phanerozoic: problems and prospects. J. Geol. Soc. 164, 731–745 (doi:10.1144/0016/76492006-184) [Google Scholar]