Abstract
Evolutionary biologists have argued that there should be a positive relationship between sperm size and sperm velocity, and that these traits influence a male's sperm competitiveness. However, comparative analyses investigating the evolutionary associations between sperm competition risk and sperm morphology have reported inconsistent patterns of association, and in vitro sperm competition experiments have further confused the issue; in some species, males with longer sperm achieve more competitive fertilization, while in other species males with shorter sperm have greater sperm competitiveness. Few investigations have attempted to address this problem. Here, we investigated the relationship between sperm morphology and sperm velocity in house mice (Mus domesticus). We conducted in vitro sperm velocity assays on males from established selection lines, and found that sperm midpiece size was the only phenotypic predictor of sperm swimming velocity.
Keywords: sperm motility, sperm design, sperm competition, ejaculate quality
1. Introduction
When females mate with more than one male in a single reproductive event, sperm from rival males are forced to compete for fertilization (Parker 1998). Theory predicts that sperm competition will favour increased spermatogenic investment, and thus can be an influential force in the evolution of testes size and sperm number (Parker 1998). Consistent with theory, experimental evolution studies have shown that sperm competition selects for larger (Hosken & Ward 2001) and more efficient testes (Firman & Simmons in press). Comparative studies across taxa have used testes size (relative to body size) as a proxy for sperm competition risk, and investigated the evolutionary associations between sperm competition and sperm morphology. These studies have provided conflicting evidence for the role of sperm competition in the evolution of sperm size. Sperm competition has been shown to have no influence on sperm length (Gage & Freckleton 2003), and to select for increased sperm length (Gomendio & Roldan 1991; Briskie et al. 1997; Byrne et al. 2003). Thus, the influence of sperm competition on sperm morphology and function remains controversial (Humphries et al. 2008).
For vertebrates, it has been suggested that increased sperm length results in greater energy reserves and faster swimming speeds, and thus may confer an advantage in sperm competition, either by reaching the site of fertilization quicker or by having better ova penetrating ability (Gomendio & Roldan 1991; Byrne et al. 2003; Fitzpatrick et al. 2009). In mammals, variation in overall sperm length is due primarily to differences in the length of the midpiece and flagellum (Cummins & Woodall 1985). The sperm midpiece contains a dense helical array of mitochondria that provides energy to propel the cell, the volume of which determines the flagellar beat frequency (Cardullo & Baltz 1991). Thus, the size of the sperm midpiece may be important in determining the outcome of sperm competition. Indeed, comparative studies among vertebrates have shown that sperm size correlates positively with sperm swimming speed (Fitzpatrick et al. 2009; Lüpold et al. 2009). Among primates, males of polygamous species have sperm with larger midpieces, and presumably higher densities of mitochondria, compared with monogamous species, suggesting that increases in midpiece volume may translate to greater swimming velocities and thus lead to an advantage in sperm competition (Anderson & Dixson 2002). This evolutionary association extends across a variety of mammalian taxa (Anderson et al. 2005). In contrast, a study of red deer (Cervus elaphas) revealed a negative association between sperm midpiece length and sperm swimming speed (Malo et al. 2006), highlighting the need for further within-species investigations of this relationship in other mammals. Here, we performed sperm velocity assays, obtained linear measurements of sperm and analysed the relationship between sperm morphology and sperm velocity in house mice populations subject to selection from sperm competition and populations subject to enforced monogamy.
2. Material and methods
In 2005, wild-derived house mice (n = 120) were obtained from an animal resources centre (Murdoch, Western Australia, Australia) and subjected to laboratory evolution (Firman & Simmons in press). Eight selection lines were established and maintained under either a polygamous (P) or a monogamous (M) mating regime. Historical information on the source population used to establish the selection lines, the mating design and selection regime has been described previously (Firman & Simmons in press). Following five generations of selection, eight-week-old males (n = 68) were sacrificed via lethal injection and used for sperm analyses. Males from four M lines and three P lines were used in this experiment. Sperm from the caudal epididymides were incubated at 37°C for 90 min in a culture medium (Firman & Simmons 2008a). A detailed description of the medium and culture conditions is described elsewhere (Murase & Roldan 1996). Sperm velocity was quantified using the CEROS computer-assisted sperm analysis system (CASA) (v.10, Hamiliton & Thorne Research) using standard mouse parameters (Nayernia et al. 2003). An approximately 10 µl aliquot of sperm suspension was loaded into a haemocytometer and five fields of view were scanned. The CASA gave measures of total sperm number and the percentage motile sperm, and provided velocity parameters including: average path velocity (VAP), curvilinear velocity (VCL), straight line velocity (VSL), beat cross frequency, amplitude of head displacement, linearity and straightness. Samples were maintained at 37°C and CASA scans were repeated at set intervals over 5 h. These data were use to construct a ‘percentage motile sperm’ decay curve, the magnitude of which was used as a measure of sperm longevity.
Following the initial 90 min incubation period, a 50 µl aliquot of the incubated sperm suspension was fixed in a 4 per cent formaldehyde solution. Sperm smears were stained with Coomassie brilliant blue. Images of stained sperm enabled linear measurements of sperm components, including sperm flagella length and total sperm length (400× magnification), and sperm head length, sperm head width and sperm midpiece length (1000× magnification) using the image analysis application ImageJ (v.132). Ten cells per individual were measured.
3. Results
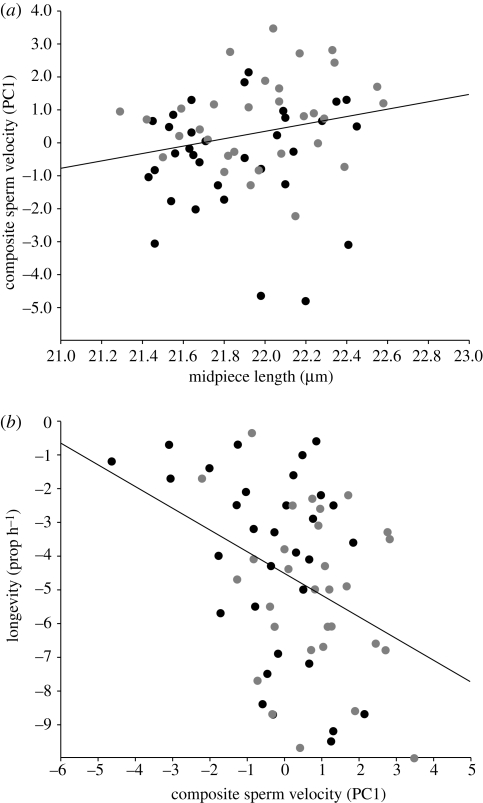
Preliminary ANOVAs showed that there was significantly more variation between individuals than within individuals in sperm number, sperm morphology measurements and sperm velocity traits (p < 0.001). As such, repeatability estimates were high (r > 0.966; Becker 1984), reflecting that we had sufficient replication to capture individual variation (table S1 in the electronic supplementary material). To summarize the variation among the highly correlated sperm velocity traits, we performed a principal component analysis, which produced a single principal component that accounted for 50 per cent of the total variation, and had a corresponding eigenvalue of 3.47 (table S2 in the electronic supplementary material). VAP, VSL and VCL contributed significantly to PC1 (hereafter referred to as composite sperm velocity) (Mardia et al. 1979) (table S2 in the electronic supplementary material). We performed statistical analyses to investigate the relationship between sperm morphology and composite sperm velocity. Our independent unit of replication was the number of lines for each selection treatment (M = 4, P = 3). Thus, we conducted nested ANOVAs with replicate line nested within selection history as a random factor. Of the five sperm components, sperm midpiece length was the only significant predictor (table 1); sperm with longer midpieces had faster swimming velocities (figure 1a). The selection history of the lines from which males were sampled also had a significant effect on sperm velocity; males from the polygamous lines had, on average, greater PC1 values (0.73 ± 0.27) compared with males from the monogamous lines (−0.67 ± 0.25) (table 1). Males from the P lines had on average higher mean values compared with males from the M lines for many of the sperm performance traits (table S1 in the electronic supplementary material). However, there was no statistically significant divergence in the total number of sperm (table 1) or sperm morphology (table S3 in the electronic supplementary material). There was a significant negative relationship between composite sperm velocity and sperm longevity (table 1; figure 1b).
Table 1.
Nested ANOVA of sperm traits for house mice, to test (a) which traits influence sperm swimming velocity, (b) whether male selection history influenced sperm number, and (c) the relationship between sperm velocity and sperm longevity. To account for non-independence of males, replicate selection line was nested within selection history (monogamous/polygamous). Significant p-values are given in italic.
| term | mean square | d.f. | F ratio | prob > F |
|---|---|---|---|---|
| (a) PC1 | ||||
| whole model | 7.07 | 11, 53 | 1.88 | 0.048 |
| sperm head length | 4.03 | 1, 53 | 1.07 | 0.303 |
| sperm head width | 0.11 | 1, 53 | 0.03 | 0.353 |
| sperm midpiece length | 22.31 | 1, 53 | 5.92 | 0.037 |
| sperm flagellum length | 0.65 | 1, 53 | 0.17 | 0.451 |
| total sperm length | 0.62 | 1, 53 | 0.17 | 0.795 |
| selection history | 2.49 | 1, 5 | 0.66 | 0.024 |
| line [selection history] | 23.70 | 5, 53 | 1.26 | 0.780 |
| (b) sperm number | ||||
| whole model | 66.86 | 6, 58 | 3.18 | 0.009 |
| selection history | 34.37 | 1, 5 | 0.53 | 0.497 |
| line [selection history] | 66.73 | 5, 58 | 3.18 | 0.013 |
| (c) sperm longevity | ||||
| whole model | 18.63 | 7, 54 | 3.36 | 0.044 |
| PC1 | 92.41 | 1, 54 | 16.65 | 0.004 |
| selection history | 0.16 | 1, 5 | 0.07 | 0.457 |
| line [selection history] | 1.91 | 5, 54 | 0.34 | 0.806 |
Figure 1.
(a) The relationship between composite sperm velocity and sperm midpiece length (y = 1.12x – 24.29, r2 = 0.070), and (b) sperm longevity (y = −0.64x – 4.52, r2 = 0.143) in house mice. The black circles represent data from males of the monogamous selection lines, and the grey circles represent data from males of the polygamous selection lines.
4. Discussion
Analyses across mammal taxa have lead to the intuitive conclusion that sperm competition may select for larger, faster sperm (Gomendio & Roldan 1991; Anderson & Dixson 2002; Fitzpatrick et al. 2009). However, there have been few direct empirical investigations to support this hypothesis. Here, we provide conclusive data on the relationship between sperm form and sperm function, and show that sperm midpiece length predicts sperm swimming velocity in house mice.
A similar investigation on red deer sperm revealed the opposite relationship between sperm midpiece size and sperm swimming velocity. Malo et al. (2006) showed that sperm with elongated heads and shorter midpieces swam faster, and found no relationship between sperm velocity and flagellum or total sperm lengths. The inconsistencies between these two mammalian species may be due to variation in sperm head morphology, a factor that may also account for the absence of a general relationship between sperm competition risk and sperm length among mammals (Gage & Freckleton 2003). Red deer sperm have an ovoid head shape, while mouse sperm bear a hooked acrosomal cap that is characteristic of muroid rodents. Thus, hydrodynamic forces imposed on morphologically varied sperm would be markedly different, and different sperm components might be expected to contribute to sperm swimming performance (Humphries et al. 2008). Mouse sperm may require large amounts of energy to overcome the drag generated by the hooked sperm head, and to propel them through the viscous microenvironment. In house mice, sperm with larger midpieces may have greater mitochondrial loads and higher ATP content, which translates into the faster velocity as we have observed here.
Our results have important implications for the role of sperm competition in the evolution of sperm quality in rodents. We found that males with a polygamous selection history had significantly greater sperm velocities, but not longer sperm, compared with males with a monogamous selection history. This result implies that sperm velocity (independent of midpiece size) responds rapidly to sperm competition during the early stages of selection, and suggests that changes in sperm size may arise further along the evolutionary pathway. Our sperm velocity data also revealed a trade-off between sperm longevity and sperm velocity. Successful fertilization is dependent upon sperm reaching the oviduct and maintaining their fertilizing ability while the ova are viable. In mice, it has been reported that as much as eight hours may lapse between copulation and fertilization (Rugh 1968). Therefore, it would be detrimental if sperm expended their ATP supplies and lost their fertilizing ability prior to ova maturation. Our own studies of house mice suggest that shorter sperm, which would have greater longevity, have a selective advantage when in competition for fertilization (Firman & Simmons 2008b). Thus, selection via sperm competition is likely to be stabilizing around an optimum morphology that maximizes sperm velocity and longevity.
Acknowledgements
This research was approved by the UWA animal ethics committee (approval no. 3/100/299).
This research was funded by the Australian Research Council. We thank Ben Roberts for animal husbandry.
References
- Anderson M. J., Dixson A. F.2002Motility and the midpiece in primates. Nature 416, 496 (doi:10.1038/416496a) [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Nyholt J., Dixson A. F.2005Sperm competition and the evolution of sperm midpiece volume in mammals. J. Zoolog. 267, 135–142 (doi:10.1017/S0952836905007284) [Google Scholar]
- Becker W. A.1984Manual of quantitative genetics. Washington, DC: Pullman [Google Scholar]
- Briskie J. V., Montgomerie R., Birkhead T. R.1997The evolution of sperm size in birds. Evolution 51, 937–945 (doi:10.2307/2411167) [DOI] [PubMed] [Google Scholar]
- Byrne P. G., Simmons L. W., Roberts J. D.2003Sperm competition and the evolution of gamete morphology in frogs. Proc. R. Soc. Lond. B 270, 2079–2086 (doi:10.1098/rspb.2003.2433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardullo R. A., Baltz J. M.1991Metabolic regulation in mammalian sperm: mitochondrial volume determines sperm length and flagellar beat frequency. Cell Motil. Cytoskeleton 19, 180–188 (doi:10.1002/cm.970190306) [DOI] [PubMed] [Google Scholar]
- Cummins J. M., Woodall P. F.1985On mammalian sperm dimensions. J. Reprod. Fertil. 75, 153–175 [DOI] [PubMed] [Google Scholar]
- Firman R. C., Simmons L. W.2008aThe frequency of multiple paternity predicts variation in testes size among island populations of house mice. J. Evol. Biol. 21, 1524–1533 (doi:10.1111/j.1420-9101.2008.01612.x) [DOI] [PubMed] [Google Scholar]
- Firman R. C., Simmons L. W.2008bPolyandry, sperm competition, and reproductive success in mice. Behav. Ecol. 19, 695–702 (doi:10.1093/beheco/arm158) [Google Scholar]
- Firman R. C., Simmons L. W.In press Experimental evolution of sperm quality via postcopulatory sexual selection in house mice. Evolution. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick J. L., Montgomerie R., Desjardins J. K., Stiver K. A., Kolm N., Balshine S.2009Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132 (doi:10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage M. J. G., Freckleton R. P.2003Relative testis size and sperm morphometry across mammals: no evidence for an association between sperm competition and sperm length. Proc. R. Soc. Lond. B 270, 625–628 (doi:10.1098/rspb.2002.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomendio M., Roldan E. R. S.1991Sperm competition influences sperm size in mammals. Proc. R. Soc. Lond. B 243, 181–185 (doi:10.1098/rspb.1991.0029) [DOI] [PubMed] [Google Scholar]
- Hosken D. J., Ward P. I.2001Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13 (doi:10.1046/j.1461-0248.2001.00198.x) [Google Scholar]
- Humphries S., Evans J. P., Simmons L. W.2008Sperm competition: linking form to function. BMC Evol. Biol. 8, 319 (doi:10.1186/1471-2148-8-319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüpold S., Calhim S., Immler S., Birkhead T. R.2009Sperm morphology and sperm velocity in passerine birds. Proc. R. Soc. B 276, 1175–1181 (doi:10.1098/rspb.2008.1645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo A. F., Gomendio M., Garde J., Lang-Lenton B., Soler A. J., Roldan E. R. S.2006Sperm design and sperm function. Biol. Lett. 2, 246–249 (doi:10.1098/rsbl.2006.0449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardia K. V., Kent J. T., Bibby J. M.1979Multivariate analysis. London, UK: Academic Press [Google Scholar]
- Murase T., Roldan E. R. S.1996Progesterone and the zona pellucida activate different transducing pathways in the sequence of events leading to diacylglycerol generation during mouse sperm acrosomal exocytosis. Biochem. J. 320, 1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayernia K., Drabent B., Adham I. M., Moschner M., Wolf S., Meinhart A., Engel W.2003Male mice lacking three germ cell expressed genes are fertile. Biol. Reprod. 69, 1973–1978 (doi:10.1095/biolreprod.103.018564) [DOI] [PubMed] [Google Scholar]
- Parker G. A.1998Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 3–54 London, UK: Academic Press [Google Scholar]
- Rugh R.1968The mouse: its reproduction and development. Minneapolis, MN: Burgess Publishing Company [Google Scholar]