Abstract
Migration remains one of the great mysteries of animal life. Small migratory birds rely on refuelling stopovers after crossing ecological barriers such as deserts or seas. Previous studies have suggested that fuel reserves may determine stopover duration but this hypothesis could not be tested because of methodological limitations. Here, we provide evidence that subcutaneous fat stores determine stopover duration by measuring the permanence of migratory garden warblers (Sylvia borin) on a small Mediterranean island during spring migration with telemetry methods. Garden warblers with large amounts of fat stores departed the island significantly sooner than lean birds. All except one fat bird left the island on the same evening after capture, with a mean total stopover estimate of 8.8 hours. In contrast, the mean estimated total stopover duration of lean birds was 41.3 hours. To our knowledge, this is the first study that measures the true minimum stopover duration of a songbird during migration.
Keywords: migration, garden warbler, stopover, subcutaneous body fat, telemetry, physiology of migration
1. Introduction
Many species of birds migrate each year and cross deserts or seas where food is not available. Migration across such ecological barriers relies on refuelling at stopover sites. The duration of a stopover is influenced by environmental factors including availability of food and weather conditions, and by endogenous programmes (reviewed in Gwinner & Helm 2003; Jenni & Schaub 2003). At the individual level, it has been suggested that subcutaneous fat deposits are the main physiological determinant of stopover duration (e.g. Bairlein 1985; Biebach 1985; Biebach et al. 1986; Cherry 1982; Gannes 2002; Moore & Kerlinger 1987, but see Kuenzi et al. 1991; Lyons & Haig 1995). This idea has never been tested properly, because actual departure from stopover sites in relation to fat stores has never been measured. Previous estimates from capture–recapture studies did not take into account the fact that lean birds are more active during the day when they search for food and hence are more likely to be recaptured than fat birds which feed less (Lindström & Alerstam 1992; Salewski & Schaub 2007). Indeed, such differences in the diurnal activity of fat and lean birds during stopover are known to occur (Bairlein 1985; Yong & Moore 1993; Titov 1999; Fusani et al. 2009). If this differential mobility is incorporated into capture–recapture studies and the entire stopover duration is estimated instead of minimum stopover, subcutaneous body fat does not appear to influence the stopover duration (Salewski & Schaub 2007).
To overcome the shortcomings of indirect estimates of stopover duration, we studied the influence of subcutaneous fat stores on stopover duration, at the same time minimizing variation in environmental parameters. In a quasi-experimental approach, we monitored radio-tagged lean and fat garden warblers (Sylvia borin) on a stopover site during consecutive days with constant optimal weather conditions. Because fat and lean birds were tagged at the same time, we were able to control for environmental variables, as any change in the latter would have equally affected the two groups.
2. Material and methods
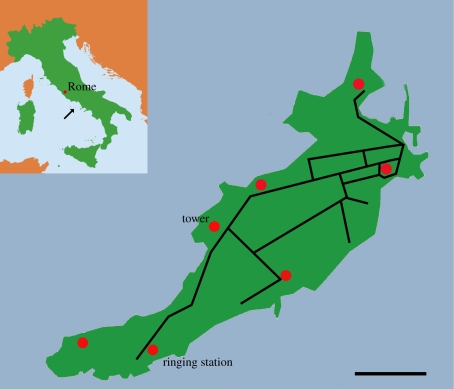
The study was conducted on Ventotene (Italy), an approximately 1.5 km2 island (figure 1) in the Tyrrhenian Sea (40°47′ N, 13°24′ E), which is visited by songbirds during migration. A ringing station has been active on the island since 1988. We caught garden warblers on 10 May (n = 18) and 11 May 2009 (n = 2), two consecutive days with constant weather conditions, which did not change until all birds had left the island (table 1). In fact, 8–13 May was the main period of broad-front garden warbler migration along the Pontinian islands (including Ventotene) in 2009.
Figure 1.
Map of Ventotene with main roads in black, red dots indicate one stationary (tower) and six mobile telemetry locations, the inlay shows Italy with a black arrow indicating the location of Ventotene (scale bar, 200 m).
Table 1.
Weather data from Ponza (40 km NE of Ventotene) and number of tagged and leaving birds (2 birds that lost their transmitters were excluded; period during which all birds left the island in italic; abbreviations: ENE, east northeast; ESE, east southeast; NE, northeast; NNE, north northeast; SSE, south southeast).
| date | temp. range (°C) | humidity (%) | air pressure range (hPa) | wind (20.00) | wind speed (20.00 h; km h−1) | clouds (20.00 h) | no. of birds tagged | no. of birds left |
|---|---|---|---|---|---|---|---|---|
| 10.05.2009 | 16–19 | 29–76 | 1020–1021 | ENE | 3.7 | no clouds | 17 | 8 |
| 11.05.2009 | 16–20 | 41–87 | 1019–1020 | NE | 16.7 | no clouds | 1 | 4 |
| 12.05.2009 | 17–21 | 30–71 | 1019–1020 | ESE | 5.6 | no clouds | 0 | 1 |
| 13.05.2009 | 17–20 | 45–79 | 1017–1020 | SSE | 7.4 | no clouds | 0 | 3 |
| 14.05.2009 | 18–23 | 41–67 | 1010–1016 | no | 0 | no clouds | 0 | 2 |
| 15.05.2009 | 17–21 | 77–91 | 1005–1010 | ESE | 27.8 | scattered | 0 | 0 |
| 16.05.2009 | 20–22 | 56–80 | 1005–1012 | SSE | 18.5 | cloudy | 0 | 0 |
| 17.05.2009 | 20–24 | 47–95 | 1014–1016 | NNE | 18.5 | cloudy | 0 | 0 |
Following the European ringing standards described in Bairlein (1995), we scored their subcutaneous fat on a 0–8 scale, the size of the pectoral muscles on a 0–3 scale, and measured body mass, tarsus and the third primary. We selected 10 lean (fat scores of 0 or 1, muscle scores 1 or 2) and 10 fat (fat scores 3 or 4, muscle scores 2 or 3) garden warblers and glued a Holohil LB-2 or LB-2N transmitter (battery life two weeks, weight 0.37 g) on their backs (skin glue, Sauer GmbH, Lobbach, Germany). The transmitters represented 1.9–2.7% of the body mass of the birds (lean birds range: 13.9–16.4 g; fat birds range: 15.4–19.1 g). We alternated tagging of fat and lean birds, so that capture time of day did not differ between lean and fat birds (Mann–Whitney U-test: U = 29.5, p = 0.12). Birds were released after attaching the transmitter, and their presence on the island monitored during day and night on a 2 h basis for the first 48 h, on a 4 h basis until the last animal had left, and a 9–12 h basis for five more days. Telemetry was conducted with one stationary receiver located at a tower and a mobile receiver at six other locations around the island (see figure 1 and the electronic supplementary material). In total, we conducted 923 scans to measure the absence or presence of radio-tagged individuals and unambiguously determined the presence of birds remaining on the island or their absence after departure to resume migration. Two garden warblers (one lean and one fat) lost their transmitters (continuous constant signal from a constant direction) and were excluded from the dataset. The signals of 14 birds were recorded during all scans, the signals of three birds were missed during one scan each, and the signal of one bird was missed during three subsequent scans, but then the signals of all these birds were detectable again until departure. Each frequency was checked for at least 72 h after the signal had disappeared, to exclude the possibility that the bird was only temporarily not detectable.
Weather data were obtained from Ponza, a neighbouring island 40 km northeast of Ventotene (table 1).
Statistical analyses were conducted with Systat 12 and Mark (White & Burnham 1999). Telemetry data were analysed with the ‘nest success model’ for radio-telemetric data implemented in Mark. Use of the nest success model was justified because of the high detection probability of radio-tagged birds (only six misses, see above). We (i) constructed a full model with survival probability depending on fat class, time (i.e. survival probability changes between different re-sightings) and the interaction between these two factors (φ[fat class * time]). We compared this full model with all other possible reduced models that considered (ii) only variation in survival probability over time independent of fat class (φ[time]), (iii) only variation in fat class independent on time (φ[fat class]), and (iv) a model constant survival model independent of fat class and time (φ[.]). The best model was selected according to the corrected Akaike information criterion (AICc) implemented in Mark, and the model fit was tested against the fit of the next best model using the likelihood ratio test implemented in Mark. Because our data were left-truncated, i.e. we did not know how long each bird had already been present on the island before capture, we estimated total stopover duration using the life expectancy formula S = −1/ln(φ) as suggested by Efford (2005; see also the electronic supplementary material for the calculations and the conversion of S into hours). Data are presented as means ± 95% CI.
3. Results
The body mass of fat birds (mean ± 95% CI: 17.3 ± 1.06 g) was significantly higher than the body mass of lean birds (15.3 ± 0.66 g; t-test: t13.4 = 3.723, p = 0.002); however, this difference was entirely due to subcutaneous fat deposits, as structural body size, measured either as the length of the tarsus (fat birds: 20.3 ± 0.52 mm; lean birds: 20.1 ± 0.23 mm) or the third outermost primary wing feather (fat birds: 62.2 ± 1.74 mm; lean birds: 62.9 ± 2.33 mm) did not differ between the two groups (tarsus: t11.1 = 0.937, p = 0.368; third primary: t14.8 = −0.573, p = 0.575).
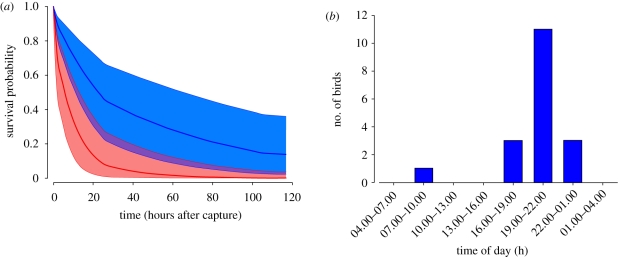
We then went on to test whether stopover duration differed between fat and lean birds. The nest success survival model including only fat class as an explanatory factor best explained the variation in the data and had a significantly better fit than the next best model with constant survival probability for all birds regardless of fat class and sampling interval (table 2; figure 2a). Thus, fat class best predicted stopover duration with fat birds having a lower mean stopover survival probability (φ = 0.833; 95% CI: 0.710–0.911) than lean birds (φ = 0.945; 0.898–0.971). Mean stopover time as estimated from the life expectancy formula (Efford 2005) was 8.8 h (3.9–19.3 h) for fat birds and 41.3 h (16.5–106.7 h) for lean birds. The result of the complex survival analysis was confirmed by a simple Mann–Whitney U-test, indicating that minimum stopover duration was significantly shorter in fat than in lean birds (U = 7.5, p < 0.004).
Table 2.
Comparison of nest survival models. The model including only fat class as predictive factor (φ[fat class]) best explained the variation in the data and had a significant better fit (χ2=5.86, p < 0.016) than the next best model in which survival was constant, i.e. independent of fat class and time (φ[.]; φ = survival probability).
| model | AICc | delta AICc | AICc weight | model likelihood | parameters | deviance |
|---|---|---|---|---|---|---|
| φ[fat class] | 122.461 | 0 | 0.871 | 1.000 | 2 | 118.405 |
| φ[.] | 126.278 | 3.82 | 0.129 | 0.148 | 1 | 124.260 |
| φ[time] | 160.136 | 37.37 | 0 | 0 | 34 | 79.130 |
| φ[fat class * time] | 229.035 | 106.57 | 0 | 0 | 60 | 62.410 |
Figure 2.
(a) Survival probability plot (mean φ ± 95% CI) of lean (blue) and fat (red) garden warblers. (b) Time histogram indicating the time of day (GMT + 1 h) at which individual garden warblers were observed for the last time on the island. All except one bird left the island during the night.
All except one bird departed from the island after sunset (figure 2b), suggesting that—although garden warblers may reach stop-over sites at any time when flying across the open Mediterranean Sea (Grattarola et al. 1999)—they resume migration at night.
4. Discussion
We conclude that fat stores predict stopover duration in migratory garden warblers. Because weather conditions were optimal for migration for the entire period of the study, any difference in stopover time between birds was likely to be due to internal factors only. Hence, although capture–recapture studies did not take into account the different mobility of fat and lean birds (Salewski & Schaub 2007) and due to right-censoring had a very low precision with respect to when individual birds really left stopover sites, they were right in concluding that stopover duration is determined mainly by fat stores (Cherry 1982; Bairlein 1985; Biebach 1985; Biebach et al. 1986; Moore & Kerlinger 1987; Gannes 2002).
We do not know exactly how long birds may already have stayed on the island before we caught and equipped them with a radiotransmitter. Thus, fat birds could have already spent more time on the island than lean birds. Because garden warblers and other passerines arrive at Ventotene with rather variable fat scores ranging from 0 to 5 (Pilastro & Spina 1997; Grattarola et al. 1999; W. Goymann, F. Spina & L. Fusani 2006–2009, personal observations) this is unlikely to be the case. Our data and those of Grattarola et al. (1999) suggest that garden warblers arriving with reasonable fat scores continue northward migration the following night, whereas birds with depleted fat stores need to stay and refuel before continuation of their journey. The life expectancy formula to calculate total stopover duration is most appropriate for populations with constant φ (Efford 2005). Because a survival model with constant φ was superior in explaining the variance in the data compared with models that assume variation of φ over time, we are confident that our life expectancy calculations resulting in different total stopover duration estimates for fat and lean birds provide a reasonable estimate of garden warblers' stopover behaviour at Ventotene.
Besides providing direct evidence that garden warblers leave stopover sites after sunset (for other migrants see Bolshakov & Chernetsov (2004) and Wikelski et al. (2003)), our study with free-living birds supports more than 200 years of studies starting with Naumann (1795–1817) which used Zugunruhe as a measure of migratory disposition in caged birds: fat reserves predict stopover duration (present study) as they do for Zugunruhe (Fusani et al. 2009).
Acknowledgements
All experimental procedures were authorized by the Regione Lazio with respect to Italian laws.
We thank Sandro Boccafogli, Lisa Carrera, Letizia Crava, Sara Lupi and Ivan Maggini for assistance in the field, and Manfred Gahr and the Max-Planck-Gesellschaft for funding and support, Martin Wikelski, Marilyn Ramenofsky and five anonymous referees for valuable comments. This is ‘Progetto Picole Isole ISPRA paper no. 41.
References
- Bairlein F.1985Body weights and fat deposition of palaearctic passerine migrants in the central Sahara. Oecologia 66, 141–146 (doi:10.1007/BF00378566) [DOI] [PubMed] [Google Scholar]
- Bairlein F.1995Manual of field methods. European–African songbird migration network. Wilhelmshaven, Germany: Institut für Vogelkunde [Google Scholar]
- Biebach H.1985Sahara stopover in migratory flycatchers: fat and food affect the time program. Experientia 41, 695–697 (doi:10.1007/BF02007727) [Google Scholar]
- Biebach H., Friedrich W., Heine G.1986Interaction of bodymass, fat, foraging and stopover period in trans-Sahara migrating passerine birds. Oecologia 69, 370–379 (doi:10.1007/BF00377059) [DOI] [PubMed] [Google Scholar]
- Bolshakov C., Chernetsov N.2004Initiation of nocturnal flight in two species of long-distance migrants (Ficedula hypoleuca and Acrocephalus schoenobaenus) in spring: a telemetry study. Avian Ecol. Behav. 12, 63–76 [Google Scholar]
- Cherry J. D.1982Fat deposition and length of stopover of migrant white-crowned sparrows. Auk 99, 725–732 [Google Scholar]
- Efford M. G.2005Migrating birds stop over longer than usually thought: comment. Ecology 86, 3415–3418 (doi:10.1890/04-1401) [Google Scholar]
- Fusani L., Cardinale M., Carere C., Goymann W.2009Stopover decision during migration: physiological conditions predict nocturnal restlessness in wild passerines. Biol. Lett. 5, 302–305 (doi:10.1098/rsbl.2008.0755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannes L. Z.2002Mass change pattern of blackcaps refueling during spring migration: evidence for physiological limitations to food assimilation. Condor 104, 231–239 (doi:10.1650/0010-5422(2002)104[0231:MCPOBR]2.0.CO;2) [Google Scholar]
- Grattarola A., Spina F., Pilastro A.1999Spring migration of the garden warbler (Sylvia borin) across the Mediterranean Sea. J. Ornithol. 140, 419–430 (doi:10.1007/BF01650986) [Google Scholar]
- Gwinner E., Helm B.2003Circannual and circadian contributions to the timing of avian migration. In Avian migration (eds Berthold P., Gwinner E., Sonnenschein E.), pp. 81–95 Berlin, Germany: Springer [Google Scholar]
- Jenni L., Schaub M.2003Behavioural and physiological reactions to environmental variation in bird migration: a review. In Avian migration (eds Berthold P., Gwinner E., Sonnenschein E.), pp. 155–171 Berlin, Germany: Springer [Google Scholar]
- Kuenzi A. J., Moore F. R., Simons R. R.1991Stopover of neotropical landbird migrants on East Ship Island following trans-gulf migration. Condor 93, 869–883 (doi:10.2307/3247722) [Google Scholar]
- Lindström Å., Alerstam T.1992Optimal fat loads in migrating birds: a test of the time-minimization hypothesis. Am. Nat. 140, 477–491 (doi:10.1086/285422) [DOI] [PubMed] [Google Scholar]
- Lyons J. E., Haig S. M.1995Fat content and stopover ecology of spring migrant semipalmated sandpipers in South Carolina. Condor 97, 427–437 (doi:10.2307/1369028) [Google Scholar]
- Moore F., Kerlinger P.1987Stopover and fat deposition by North American wood-warblers (Parulinae) following spring migration over the gulf of Mexico. Oecologia 74, 47–54 (doi:10.1007/BF00377344) [DOI] [PubMed] [Google Scholar]
- Naumann J. A.1795–1817Naturgeschichte der Land- und Wasser-Vögel des nördlichen Deutschlands und angränzender Länder. Aue, Germany: Köthen [Google Scholar]
- Pilastro A., Spina F.1997Ecological and morphological correlates of residual fat reserves in passerine migrants at their spring arrival in southern Europe. J. Avian Biol. 28, 309–318 (doi:10.2307/3676944) [Google Scholar]
- Salewski V., Schaub M.2007Stopover duration of Palearctic passerine migrants in the western Sahara—independent of fat stores? Ibis 149, 223–236 (doi:10.1111/j.1474-919X.2006.00608.x) [Google Scholar]
- Schmaljohann H., Liechti F., Bruderer B.2007Songbird migration across the Sahara: the non-stop hypothesis rejected! Proc. R. Soc. B 274, 735–739 (doi:10.1098/rspb.2006.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titov N.1999Fat level and temporal pattern of diurnal movements of robins (Erithacus rubecula) at an autumn stopover site. Avian Ecol. Behav. 2, 89–99 [Google Scholar]
- Tsvey A., Bulyuk V., Kosarev V.2007Influence of body condition and weather on departures of first-year European robins, Erithacus rubecula, from an autumn migratory stopover site. Behav. Eco. Sociobiol. 61, 1665–1674 (doi:10.1007/s00265-007-0397-z) [Google Scholar]
- White G. C., Burnham K. P.1999Program Mark: survival estimation from populations of marked animals. Bird Study 46(Suppl.), 120–138 [Google Scholar]
- Wikelski M., Tarlow E. M., Raim A., Diehl R. H., Larkin R. P., Visser G. H.2003Avian metabolism: costs of migration in free-flying songbirds. Nature 423, 704 (doi:10.1038/423704a) [DOI] [PubMed] [Google Scholar]
- Yong W., Moore F. R.1993Relation between migratory activity and energetic condition among thrushes (Turdinae) following passage across the Gulf of Mexico. Condor 95, 934–943 (doi:10.2307/1369429) [Google Scholar]