Abstract
Recent discoveries of tetrapod trackways in 395 Myr old tidal zone deposits of Poland (Niedźwiedzki et al. 2010 Nature 463, 43–48 (doi:10.1038/nature.08623)) indicate that vertebrates had already ventured out of the water and might already have developed some air-breathing capacity by the Middle Devonian. Air-breathing in lungfishes is not considered to be a shared specialization with tetrapods, but evolved independently. Air-breathing in lungfishes has been postulated as starting in Middle Devonian times (ca 385 Ma) in freshwater habitats, based on a set of skeletal characters involved in air-breathing in extant lungfishes. New discoveries described herein of the lungfish Rhinodipterus from marine limestones of Australia identifies the node in dipnoan phylogeny where air-breathing begins, and confirms that lungfishes living in marine habitats had also developed specializations to breathe air by the start of the Late Devonian (ca 375 Ma). While invasion of freshwater habitats from the marine realm was previously suggested to be the prime cause of aerial respiration developing in lungfishes, we believe that global decline in oxygen levels during the Middle Devonian combined with higher metabolic costs is a more likely driver of air-breathing ability, which developed in both marine and freshwater lungfishes and tetrapodomorph fishes such as Gogonasus.
Keywords: Dipnoi, air-gulping, Rhinodipterus, Gogo formation, Devonian
1. Introduction
Dipnoans, or lungfish, are an ancient lineage of osteichthyan fish that first appeared in the Early Devonian (Chang & Yu 1984). Today they are represented by three genera, all of which respire bimodally to some extent (Graham 1997). All exist in freshwater environments exposed to seasonal drying or anoxic conditions. Lungfish take in air by gulping a bubble in the mouth, thus a large buccal cavity is an intrinsic feature of all modern air-breathing species.
The incidence of air-gulping in extinct taxa is more poorly understood. Because of the paucity of soft tissue preservation, we must rely on osteological features associated with air-breathing as indicators of behaviour in archaic forms. Cranial ribs, paired structures that attach to the base of the neurocranium, are important for air-gulping in extant species by assisting in hyoid depression and anchoring the pectoral girdle (Bishop & Foxon 1968). Other features associated with air gulping include the possession of a large buccal cavity (indicated by an elongated parasphenoid), a slot between tooth plates for a tongue pad, and a highly mobile ceratohyal and pectoral girdle. Strongly curved pleural ribs like those seen in Howidipterus and Barwickia from the Middle Devonian of Australia have been interpreted as an adaptation for enlarged lungs (Long & Clement 2009), unlike the stout, straighter ribs of the marine Gogo lungfishes (Campbell & Barwick 2002). The ribs of Rhinodipterus ulrichi from Germany were interpreted as being gently curved (Schultze 1975).
The distribution of cranial ribs is known from the Middle Devonian to recent lungfish. Recorded from Howidipterus and Barwickia from a Middle Devonian lacustrine site in Australia (Long 1992), they are also present in later taxa such as Sagenodus (Schultze & Chorn 1997) and Gnathorhiza (Berman 1976). Like the extant taxa, these fishes inhabited freshwater systems exposed to seasonal drying or anoxia, possibly caused by rotting vegetation. Fleurantia and Scaumenacia from the marginal marine Late Devonian deposits of Quebec also possess cranial ribs (Cloutier 1996), their presence in Dipterus from the fluvial Old Red Sandstone of Scotland is not confirmed (Ahlberg & Trewin 1995). Schultze (1975) was first to record the presence of cranial ribs in Rhinodipterus.
It is popularly believed that vertebrate air-gulping originated in a tropical lowland setting (Thomson 1969). Most fossil lungfish inferred to be air-gulpers have all been from freshwater deposits. The exceptions are the Escuminac Bay fauna, and partial remains of Rhinodipterus from marginal marine environments from the Late Devonian of Europe (Gross 1956; Ørvig 1961; Cloutier 1996). Usually, marine Dipnoi lack the morphological features that would indicate that they were air-breathers (den Blaauwen et al. 2005), and no evidence of cranial ribs in the common Late Devonian marine Gogo fauna (Holodipterus, Chirodipterus, Griphognathus) (Miles 1977). However, cranial rib articulations and other features suggesting air-gulping behaviour have been found in a new species of Rhinodipterus recently discovered from the shallow marine reef environment of the Gogo Formation, Western Australia.
2. Material and methods
(a). Site information
(i). The Gogo formation
The Gogo formation is an early to mid-Frasnian shallow marine inter-reef shale deposit within a large reef complex in the Canning Basin, Western Australia (Playford 1980). Gogo has yielded an incredibly diverse fish fauna with over 50 species described so far, more than 10 of which are dipnoans.
(b). Methods
A new species of Rhinodipterus from the Gogo formation of Western Australia is known from a single incomplete specimen (WAM 09.6.149) and will be described in detail in a separate paper (Clement in preparation). The specimen was prepared by one of us (J.L.) using weak acetic acid (7–10%), fortified by Mowital B30 consolidant, and coated in a sublimate of ammonium chloride for photography.
3. Morphological observations
(a). Air-gulping features
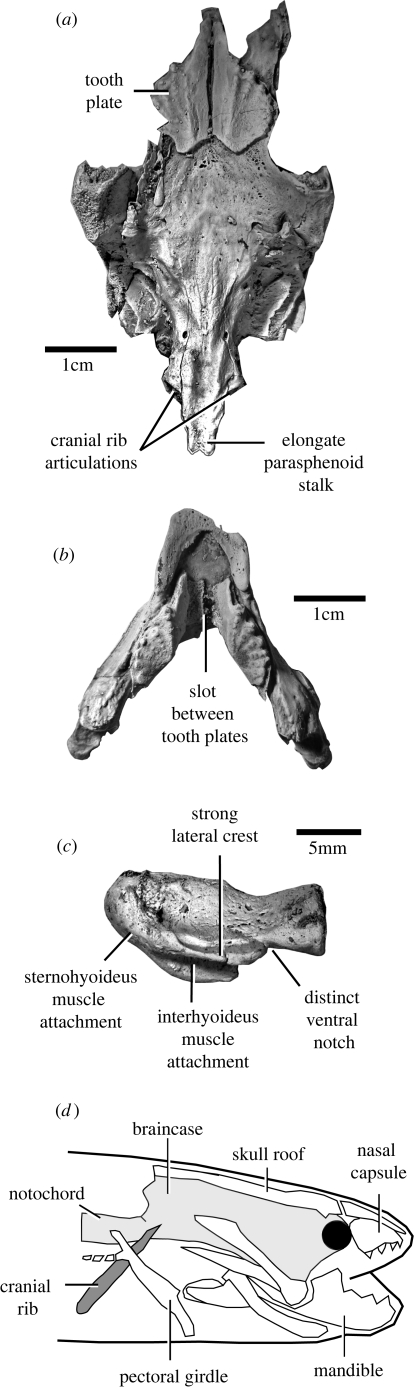
Figure 1 shows the characteristic elongated parasphenoid and articulations for cranial ribs of Rhinodipterus. The lengthening of the parasphenoid extending posterior to the neurocranium significantly enlarges the buccal cavity, would have enabled the animal to hold a large air bubble. The slot between the tooth plates for a tongue pad (figure 1b) acts as a stop-valve when air is being forced into the lungs (Campbell & Barwick 1988).
Figure 1.
(a) Rhinodipterus sp. skull in ventral view. (b) Rhinodipterus sp. mandible in dorsal view. (c) Rhinodipterus sp. ceratohyal in lateral view. (d) The extant Protopterus showing arrangement of cranial ribs attached to neurocranium (adapted from Bemis 1986).
The distinct ‘stepped’ shape of the ceratohyal (indicated by the ventral notch) in Rhinodipterus (figure 1c) is more similar to Neoceratodus (Günther 1871) than other Devonian genera. The strong posterior margin of this bone indicates a robust connection of the sternohyoideus muscle to the pectoral girdle. The large lateral crest on this bone provides a larger area for interhyoideus muscle attachment, and these features indicate highly controlled mobility of the hyoid arch. As Neoceratodus uses its ceratohyals to push air from the branchial chambers into the lung when breathing (den Blaauwen et al. 2005), we suggest that Rhinodipterus also had increased capacity for this action when compared with other marine dipnoans.
The presence of the large articulations for cranial ribs, an elongate parasphenoid (figure 1a) and a mobile ceratohyal and pectoral girdle in Rhinodipterus indicate air-gulping behaviour. Cranial ribs anchor the pectoral girdle during air-gulping in extant forms (figure 1d) and are a key feature in all air-breathing forms (Long 1993).
4. Discussion
(a). Drivers of aerial respiration
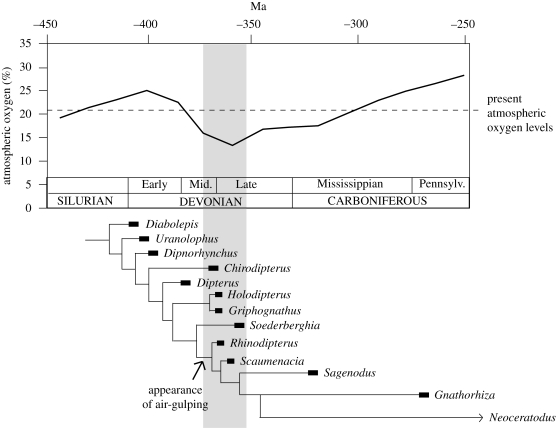
Air-gulping specializations first appeared within the Dipnoi during the Middle Devonian (figure 2), a time of maximum low global oxygen levels (Berner 2006). Air-gulping is thought to have evolved to supplement conventional aquatic respiration during this time of low oxygen (Thomson 1969). Their adaptations have also been interpreted as for buoyancy control (Graham 1997), to allow the expulsion of excessive carbon dioxide (Thomson 1969), or to supply the heart with an additional source of oxygen (Farmer 1999). However, in many extant fishes including Neoceratodus, activity is a stronger stimulus for air breathing than aquatic hypoxia (Johansen et al. 1967). The question remains as to whether such adaptations were environmentally or metabolically induced.
Figure 2.
Palaeozoic global oxygen levels (Berner 2006) and the rise of air-gulping. Shaded area represents global oxygen levels of 15% or less. Rhinodipterus has been added to the pruned tree of Ahlberg et al. (2006) to show monophyly of air-gulping dipnoans.
(b). Where did air-gulping evolve?
Marine waters are usually better oxygenated than most freshwater environments because of increased wind-driven mixing and tidal flushing (Graham 1997). This trend contributed to the belief that air-gulping must have evolved in hypoxic or anoxic freshwater conditions. However, while hypoxia in marine environments is generally not as extreme, widespread or prolonged as in freshwater habitats, it does still exist. An expansion of hypoxic waters across continental shelves occurred during the warm climate of the Palaeozoic (Berry et al. 1989), with hypoxic conditions common in shallow marine and estuarine areas. Despite this, few workers considered that aerial respiration could have evolved in marine waters.
The presence of Rhinodipterus in marine conditions might indicate that these environments were oxygen stressed. Many of the exceptionally preserved specimens from Gogo show asymmetry (e.g. variable shape and number of dermal skull bones) with high levels of variability often present within the same individual (Long & Trinajstic in press). Increasing asymmetry has been shown to occur in animals in stressed habitats (Parsons 1992). The superb state of preservation of the Gogo fossils also indicates quick burial and low oxygen levels. While marine conditions were generally better oxygenated than many freshwater environments, there was still sufficient hypoxia in some marine environments to select for air-gulping (Playford & Wallace 2001). A large spiracular notch suggests accessory air intake by comparison with extant forms like Polypterus. The tetrapodomorph fish Gogonasus shows large spiracular openings on top of the skull (Long et al. 2006), indicating that some other Gogo fishes may also have been adapting to hypoxic conditions.
Ecological radiations of fishes in the Devonian are likely to have been associated with increased metabolic costs for greater mobility, and more intensive competition and predation pressures (Graham 1997). The ability to extract additional oxygen could have afforded air-gulping lungfishes the selective advantage of higher rates of metabolic activity, and does not confine their evolution to specific ecological conditions.
5. Conclusions
Rhinodipterus provides the earliest unequivocal evidence of air-gulping adaptations in a marine lungfish. Extant lungfish shed little light on the selective pressures of the earliest air-breathing dipnoans. Despite the ambiguity of the environment in which air-gulping evolved, this observation raises some interesting phylogenetic implications. Do all the air-gulpers belong to a single lineage or did air-gulping evolve more than once in the Dipnoi? Such a complex suite of characters as those involved in aerial respiration most probably evolved only once, suggesting that these fishes comprise a monophyletic group (figure 2), based on the recent phylogenetic analysis of lungfishes by Ahlberg et al. (2006). We suggest that it was a combination of increased metabolic activity combined with low global oxygen levels that drove the evolution of air-gulping in lungfishes during the Devonian Period.
Acknowledgements
We thank K. Campbell for useful discussion, G. Young for comments on the manuscript, and A. Warren who discovered the specimen. This work was funded by ARC Discovery Grant DP0772138.
References
- Ahlberg P. E., Trewin N. H.1995The postcranial skeleton of the Middle Devonian lungfish Dipterus valenciennesi. Trans. R. Soc. Edin. Earth Sci. 85, 159–175 [Google Scholar]
- Ahlberg P. E., Smith M. M., Johanson Z.2006Developmental plasticity and disparity in early dipnoan (lungfish) dentitions. Evol. Dev. 8, 331–349 [DOI] [PubMed] [Google Scholar]
- Bemis W. E.1986Feeding systems of living Dipnoi: anatomy and function. J. Morphol. Suppl. 1, 249–275 [Google Scholar]
- Berman D. S.1976Cranial morphology of the Lower Permian lungfish Gnathorhiza (Osteichthyes: Dipnoi). J. Paleontol. 50, 1020–1033 [Google Scholar]
- Berner R. A.2006GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmoshim. Acta 70, 5653–5664 (doi:10.1016/j.gca.2005.11.032) [Google Scholar]
- Berry W. B. N., Wilde P., Quinby-Hunt M. S.1989Paleozoic (Cambrian through Devonian) anoxitropic biotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 74, 3–13 (doi:10.1016/0031-0182(89)90016-3) [Google Scholar]
- Bishop I. R., Foxon G. E.1968The mechanism of breathing in the South American lungfish, Lepidosiren paradoxa, a radiological study. J. Zool. 154, 263–271 (doi:10.1111/j.1469-7998.1968.tb01663.x) [Google Scholar]
- Campbell K. S. W., Barwick R. E.1988Geological and palaeontological information and phylogenetic hypotheses. Geol. Mag. 125, 207–227 (doi:10.1017/S0016756800010165) [Google Scholar]
- Campbell K. S. W., Barwick R. E.2002The axial postcranial structure of Griphognathus whitei from Gogo; comparisons with other Devonian dipnoans. Record West. Aust. Mus. 21, 167–201 [Google Scholar]
- Chang M. M., Yu X.1984Structure and phylogenetic significance of Diabolichthys speratus gen. et sp. nov., a new dipnoan-like form from the Lower Devonian of eastern Yunnan, China. Proc. Linn. Soc. NSW 107, 171–184 [Google Scholar]
- Clement A. M.In preparation A new species of Rhinodipterus: an air-gulping lungfish from the Late Devonian of Australia and its biogeographical implications. [Google Scholar]
- Cloutier R.1996Dipnoi (Akinetia: Sarcopterygii). In Devonian fishes and plants of Miguasha, Quebec, Canada (eds Schultze H. P., Cloutier R.), pp. 198–226 Munich, Germany: Pfeil, Verlag [Google Scholar]
- den Blaauwen J. L., Barwick R. E., Campbell K. S. W.2005Structure and function of the tooth plates of the Devonian lungfish Dipterus valenciennesi from Caithness and the Orkney Islands. Record West. Aust. Mus. 23, 91–113 [Google Scholar]
- Farmer C. G.1999Evolution of the vertebrate cardio-pulmonary system. Ann. Rev. Physiol. 61, 573–592 (doi:10.1146/annurev.physiol.61.1.573) [DOI] [PubMed] [Google Scholar]
- Graham J. B.1997Air-breathing fishes: evolution, diversity and adaptation. San Diego, CA: Academic Press [Google Scholar]
- Gross W.1956Über Crossopterygier und Dipnoer aus dem baltischen Oberdevon im Zusammenhang einer vergleichenden Untersuchung des Porenkanalsystems paläozoischer Agnathen und Fische. Stockholm, Sweden: Almqvist and Wiksell [Google Scholar]
- Günther A.1871Description of Ceratodus, a genus of ganoid fishes recently discovered in rivers of Queensland, Australia. Phil. Trans. R. Soc. Lond. 161, 511–571 (doi:10.1098/rstl.1871.0020) [Google Scholar]
- Johansen K., Lenfant C., Grigg G. C.1967Respiratory control in the lungfish Neoceratodus forsteri (Krefft). Comp. Biochem. Physiol. 20, 835–854 (doi:10.1016/0010-406X(67)90057-6) [Google Scholar]
- Long J. A.1992Cranial anatomy of two new Late Devonian lungfishes, from Mt Howitt, Victoria. Record Aust. Mus. 44, 299–318 (doi:10.3853/j.0067-1975.44.1992.37) [Google Scholar]
- Long J. A.1993Cranial Ribs in Devonian lungfishes and the origin of dipnoan air-breathing. Mem. Assoc. Austral. Palaeontol. 15, 199–209 [Google Scholar]
- Long J. A., Clement A. M.2009The postcranial anatomy of two Middle Devonian lungfishes (Osteichthyes, Dipnoi) from Mt. Howitt, Victoria, Australia. Mem. Mus. Victoria 66, 189–202 [Google Scholar]
- Long J. A., Trinajstic K.In press The Late Devonian Gogo Formation lägerstatten of Western Australia– exceptional vertebrate preservation and diversity. Ann. Rev. Earth Planet. Sci. 38 [Google Scholar]
- Long J. A., Young G. C., Holland T., Senden T. J., Fitzgerald E. M. G.2006An exceptional Devonian fish from Australia sheds light on tetrapod origins. Nature 444, 199–202 (doi:10.1038/nature05243) [DOI] [PubMed] [Google Scholar]
- Miles R. S.1977Dipnoan (lungfish) skulls and the relationships of the group: a study based on new species from the Devonian of Australia. Zool. J. Linn. Soc. 61, 1–328 [Google Scholar]
- Niedźwiedzki G., Szrek P., Narkiewicz K., Narkiewicz M., Ahlberg P. E.2010Tetrapod trackways from the early Middle Devonian period of Poland. Nature 463, 43–48 (doi:10.1038/nature08623) [DOI] [PubMed] [Google Scholar]
- Ørvig T.1961New finds of acanthodians, arthrodires, crossopterygians, ganoids and dipnoans in the upper Middle Devonian calcareous flags (Oberer Plattenkalk) of the Bergisch Gladbach-Paffrath trough. Palaontol. Zeitschr. 35, 10–27 [Google Scholar]
- Parsons P. A.1992Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity 68, 361–364 [DOI] [PubMed] [Google Scholar]
- Playford P. E.1980Devonian ‘great barrier reef’ of Canning basin, Western Australia. Bull. Am. Assoc. Petrol. Geol. 64, 814–840 [Google Scholar]
- Playford P. E., Wallace C. J. K.2001Exhalative mineralization in Devonian reef complexes of the Canning Basin, Western Australia. Econ. Geol. 96, 1595–1610 (doi:10.2113/96.7.1595) [Google Scholar]
- Schultze H. P.1975Das Axialskelett der Dipnoer aus dem Overdevon von Bergisch-Gladbach (Westdeutschland). Colloques int. Cent. natn. Res. scient. 218, 149–159 [Google Scholar]
- Schultze H. P., Chorn J.1997The permo-carboniferous genus Sagenodus and the beginning of modern lungfish. Contrib. Zool. 67, 9–70 [Google Scholar]
- Thomson K. S.1969The biology of the lobe-finned fishes. Biol. Rev. 44, 91–154 (doi:10.1111/j.1469-185X.1969.tb00823.x) [DOI] [PubMed] [Google Scholar]