Abstract
Frogs have highly conserved hand and foot morphology, possessing four fingers and five toes. As an exception, two Japanese ranid frog species, the Otton frog Babina subaspera and the dagger frog Babina holsti, possess a unique thumb-like structure (the pseudothumb) in the forelimb, giving an appearance of a total of five fingers on the hand. To obtain insights into the developmental mechanisms that generate this novel character, we investigated the hand morphogenesis of the Otton frog. The unique morphological pattern of the pseudothumb was already established in juveniles. Surprisingly, the bud-like structure, which is similar to the area of inductive activity (e.g. feather buds in birds and the carapacial ridge in turtles), was detected over the site where the future prepollex develops in larvae. By contrast, this bud-like structure was not found in larvae of other ranid species. We discuss possible scenarios that would favour the evolution of this very unusual trait in frogs.
Keywords: Anura, limb morphogenesis, morphological novelty, prepollex, Ranidae
1. Introduction
Frogs and toads (order Anura) usually have four-fingered hands and five-toed legs. As an exception, two ranid species of the genus Babina, the Otton frog Babina subaspera (figure 1a) and the dagger frog Babina holsti that are, respectively, endemic to the Amami Island Group and the Okinawa Island Group in the central part of the Ryukyu Archipelago, Japan, possess a thumb-like structure medial to the first finger, giving the appearance of a total of five fingers on the hand (Maeda & Matsui 1999). The genus Babina (sensu Frost 2009) consists of nine species, of which the other seven species occurring in Vietnam, southern China, Taiwan and the southern Ryukyus have no such structure (Frost et al. 2006). As an initial step in understanding the developmental mechanisms underlying the generation of this unique character, we describe hand morphogenesis of B. subaspera.
Figure 1.
Comparison of prepollex morphology between the Otton frog, Babina subaspera and other anuran species. (a) An adult Otton frog in the field of Amamioshima, Japan. (b) Radiograph of an adult male B. subaspera (snout-vent length = 132.9 mm), focusing on completely ossified prepollical spine (arrowhead). (c) Ventral view of right-hand skeleton stained with Alcian blue (for cartilage) and Alizarin red (for bone) of Glandirana rugosa at stage 45. (d) Ventral view of right-hand skeleton of B. subaspera at the same ontogenetic stage. (e,f) Schematic illustrations of the pictures given in (c) and (d), respectively. Red, ossified metacarpals I–IV; blue, cartilaginous elements represented by the complex of carpals I–IV, ulnare, intermedium, centrale, radiale, Element Y (cpl) and prepollex (asterisk); yellow, tissues overlaying the skeletal elements. Arrows indicate the direction in which the prepollex curves. In juveniles of typical frog species, the prepollex is never beyond the distal end of metacarpal I and bends towards the first finger. In juvenile B. subaspera, the tip of the prepollex reaches to the end of metacarpal I and turns to the opposite direction of the first finger. Scale bars, (c,d) 1 mm.
2. Material and methods
We adopted three approaches. First, to confirm the existence of thumb-like structure in B. subaspera, adults were captured in the field for observations of superficial hand morphology. Sex was determined based on the presence of a vocal opening in males. Second, to compare the morphology of forelimb skeletons between B. subaspera and other anuran species, juveniles and adults were examined by radiograph or whole-mount skeletal preparation (Klymkowsky & Hanken 1992). Third, to examine the internal structure of the hand in larval and juvenile stages, histological sections were prepared. Forelimb anlagen were extracted from the gill chambers, and the tissues were fixed with Bouin's fixative or 4 per cent paraformaldehyde and embedded in paraffin. Then, they were cut into 6–8 µm thick sections with microtome and stained with haematoxylin–eosin or Milligan's trichrome, following standard histological protocols. Developmental stages of larvae and juveniles were determined following the criteria of Gosner (1960). A total of eight species: B. holsti, Babina okinavana, B. subaspera, Glandirana rugosa, Lithobates catesbeiana, Rana japonica, Rana ornativentris (Ranidae) and Rhacophorus arboreus (Rhacophoridae) were examined (the taxonomic system follows Frost 2009). A list of frog specimens used for each investigation is given in the electronic supplementary material 1. In this study, we describe the thumb-like structure that is located medial to the first finger: a complex of skeletal elements, muscles, connective tissues and skin that is possessed by B. holsti and B. subaspera as a ‘pseudothumb’. Skeleton (bone or cartilage) that exists inside the pseudothumb is defined as a ‘prepollex’, following the standard convention (e.g. Duellman & Trueb 1994; Fabrezi 2001), the transformed prepollex, whose tip is very sharp and curves in the opposite direction to the first finger, which is observed in some anuran species including B. holsti and B. subaspera, is defined as the ‘prepollical spine’.
3. Results
All 140 adult B. subaspera (male: n = 76 and female: n = 64) captured in the field possessed the pseudothumb that was separated from the first finger in the hand. Radiographs and whole-mount skeletal preparations of adult specimens (n = 5) revealed that well-developed prepollex was completely ossified, in addition to skeletal elements of each finger (figure 1b). The tip of the prepollex was very sharp, approaching the condition of a prepollical spine. A prepollex consists of two articulated bones: the distal one that constitutes main part of the prepollical spine and the proximal one that articulates with more proximal Element Y and carpal 1, which has a spherical surface (electronic supplementary material 2). Anatomical comparison of architectural patterns of the hand skeletal muscles showed no obvious differences among species. In B. subaspera, the distal part of the prepollex was accompanied by a large amount of connective tissue. By examining museum specimens, we confirmed that B. holsti also possessed the pseudothumb and prepollical spine (n = 2; electronic supplementary material 3).
Subsequently, skeletal anatomy in juveniles (stage 45) was compared between non-Babina frog species and B. subaspera. In both groups, the shaft of the radioulna, metacarpals and phalanges were already ossified, as indicated by Alizarin uptake (figure 1c,d). The prepollex was present in all species examined, but was still cartilaginous, stained by Alcian blue, as well as carpal elements. The size of the prepollex varied among species. In non-Babina species, the tip of the prepollex never extended beyond the distal end of the first metacarpal (figure 1c,e). By contrast, in B. subaspera, the prepollex was elongated and its tip extended to the level of the distal end of the first metacarpal (figure 1d,f). The most striking difference between the two groups was the direction in which the prepollex curved. In non-Babina species, the prepollex was bent in the direction of the first finger (figure 1c,e). On the other hand, in B. subaspera, the prepollex was curved in the opposite direction of the first finger, separated from it (figure 1d,f and electronic supplementary material 4).
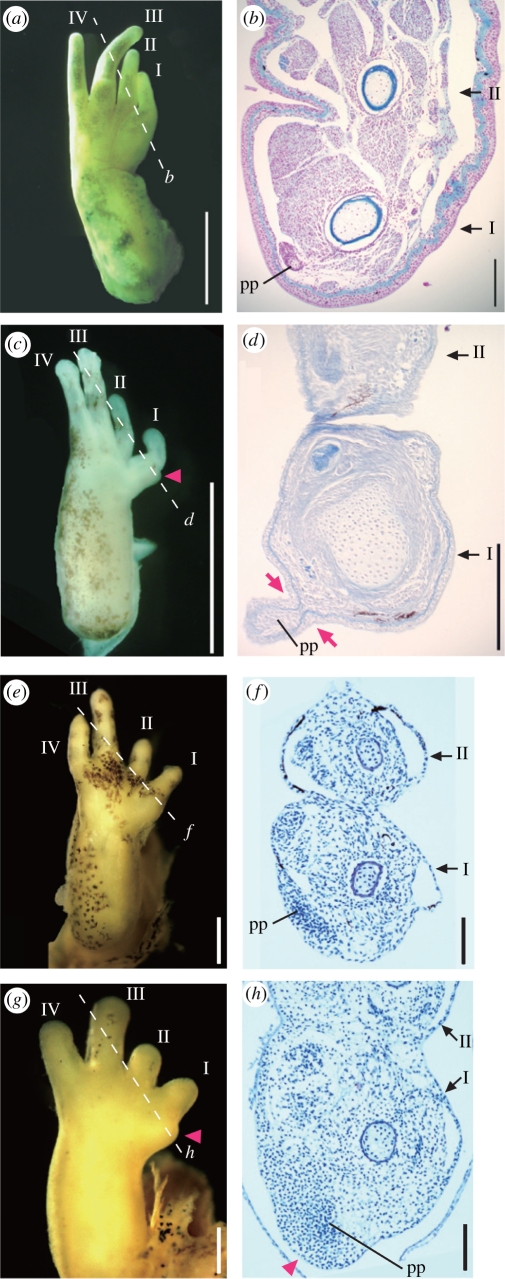
In larvae (stage 37), the whole prepollex was encased in the sheath of the first finger in non-Babina ranids (G. rugosa, L. catesbeiana, R. japonica; figure 2a,b). By contrast, the future pseudothumb region was separated from the first finger by a notch of thickened epidermis in B. subaspera (figure 2c,d). Surprisingly, the domain where the future prepollex should be established had already swelled in the forelimb anlagen of B. subaspera at the earlier larval stage (stage 36), whereas no such bud-like structure was observed at the corresponding domain in non-Babina ranids (G. rugosa and L. catesbeiana) at the same ontogenetic stage (figure 2e,g). Histological sections revealed that the newly developed cartilaginous prepollex was anteriorly covered with a thin epidermal layer and a small number of mesenchymal cells in non-Babina ranids (figure 2f). On the other hand, the newly formed prepollex was overlaid by a large number of mesenchymal cells that occupies the inside of the bud-like structure in B. subaspera (figure 2h).
Figure 2.
Comparison of early prepollex development between non-Babina ranids and Babina subaspera. (a) Ventral view of right forelimb anlage of Lithobates catesbeiana larva at stage 37. (b) Histological section showing the transverse plane of the first and second fingers of the specimen in (a). The left is the ventral side. (c) Right forelimb anlage of B. subaspera larva at stage 37. Arrowhead indicates a bud-like structure medial to the first finger. (d) Histological section showing the transverse plane in (c). Arrows indicate thickened epidermis that separates the pseudothumb from the first finger. (e) Right forelimb anlage of Glandirana rugosa larva at stage 36. (f) Histological section showing the transverse plane in (e). (g) Right forelimb anlage of B. subaspera larva at stage 36. (h) Histological section showing the transverse plane in (g). A bud-like structure (arrowhead) is composed of the ectoderm underlain by a large amount of mesenchymal cells over the just-formed cartilaginous condensation of the prepollex (pp). No corresponding structure is observed in the larval hand of non-Babina ranids. I–IV indicate each finger. Scale bars, (a,c) 2.5 mm, (b,d) 0.2 mm, (e,g) 0.5 mm, and (f,h) 0.1 mm.
4. Discussion
In B. subaspera, the domain where the future pseudothumb emerges was already swollen at the larval stage, but such a bud-like structure was not found in stage- and size-matched forelimbs of G. rugosa and stage-matched but larger forelimbs of L. catesbeiana. This feature suggests that the pseudothumb of B. subaspera is a novel trait in frogs. A heterochronic shift in the activation timing of genetic pathways that enlarge the prepollex (e.g. in adult males of Hyla rosenbergi (Kluge 1981) reviewed in Wells 2007) in larval stages might be a possible mechanism underlying the formation of the pseudothumb in Babina.
Although epidermal thickening is not seen in its apical domain, the bud-like structure resembles the area of epithelial–mesenchymal interactions, such as early-stage feather buds in avian embryos (Eames & Schneider 2005) and the carapacial ridge in chelonian embryos (Nagashima et al. (2007) and references therein). We speculate that the mesenchymal cells which occupy the inside of the bud (figure 2h) are highly proliferative and secrete chemotactic factors (e.g. fibroblast growth factors) and regulate the outgrowth and patterning of the underlying cartilaginous prepollex uniquely in this anuran species, as in the carapacial ridge that establishes the characteristic rib pattern in turtles. Molecular mechanisms that spatially regulate the development of endochondral bones in the vertebrate limb are poorly understood. The Otton frog, B. subaspera, would be interesting material for elucidating potential mechanisms that regulate the outgrowth and patterning of appendicular bones in vertebrates.
Fabrezi (2001) critically discussed the homology of the anuran prepollex and concluded that it was non-homologous with true digits based on developmental criteria. In this study, we observed that the developmental pattern was different between the prepollex and so-called digits: ossification is substantially delayed in the former. Considering the above, we have treated the anuran prepollex as non-homologous with the true digits and called the thumb-like structure, which is mainly composed of the prepollex and is seen in two Japanese Babina species, a ‘pseudothumb’. Of course, these data are not enough to provide a clear answer to digit homology. Expression analysis of genes that specify the first finger of tetrapods such as Hoxd12-9 (reviewed in Wagner & Vargas 2008; Young et al. 2009) in anuran larval manus would be helpful.
A thumb-like structure that contains a prepollical spine is found in males of several lineages of Anura (reviewed in Fabrezi 2001; Wells 2007). Prepollical spines are thought to have evolved for use in male–male combat as a weapon (Shine 1979) or in amplexus (Kluge 1981). However, B. subaspera possesses prepollical spines in both sexes. When disturbed by humans, they medially flex their forearms to attack with the sharp, ossified prepollical spine projected from the fleshy sheath. Although no direct observations exist, this defensive behaviour may also be employed to protect the frog from predators such as snakes. The function of this unique character in frogs together with its developmental mechanism will be an interesting topic both in morphological and ecological studies in the future.
Acknowledgements
The study was carried out under permission no. 87 from Kagoshima education commission issued to Prof. Hiroyoshi Higuchi.
The authors would like to thank Yuya Watari in helping collection of frog samples, Tomoaki Ueno (Aleph Animal Hospital, Tsukuba) for taking radiographs, Shin-ichiro Kawada (National Science Museum, Tokyo) for access to Museum collections, Matthew Brandley, Tsutomu Hikida, Atsushi Kurabayashi, Masafumi Matsui, Richard Schneider, Mamoru Toda and two anonymous referees for discussions. This study was partly financially supported by PRO NATURA FUND 2004.
References
- Duellman W. E., Trueb L.1994Biology of amphibians. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Eames B. F., Schneider R. A.2005Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development 132, 1499–1509 (doi:10.1242/dev.01719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrezi M.2001A survey of prepollex and prehallux variation in anuran limbs. Zool. J. Linn. Soc. 131, 227–248 (doi:10.1111/j.1096-3642.2001.tb01316.x) [Google Scholar]
- Frost D. R.2009Amphibian species of the world: an online reference, version 5.3 New York, NY: American Museum of Natural History; See http://research.amnh.org/vz/herpetology/amphibia/index.php [Google Scholar]
- Frost D. R., et al. 2006The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 297, 1–371 (doi:10.1206/0003-0090(2006)297[0001:TATOL]2.0.CO;2) [Google Scholar]
- Gosner K. L.1960A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 [Google Scholar]
- Kluge A. G.1981The life history, social organization, and parental behavior of Hyla rosenbergi Boulenger, a nest-building gladiator frog. No. 160 Ann Arbor, MI: Museum of Zoology, University of Michigan [Google Scholar]
- Klymkowsky M. W., Hanken J.1992Whole-mount staining of Xenopus and other vertebrates. Meth. Cell Biol. 36, 419–441 (doi:10.1016/S0091-679X(08)60290-3) [DOI] [PubMed] [Google Scholar]
- Maeda N., Matsui M.1999Frogs and toads of Japan. Tokyo, Japan: Bun-Ichi Sogo Shuppan [Google Scholar]
- Nagashima H., Kuraku S., Uchida K., Ohya K. Y., Narita Y., Kuratani S.2007On the carapacial ridge in turtle embryos: its developmental origin, function and the chelonian body plan. Development 134, 2219–2226 (doi:10.1242/dev.002618) [DOI] [PubMed] [Google Scholar]
- Shine R.1979Sexual selection and the sexual dimorphism in the Amphibia. Copeia 1979, 297–306 (doi:10.2307/1443418) [Google Scholar]
- Wagner G. P., Vargas A. O.2008On the nature of thumbs. Genome Biol. 9, 213 (doi:10.1186/gb-2008-9-3-213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K. D.2007The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press [Google Scholar]
- Young R. L., Caputo V., Giovannotti M., Kohlsdorf T., Vargas A. O., May G. E., Wagner G. P.2009Evolution of digit identity in the three-toed Italian skink Chalcides chalcides: a new case of digit identity frame shift. Evol. Dev. 11, 647–658 (doi:10.1111/j.1525-142X.2009.00372.x) [DOI] [PubMed] [Google Scholar]