Abstract
Disentangling the relative contribution of predation avoidance and increased foraging efficiency in the evolution of sociality in animals has proven difficult given that the two types of benefits often operate concurrently. I identified different types of refuges from predation in birds related to morphological and ecological traits, providing an opportunity to examine concomitant changes in sociality over evolutionary times. Results of a matched-species comparative analysis indicated a reduction in the size of foraging or non-foraging groups but not complete disappearance under negligible predation risk. The results suggest that while predation avoidance is an important component in the evolution of sociality in birds, it is most probably not acting alone but rather in conjunction with other benefits such as increased foraging efficiency.
Keywords: birds, group size, predation risk, relaxed selection, sociality
1. Introduction
Many species of animals perform their activities in groups, whether it be searching and exploiting food resources or resting. Adaptive explanations for sociality usually emphasize predation avoidance or enhanced foraging efficiency (Krause & Ruxton 2002). By living in groups, animals can detect and avoid predators more easily and can also locate and exploit food patches more efficiently. In most species, the two types of benefit are probably operating together and teasing apart their relative contribution has been remarkably difficult.
Comparative analyses can be useful to disentangle the two types of benefit by contrasting populations or species exposed to different levels of predation risk. For instance, island populations or island species have often evolved with little predation risk and recent comparative analyses with their mainland counterparts suggest that many anti-predation adaptations, such as foraging in groups, have been lost or maintained at a lower level when predation risk is negligible (van Schaik & van Noordwijk 1986; Beauchamp 2004; Blumstein & Daniel 2005).
The above studies have focused on island populations or species but isolation from predation over evolutionary times can also occur in other contexts that have received less attention. A refuge from predation has been suggested to occur in larger species, which can be attacked by a reduced array of predators (Cohen et al. 1993). Species can also avoid predators in time and space (Sih 1987). In rodents, for instance, species that can avoid predators more easily, as is the case for nocturnal species, live in smaller groups (Ebensperger & Blumstein 2006). Here, I use a comparative analysis to contrast species exposed to differential predation risk to examine consequences for sociality in birds in foraging and non-foraging contexts. I tested the predictions that species facing relatively little predation would occur in smaller groups and that one mechanism responsible for negligible predation risk is a relatively larger body mass.
The prediction that sociality should be reduced when predation risk is negligible should be, at first sight, strongest in non-foraging groups, such as roosts, because obtaining food is not a direct function of these groups. However, social foraging is strongly associated with communal roosting in animals (Beauchamp 1999; Kerth & Reckardt 2003) suggesting an indirect role for locating food resources and thus imposing a limit on the expected reduction in sociality.
In a broader context, comparative analyses have been before used to examine the role of predation risk in the evolution of sociality in animals (Arnold & Owens 1999; Ebensperger & Blumstein 2006; Varela et al. 2007). Instead of a sweeping analysis of a large number of clades, I chose to focus on narrow but well-defined evolutionary transitions involving closely related species that differ in predation risk offering a unique opportunity to examine concomitant changes in sociality.
2. Material and methods
I searched the literature to identify avian species where the extent of predation could be assessed. I only considered predation on adult foraging birds in the non-breeding season given that parent birds and their offspring are very vulnerable to predation while at the nest. In addition, most species do not form groups in the breeding season except for some species breeding communally or in colonies. For these species, I also considered information about predation on foraging birds away from the colony or nest. The main sources of information about predation were predator diet analysis and accounts of predator attacks. Personal observations by researchers confirmed and complemented this information on predation.
Accounts revealed negligible predation risk on adult foraging birds in a number of species. For these species, I tallied mean (or the median or mode) and maximum group size to provide quantitative estimates of sociality. I distinguished two different types of groups: foraging and non-foraging groups, with the latter consisting of resting groups, known as rafts or roosts. I also tallied body mass for each species with a preference for male body mass in the non-breeding season given that males typically show less annual fluctuation in body mass. Finally, I noted the reason(s) why the authors thought predation was negligible for each species.
The final step consisted in pairing each species with relaxed predation risk with the most closely related species that I could find where the extent of predation was judged non-negligible. To select sister species, I used recent phylogenetic trees based mostly on molecular traits (see the electronic supplementary material). By choosing closely related species for matching, I increased the ecological similarity between members of each pair, thus reducing the possibility that divergence in other ecological traits was responsible for putative differences in sociality. Sociality of these sister species was determined as described above. I ensured that the pairings were independent by drawing a path along the branches of the phylogeny linking each species pair, and making sure that none of the paths crossed one another (Maddison 2000).
I excluded from the analysis all pairs that consisted of only solitary species since lack of sociality cannot be related exclusively to relaxed predation in such cases (Read & Nee 1995). I used Wilcoxon signed-rank tests to examine hypotheses related to foraging group size and body mass. I could not always obtain quantitative estimates of non-foraging group sizes but using available qualitative information, I could usually rank members of a pair in terms of relative group size. In this case, I used the sign test. Given that I tested a priori hypotheses, I used one-tailed tests throughout.
3. Results
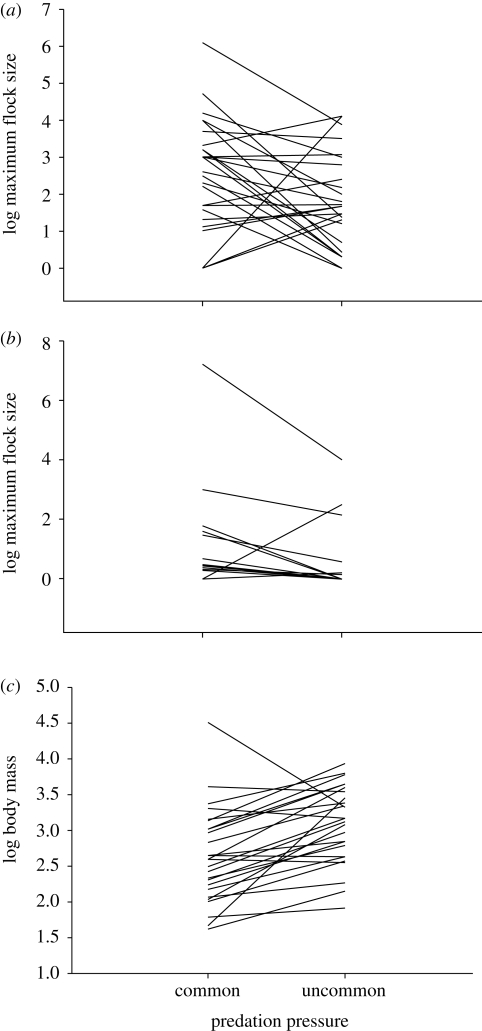
I uncovered 28 pairs of species with divergent predation risk across a large range of body sizes and avian families (see the electronic supplementary material). Sociality was reduced in pair members with negligible predation risk as indicated by a smaller maximum group size (S = 78.5, p = 0.01, n = 27; figure 1a) and a smaller mean group size (S = 32.5, p = 0.01, n = 18; figure 1b). In addition, a smaller relative size for non-foraging groups occurred in 18 of the 24 pairs with available data (M = 6, p = 0.01). Body mass was larger in the pair members less exposed to predation (S = 159, p < 0.0001, n = 28; figure 1c).
Figure 1.
Comparison of (a) maximum group size (n = 24), (b) mean group size (n = 18) and (c) body mass (n = 28) in species facing negligible predation (uncommon predation pressure) and matched species with non-negligible predation (common predation pressure).
Solitary foraging occurred in only three species with negligible predation risk (11%, see the electronic supplementary material). Solitary resting occurred in nine species with negligible risk (35%, see the electronic supplementary material).
Negligible predation risk was reported in the literature or thought to occur by the researchers in response to the following factors: large size and weaponry (n = 16, 57%), foraging in areas devoid of predators (n = 11, 39%) and foraging at night when predators are absent (n = 2, 7%), with some factors present together (see the electronic supplementary material).
4. Discussion
The reduction in foraging and non-foraging group size in birds is compatible with the hypothesis that predation risk is an important component of sociality. However, the fact that sociality did not completely disappear in most species suggests that while anti-predation is a major component of sociality in birds, it is not the only one.
In many tallied species with negligible predation risk, social foraging increased foraging efficiency by increasing the chances to locate ephemeral food patches and/or by increasing prey capture rate while in a food patch (e.g. Fleming et al. 1992; McMahon & Evans 1992; Bélisle 1998). This is particularly striking in the Swainson's hawk (Buteo swaisoni), a large raptor with negligible predation risk, but that nonetheless roosts and forages in large groups, presumably in response to patchily distributed food (England et al. 1997).
These results corroborate findings from an earlier study indicating that island species of birds with few predation threats also foraged in smaller groups (Beauchamp 2004). The results on sociality in birds are thus in line with the general finding from relaxed selection studies that traits under relaxed selection persist in their current form or in an intermediate form when other functions are still operating (Lahti et al. 2009). In the case of sociality in birds, it would appear that the foraging benefits associated with sociality are important in its maintenance.
The association between a relatively larger body mass and reduced sociality raises interesting evolutionary issues. Perhaps solitary species of birds did not evolve greater sociality because they already possessed a large size and were thus less vulnerable to predators. Alternatively, sociality was lost as greater size was evolved. Future work could identify the probable sequence of evolutionary events pertaining to the association between body mass and sociality. Nevertheless, the results do suggest a negative relationship between body mass and sociality in birds. In contrast, a positive relationship between sociality and body size was reported in rodents (Ebensperger & Cofré 2001) but not in ungulates (Brashares et al. 2000). While more work is definitely needed to address the coevolution of body mass and sociality, it is conceivable that larger body mass may deter predation to different extent in different taxa.
It is interesting to note that the decrease in sociality with negligible predation risk occurred in non-foraging as well as in foraging groups. Non-foraging groups, which in most cases include resting or roosting birds, have often been thought to form to reduce predation risk (Beauchamp 1999), but the fact that non-foraging groups can persist in the face of negligible predation risk suggests that other functions for these groups are conceivable and currently operating. Such functions may include transfer of information about distant food sources (Ward & Zahavi 1973). Nevertheless, when faced with negligible predation risk, more non-foraging groups than foraging groups consisted of solitary individuals suggesting either that avoidance of predation is more relevant in non-foraging groups or that current functions are fewer or acting less strongly. Future comparative in birds and other taxa as well will be needed to assess the generality of these findings.
Acknowledgements
I thank two referees for their insightful comments and all researchers who discussed with me the relevance of predation in their study species: Y. Chérel, A. Chiaradia, T. Gaston, J. Hatch, K. Hobson, B. Larned, Z. Li, L. MacKinnon, M. Mallory, B. Montevecchi, J. Olsen, J. Paruk, M. Petersen, R. Pitman, Y. Ropert-Coudert, P. Schwemmer, F. Sergio, M. J. Ueta and Z. Ma.
References
- Arnold K. E., Owens I. P. F.1999Cooperative breeding in birds: the role of ecology. Behav. Ecol. 10, 465–471 (doi:10.1093/beheco/10.5.465) [Google Scholar]
- Beauchamp G.1999The evolution of communal roosting in birds: origins and secondarily losses. Behav. Ecol. 10, 675–687 (doi:10.1093/beheco/10.6.675) [Google Scholar]
- Beauchamp G.2004Reduced flocking by birds on islands with relaxed predation. Proc. R. Soc. Lond. B 271, 1039–1042 (doi:10.1098/rspb.2004.2703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélisle M.1998Foraging group size: models and a test with jaegers kleptoparasitizing terns. Ecology 79, 1922–1938 [Google Scholar]
- Blumstein D. T., Daniel J. C.2005The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 272, 1663–1668 (doi:10.1098/rspb.2005.3147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashares J. S., Garland T., Arcese P.2000Phylogenetic analysis of coadaptation in behavior, diet, and body size in the African antelope. Behav. Ecol. 11, 452–463 (doi:10.1093/beheco/11.4.452) [Google Scholar]
- Cohen J. E., Pimm S. L., Yodzis P., Sadana J.1993Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 62, 67–78 [Google Scholar]
- Ebensperger L. A., Blumstein D. T.2006Sociality in New World hystricognath rodents is linked to predators and burrow digging. Behav. Ecol. 17, 410–418 (doi:10.1093/beheco/arj048) [Google Scholar]
- Ebensperger L. A., Cofré H.2001On the evolution of group-living in the New World cursorial hystricognath rodents. Behav. Ecol. 12, 227–236 (doi:10.1093/beheco/12.2.227) [Google Scholar]
- England A. S., Bechard M. J., Houston C. S.1997Swainson's hawk. In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; Retrieved from the birds of North America online: http://bna.birds.cornell.edu/bna/species/265. (doi:10.2173/bna.265) [Google Scholar]
- Fleming S. P., Smith P. C., Seymour N. R., Bancroft R. P.1992Ospreys use local enhancement and flock foraging to locate prey. Auk 109, 649–654 [Google Scholar]
- Kerth G., Reckardt K.2003Information transfer about roosts in female Bechstein's bats: an experimental field study. Proc. R. Soc. Lond. B 270, 511–515 (doi:10.1098/rspb.2002.2267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups. Oxford, UK: Oxford University Press [Google Scholar]
- Lahti D. C., Johnson N. A., Ajie B. C., Otto S. P., Hendry A. P., Blumstein D. T., Coss R. G., Donohue K., Foster S. A.2009Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496 (doi:10.1016/j.tree.2009.03.010) [DOI] [PubMed] [Google Scholar]
- Maddison W. P.2000Testing character correlation using pairwise comparisons on a phylogeny. J. Theor. Biol. 202, 195–204 (doi:10.1006/jtbi.1999.1050) [DOI] [PubMed] [Google Scholar]
- McMahon B. F., Evans R. M.1992Foraging strategies of American white pelicans. Behaviour 120, 69–89 (doi:10.1163/156853992X00219) [Google Scholar]
- Read A. F., Nee S.1995Inference from binary comparative data. J. Theor. Biol. 173, 99–108 (doi:10.1006/jtbi.1995.0047) [Google Scholar]
- Sih A.1987Prey refuges and predator–prey stability. Theor. Popul. Biol. 31, 1–12 (doi:10.1016/0040-5809(87)90019-0) [Google Scholar]
- van Schaik C. P., van Noordwijk M. A.1986The evolutionary effect of the absence of felids on the social organization of the Simeulue monkey (Macaca fascicularis Fusca). Folia Primatol. 44, 138–147 (doi:10.1159/000156208) [Google Scholar]
- Varela S. A. M., Danchin E., Wagner R. H.2007Does predation select for or against avian coloniality? A comparative analysis. J. Evol. Biol. 20, 1490–1503 (doi:10.1111/j.1420-9101.2007.01334.x) [DOI] [PubMed] [Google Scholar]
- Ward P., Zahavi A.1973The importance of certain assemblages of birds as information-centres. Ibis 115, 517–534 (doi:10.1111/j.1474-919x.1973.tb01990.x) [Google Scholar]