Abstract
Many studies have focused on the effects of anthropogenic noise on animal communication, but only a few have looked at its effect on other behavioural systems. We designed a playback experiment to test the effect of noise on predation risk assessment. We found that in response to boat motor playback, Caribbean hermit crabs (Coenobita clypeatus) allowed a simulated predator to approach closer before they hid. Two hypotheses may explain how boat noise affected risk assessment: it masked an approaching predator's sound; and/or it reallocated some of the crabs' finite attention, effectively distracting them, and thus preventing them from responding to an approaching threat. We found no support for the first hypothesis: a silent looming object still got closer during boat motor playbacks than during silence. However, we found support for the attentional hypothesis: when we added flashing lights to the boat motor noise to further distract the hermit crabs, we were able to approach the crabs more closely than with the noise alone. Anthropogenic sounds may thus distract prey and make them more vulnerable to predation.
Keywords: attention, anthropogenic noise, distraction, hermit crab, risk assessment
1. Introduction
Human populations are increasing, and so is their impact on wildlife. One factor we are systematically changing is the acoustic environment; humans are creating many novel sounds (Warren et al. 2006; Slabbekoorn & Ripmeester 2008), and these sounds may have large effects (Warren et al. 2006; Hatch & Wright 2007; Wright et al. 2007). A variety of studies have shown how anthropogenic noise may affect mating, communication and antipredator behaviour.
Human noises may change reproductive behaviour. For example, it has been suggested that male ovenbirds (Seiurus aurocapilla) on quieter territories may be more likely to have a mate than those on louder ones. If females base mate choice on male territory noise levels, instead of size or age, anthropogenic sounds may negatively affect male mate pairing success (Habib et al. 2007).
The way animals communicate can also be changed by anthropogenic sounds. Certain species of acoustically active, pond-dwelling frogs decrease their call rate when exposed to airplane flyby or motorcycle engine playbacks (Sun & Narins 2005). This finding suggests that frogs changed their calling behaviour to avoid acoustic masking.
Animals may also increase antipredator vigilance in the presence of loud noises. For instance, California ground squirrels (Spermophilus beecheyi) in areas with loud wind turbines exhibited higher rates of vigilance after hearing conspecific alarm calls than those in quieter areas (Rabin et al. 2006).
We suspect predation risk assessment may also be affected by anthropogenic noise, and we are aware of only one study conducted to assess this. Karp & Root (2009) found that loud ecotourist conversation increased alertness and flight initiation distance in hoatzin birds (Opisthocomus hoazin). However, they did not provide a mechanism for this observed pattern.
We evaluated two hypotheses that may explain how anthropogenic sounds affect risk assessment. The first is that the anthropogenic noise masks auditory cues of the approaching threat. The second is that the anthropogenic sound reallocates an animal's finite attention, effectively distracting it and preventing it from responding to predatory threats.
Attention is the process that filters out all but a few stimuli from an individual's environment, letting in only as much as it can process (Bushnell 1998; Dukas 2004). Individuals can process only a finite amount, though total attention can be divided or reallocated among various tasks or stimuli (Washburn & Taglialatela 2006). The choice of what to focus attention on is both voluntary and involuntary; distraction is commonly understood to be the animal's attention suddenly shifting involuntarily. Many animals must split their attention (Dukas 2004) or time (Lima & Bednekoff 1999) between a necessary task (e.g. foraging) and antipredator behaviour (e.g. vigilance). Consequently, distracting animals could enhance vulnerability to predation (Dukas 2004), and thus be detrimental.
We investigated whether anthropogenic noise affects a model species, the Caribbean hermit crab (Coenobita clypeatus), and if so, how. Terrestrial hermit crabs are an ideal species with which to study the effects of anthropogenic stimuli for several reasons. First, terrestrial hermit crabs are conspicuous and locally abundant. Second, they have an unambiguous and easy-to-measure antipredator behaviour (they hide in their shells). The same logic used to study flight initiation distance (Cooper & Frederick 2007) can be applied to study what we define as hiding initiation distance (HID) because, as an alternative to flight, hiding is the hermit crab antipredator response. Finally, they rely on both sight and sound (Burggren & McMahon 1988), and thus we could test risk assessment using both modalities.
2. Material and methods
Between 10 and 26 October 2009, we conducted three experiments on crabs 2–7 cm in shell length within 3 km of the Virgin Islands Environmental Resource Station (18°19′19.45′′ N, 64°43′22.58′′ W), St John, US Virgin Islands. We conducted all of the experiments within earshot (0.1–2 km) of the ocean along forest trails during the nadir of the tourist season.
(a). Does noise influence risk assessment?
Boat motor recordings were obtained through a Hollywood sound engineer. We used five exemplars (figure 1) to prevent pseudoreplication, and broadcast them at 98.1 ± 2.6 (s.d.) dB SPL (measured 1 m from the speaker). Sounds were stored in AIF format on an iPod and broadcast through a Pignose Industries speaker (model 7–100) that a person held 85 cm above the ground to eliminate vibrational cues.
Figure 1.
Spectrograms (512 point FFT, 75% overlap) of the top 40 dB of boat motor sounds broadcast to hermit crabs.
To standardize each crab's initial behaviour, we walked towards the focal subject until it hid in its shell, then waited 3 m from the subject. Once the crab re-emerged, we waited 30 s before broadcasting either a boat motor noise recording or silence (our control) for another 30 s. Then, with the noise or control still broadcast, we walked directly towards the crab until it hid, at a pace of 1.5 m s−1. The walker's (P.G.-P.) initial position was immediately adjacent to the person holding the speaker. We measured first-reaction distance (FRD) as the distance between the person and the hermit crab when it responded by freezing or antennopating. We then measured the HID as the distance the person was from the hermit crab when it hid. If the hermit crab did not have a detectable first reaction, we considered FRD to be equal to its HID. Finally, we measured (with a tape measure) the hermit crab's shell length to the nearest millimetre.
(b). Does noise mask predator sounds?
We conducted this experiment similarly to the first, but used a silent looming object (a black t-shirt covering an inflatable donut) to flush the crab instead of a person, to remove any acoustic or vibrational cues associated with the approach. The looming object was presented on a horizontal 3 m pole that swung, silently, towards it from a 2.6 m tall pole. We were able to accurately record HID, not FRD, for this experiment.
(c). Are crabs distracted by noise?
We conducted the third experiment similarly to the first as well, except our acoustic treatments were boat motor noise with flashing lights, or boat motor noise only. We used the lights as a distraction for two reasons. First, we might not have detected a change in behaviour if the boat noise had already exceeded the crabs’ finite auditory capacity. Second, we wanted to avoid masking the new stimulus with the boat motor noise. We used two headlamps (a Petzl Zipka Plus 2 and a Princeton Tech Quad) that flashed at different rates and attached them 90 cm from the ground 1 m away from the focal crab.
(d). Statistical analysis
We used unpaired, two-tailed t-tests to see whether crabs discriminated between the control and treatment, and to see whether FRD and HID were influenced by the treatment. We calculated Cohen's d-scores (Cohen 1988), using the pooled variance, for HID and FRD.
3. Results
(a). Does noise influence risk assessment?
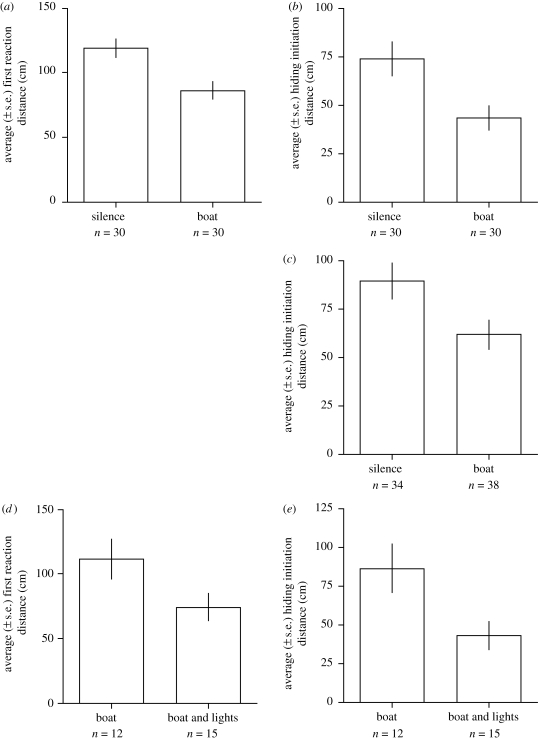
We could get significantly closer to the crabs before they first reacted with the boat motor noise than with the control silence (figure 2a). We also could get significantly closer to the crabs before they hid when we broadcast the boat motor noise than when we used the control silence (figure 2b). The effect sizes for both were large (figure 2a,b).
Figure 2.
The response (average ± s.e. of FRD or average ± s.e. of HID) of hermit crabs to approaching threats. (a,b) A human approached with either boat motor noise or silence. (c) A looming object (a black t-shirt put over an inflated donut) approached with either boat motor noise or silence. (d,e) A human approached with either boat motor noise combined with flashing lights or boat motor noise only. (a) d-score = 0.814, p = 0.003; (b) d-score = 0.709, p = 0.008; (c) d-score = 0.532, p = 0.027; (d) d-score = 0.717, p = 0.084; (e) d-score = 0.898, p = 0.030.
(b). Does noise mask predator sounds?
The looming object was able to get significantly closer to the crabs before they hid when we broadcast the boat motor noise than when we broadcast silence (figure 2c).
(c). Are crabs distracted by noise?
The addition of the flashing lights to the boat motor noise decreased, but not significantly, the distance we could approach before the crabs first reacted (figure 2d). The addition of the flashing lights significantly decreased the HID (figure 2e).
4. Discussion
We found a large effect of boat motor noise on hermit crab behaviour; simulated predators could get closer during noise playback. This suggests that crabs have an impaired ability to respond to a potential predator in the presence of boat motor noise.
We evaluated two alternative mechanisms to explain the boat motor noise's effect on hermit crabs’ risk assessment: acoustic masking and attentional deficits. Results from the second experiment were inconsistent with the acoustic masking hypothesis, but results from the third experiment were consistent with the second, attentional hypothesis. Our experimental design does not allow us to completely reject a masking effect because while the boat motor noise was reasonably broad-band, we neither recorded our quiet footfalls (which were likely to be relatively low frequency) nor do we have hermit crab audiograms to demonstrate conclusively that the noise blocked their hearing. Nevertheless, we hypothesize that the primary mechanism behind our results is the attentional hypothesis both because the loud broadcast sounds clearly influenced crab behaviour and because the results of the second experiment showed there were few, if any, acoustic masking effects. We expand on this hypothesis and propose the ‘distracted prey hypothesis’, which states that any stimulus an animal can perceive is capable of distracting it by reallocating part of its finite attention and thus preventing it from responding to an approaching threat.
Anthropogenic noises thus could reduce an animal's fitness by increasing vulnerability to predation. Krause & Godin (1996) suggested that predators might be more likely to take advantage of less vigilant prey. Our findings that prey are less responsive to predators, combined with the possibility that predators are more likely to attack, may increase vulnerability to predation.
As anthropogenic sounds become more frequent, the strength of an animal's response to them may decrease owing to habituation (Thompson & Spencer 1966). This process is common to many animals (Chace & Walsh 2006; Nowacek et al. 2007). In the context of our experiment, habituation implies that with long-term exposure animals may become less distracted by noise and would then be able to assess risk properly, regardless of whether the noise is present. While this is an empirical question, noise effects on risk assessment may be more pronounced in areas with few anthropogenic noises.
We expect the distracted prey hypothesis to be applicable to all taxa with attentional abilities. Many studies on attention use birds, which have been shown to divide time (Lima & Bednekoff 1999) and attention (Dukas 2004) between antipredator vigilance and foraging. Birds assess risk using both acoustic and visual cues (Caro 2005), and we know that anthropogenic sounds (Warren et al. 2006) and lights (Rich & Longcore 2006) interfere with a variety of their behaviours. Thus, the two modalities we used on hermit crabs also seem appropriate for testing the distracted prey hypothesis on birds. Further studies like this may illustrate that multi-modal distraction reduces attention to biologically important tasks in other taxa as well.
Acknowledgements
We thank the UCLA Office of Instructional Development, and the Department of Ecology and Evolutionary Biology for partial support, Rafe Boulon for facilitating our field research permits (no. VIIS-2009-SCI-0028) and Jonathan Drury, Robert Phillips, Randy Brown and Jamie Irving for support and assistance.
References
- Burggren W. W., McMahon B. R.1988Biology of land crabs. New York, NY: Cambridge University Press [Google Scholar]
- Bushnell P. J.1998Behavioral approaches to the assessment of attention in animals. Psychopharmacology 138, 231–259 (doi:10.1007/s002130050668) [DOI] [PubMed] [Google Scholar]
- Caro T.2005Antipredator defenses in birds and mammals. Chicago, IL: University of Chicago Press [Google Scholar]
- Chace J. F., Walsh J. J.2006Urban effects on native avifauna: a review. Landscape Urban Plan. 74, 46–69 (doi:10.1016/j.physletb.2003.10.071) [Google Scholar]
- Cohen J.1988Statistical power analysis for the behavioral sciences, 2nd edn Hillsdale, New Jersey: Lawrence Erlbaum Associates, Inc [Google Scholar]
- Cooper W. E., Jr, Frederick W. G.2007Optimal flight initiation distance. J. Theor. Biol. 244, 59–67 (doi:10.1016/j.jtbi.2006.07.011) [DOI] [PubMed] [Google Scholar]
- Dukas R.2004Causes and consequences of limited attention. Brain Behav. Evol. 63, 197–210 (doi:10.1159/000076781) [DOI] [PubMed] [Google Scholar]
- Habib L., Bayne E. B., Boutin S.2007Chronic industrial noise affects pairing success and age structure of ovenbirds Seiurus aurocapilla. J. Appl. Ecol. 44, 176–184 (doi:10.1111/j.1365-2664.2006.01234.x) [Google Scholar]
- Hatch L. T., Wright A. J.2007A brief review of anthropogenic sound in the oceans. Int. J. Comp. Psychol. 20, 121–133 [Google Scholar]
- Karp D. S., Root T. L.2009Sound the stressor: how hoatzins (Opisthocomus hoazin) react to ecotourist conversation. Biodivers. Conserv. 18, 3733–3742 (doi:10.1007/s10531-009-9675-6) [Google Scholar]
- Krause J., Godin J. J.1996Influence of prey foraging posture on flight behavior and predation risk: predators take advantage of unwary prey. Behav. Ecol. 7, 264–271 (doi:10.1093/beheco/7.3.264) [Google Scholar]
- Lima S. L., Bednekoff P. A.1999Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659 (doi:10.1086/303202) [DOI] [PubMed] [Google Scholar]
- Nowacek D. P., Thorne L. H., Johnston D. W., Tyack P. L.2007Responses of cetaceans to anthropogenic noise. Mammal Rev. 37, 81–115 (doi:10.1111/j.1365-2907.2007.00104.x) [Google Scholar]
- Rabin L. A., Coss R. G., Owings D. H.2006The effects of wind turbines on antipredator behavior in California ground squirrels (Spermophilus beecheyi). Biol. Conserv. 131, 410–420 (doi:10.1016/j.biocon.2006.02.016) [Google Scholar]
- Rich C., Longcore T.(eds)2006Ecological consequences of artificial night lighting. Washington, DC: Island Press [Google Scholar]
- Slabbekoorn H., Ripmeester E. A. P.2008Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83 (doi:10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- Sun J. W. C., Narins P. M.2005Anthropogenic sounds differentially affect amphibian call rate. Biol. Conserv. 121, 419–427 (doi:10.1016/j.biocon.2004.05.017) [Google Scholar]
- Thompson R. F., Spencer W. A.1966Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 73, 16–43 (doi:10.1037/h0022681) [DOI] [PubMed] [Google Scholar]
- Warren P. S., Katti M., Ermann M., Brazel A.2006Urban bioacoustics: it's not just noise. Anim. Behav. 71, 491–502 (doi:10.1016/j.anbehav.2005.07.014) [Google Scholar]
- Washburn D. A., Taglialatela L. A.2006Attention as it is manifest across species. In Comparative cognition: experimental explorations of animals intelligence (eds Wasserman E. A., Zentall T. R.), pp. 127–142 New York, NY: Oxford University Press [Google Scholar]
- Wright A. J., et al. 2007Anthropogenic noise as a stressor in animals: a multidisciplinary perspective. Int. J. Comp. Psychol. 20, 250–273 [Google Scholar]