Abstract
Host behaviour towards infectious conspecifics is a crucial yet overlooked component of pathogen dynamics. Selection is expected to favour individuals who can recognize and avoid infected conspecifics in order to reduce their own risk of infection. However, evidence is scarce and limited to species employing chemical cues. Here, we experimentally examine whether healthy captive house finches (Carpodacus mexicanus) preferentially forage near a same-sex, healthy conspecific versus one infected with the directly transmissible pathogen Mycoplasma gallisepticum (MG), which causes lethargy and visible conjunctivitis. Interestingly, male house finches strongly preferred feeding near diseased conspecifics, while females showed no preference. This sex difference appeared to be the result of lower aggression rates in diseased males, but not in females. The reduced aggression of diseased males may act as an ‘evolutionary trap’ by presenting a historically beneficial behavioural cue in the context of a new environment, which now includes a recently emerged, potentially fatal pathogen. Since MG can be directly transmitted during feeding, healthy males may inadvertently increase their risk of contracting MG. This behaviour is likely to significantly contribute to the continued persistence of MG epidemics in wild populations.
Keywords: house finch Carpodacus mexicanus, Mycoplasma gallisepticum, disease avoidance, aggression, pathogen transmission, foraging behaviour
1. Introduction
Pathogen transmission is a costly and sometimes deadly consequence of conspecific interaction, particularly for group-living organisms (Møller et al. 1993). Selection should therefore favour individuals who can recognize and avoid conspecifics infected with directly transmitted pathogens (Loehle 1995). However, surprisingly little is known as to whether and how individuals alter their behaviour in response to infected conspecifics, particularly outside the context of mate choice where avoidance behaviours carry both direct and indirect benefits (e.g. Rosenqvist & Johansson 1995). To date, evidence for behavioural avoidance of infected conspecifics, documented in both bullfrog tadpoles (Kiesecker et al. 1999) and social lobsters (Behringer et al. 2006), appears to result from chemical cues. Many types of pathogens, however, result in visible changes in their hosts ranging from behavioural modifications (lethargy, posture) to physical signs (e.g. inflammation, lesions, hair loss; Loehle 1995). Birds, in particular, rely heavily upon visual cues for behavioural decisions. However, no study outside of the context of mate choice has examined whether individuals use visible cues in order to avoid diseased conspecifics and reduce their own risk of infection.
House finches (Carpodacus mexicanus) suffer from the directly transmissible bacterial pathogen Mycoplasma gallisepticum (MG) which causes visible conjunctival symptoms and behavioural changes (Kollias et al. 2004). Since its emergence in 1994 (Ley et al. 1996), MG has caused annual seasonal epidemics in eastern North American house finches. MG-infected finches have significantly lower over-winter survival rates (Faustino et al. 2004) and the initial epidemic resulted in detectable population declines (Hochachka & Dhondt 2000), suggesting that MG has sufficient fitness consequences to select for avoidance of infected individuals. Furthermore, MG epidemics occur during periods of gregarious flocking (Altizer et al. 2004), and flocking behaviour increases disease prevalence (Hosseini et al. 2004). Therefore, free-living house finches in eastern North America regularly encounter visibly infected conspecifics while feeding.
Here, we use an experimental feeding choice arena to investigate whether healthy house finches preferentially feed near a healthy conspecific or one exhibiting behavioural and physical signs of MG infection. In addition, we examined behavioural differences between healthy and diseased house finches to determine potential cues healthy individuals may use to alter their preference towards conspecifics.
2. Material and methods
Experimental cages were divided into three compartments with a small food dish on either side of two wire-mesh dividers, allowing adjacent individuals to interact and aggressively displace each other without physical contact (figure 1 inset; electronic supplementary material S1). Focal individuals (17 males; 22 females) in the middle compartment were tested once against a unique, same-sex combination of one healthy (out of five males and six females) and one diseased conspecific (out of five males and 12 females) placed in either side compartment. Diseased conspecifics were experimentally inoculated with MG as part of an ongoing infection experiment and all showed visible conjunctival lesions (electronic supplementary material S2).
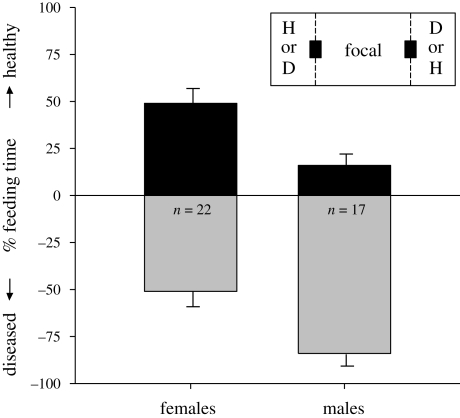
Figure 1.
Mean (±s.e.) percentage of time spent feeding by focal house finches near a healthy (‘H’; black bars) or diseased conspecific (‘D’; grey bars). The inset shows the experimental set-up with the placement of mesh barriers (dashed lines) and food dishes (black squares).
Trials were recorded on video between 8.00 and 15.30 EST in March 2009. Focal birds were food-deprived for 3 h prior to trial commencement in order to ensure standardized motivation to feed (table 1). During the first 20 min following focal bird entry (T0; table 1), we quantified at 30 s intervals the conspecifics’ levels of activity and aggression (table 2) towards the focal bird (i.e. (i) total number of interactions, and percentage of interactions where conspecific (ii) showed aggression and (iii) won). Next, the focal bird was provided with both food sources (T + 0.5 h; table 1) and its feeding behaviour quantified for 60 min at 30 s intervals by scoring when and where it was feeding.
Table 1.
Experimental timeline.
| food present? |
|||
|---|---|---|---|
| time | action | focal bird | conspecifics |
| T − 3 h | remove food of focal bird in its home cage | no | yes |
| T − 0.5 h | move conspecifics to experimental cage | no | yes |
| T0 | move focal bird to experimental cage | no | yes |
| start observation of conspecific behaviour | |||
| T + 0.5 h | provide focal bird with food in both food dishes | yes | yes |
| start observation of focal bird feeding preference | |||
| T + 1.5 h | end of observation | ||
Table 2.
Definitions of scored behaviour.
| behaviour | definition |
|---|---|
| activity level | total number of movements between perches, floor or sides of the compartment |
| aggressive interaction | peck, attack (i.e. a fast, direct flight towards the other bird that is perched next to the cage divider) or combat (i.e. both birds repeatedly peck and aggressively vocalize at each other, often when hovering in mid-air) between focal bird and conspecific |
| winning an interaction | displacement with a winner and a loser (i.e. loser moves away while winner stays put) |
We analysed sources of variation in focal bird feeding preference (i.e. time feeding near healthy–diseased conspecific) using a general linear model including sex, differences in conspecific behaviour (healthy–diseased for activity, aggression and wins) and the two-way interactions with sex. Second, we analysed conspecific behaviour (activity, aggression and wins; table 2) as a function of sex, treatment (healthy versus diseased) and their interaction using general linear mixed models including trial as a random effect. For further details on the statistical analyses, see electronic supplementary material 3.
3. Results
(a). Focal bird behaviour
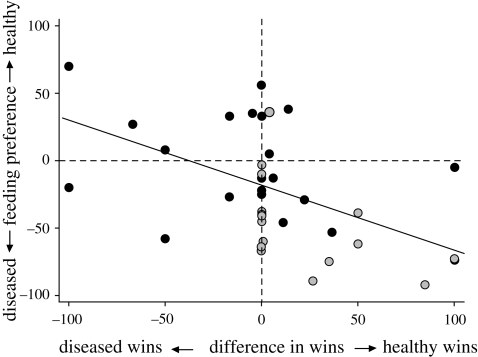
Feeding preferences significantly differed by sex (F1,34 = 8.63, p = 0.006; figure 1): male focal house finches strongly preferred to feed near diseased conspecifics (t16 = 6.15, p < 0.001), whereas females fed for similar amounts of time near each conspecific (t21 = 0.51, p = 0.62). Focal bird feeding preference was negatively related to the difference in wins between healthy and diseased conspecifics (figure 2; F1,34 = 5.54, p = 0.024), but unrelated to difference in activity (F1,34 = 0.19, p = 0.67) or aggression (F1,34 = 0.50, p = 0.49). All pair-wise interactions between behavioural differences and sex were non-significant (all p > 0.74).
Figure 2.
The extent of focal house finch feeding preference (i.e. feeding time near healthy–diseased conspecific; y-axis) for diseased conspecifics was related to the reduced percentage of wins (x-axis) by diseased male (n = 17; grey dots), but not female conspecifics (n = 22; black dots). As there was no interaction between sex and difference in wins (healthy–diseased conspecifics), the sexes were pooled for the regression analysis (solid line). The dashed lines indicate no feeding preference (horizontal) or no difference in wins (vertical).
(b). Conspecific behaviour
The effect of being healthy or diseased on conspecific behaviour varied with sex for the percentage of wins (figure 2; sex × treatment; χ21 = 5.06, p = 0.024), but not for activity (χ21 = 0.67, p = 0.41) or aggression level (χ21 = 0.41, p = 0.52). Healthy males won a higher percentage of interactions with the focal bird than diseased males (χ21 = 9.19, p = 0.002), while this difference was absent in females (χ21 = 0.24, p = 0.62; electronic supplementary material, figure S4c). Regardless of sex, healthy conspecifics were more active than diseased ones (χ21 = 42.75, p < 0.001; electronic supplementary material, figure S4a), and there was no difference in aggression (χ21 = 1.83, p = 0.18; electronic supplementary material, figure S4b).
4. Discussion
Behavioural changes such as lethargy, often referred to as ‘sickness behaviours’, are generalized responses to infection (Hart 1988) that may serve as informative cues for avoidance of conspecifics infected with a broad range of pathogens. However, in this study, healthy male house finches responded to a sickness behaviour—the reduced potential to win—by increasing time spent feeding near diseased individuals: the more likely the healthy conspecific in a given trial was to win aggressive interactions with the focal bird, the stronger the feeding preference towards the less competitive, diseased conspecific. These results suggest that healthy males seek to avoid costly social defeats, which have been shown to dynamically suppress immune responses in this species (Hawley 2006), by foraging in the vicinity of individuals with the lowest potential for winning aggressive interactions. This study is the first demonstration of individuals using visual cues (i.e. behaviour) to alter their behaviour towards diseased conspecifics, although the additional use of chemosensory cues (Hagelin & Jones 2007) cannot be excluded by our experimental design.
The sickness behaviour of infected male house finches may act as an ‘evolutionary trap’ by presenting a historically beneficial cue (i.e. reduced aggression) in the context of a new environment (Schlaepfer et al. 2002), in this case, a recently emerged pathogen. Before the emergence of MG in house finches, the risk of exposure to pathogens carried by non-aggressive individuals may have been outweighed by the benefits of access to resources, whereas the behavioural cue is now likely to carry fitness costs (Faustino et al. 2004). Since associations between this host and the pathogen have been of relatively short evolutionary duration (approx. nine finch generations), sufficient time for the selection of avoidance of infected conspecifics may not yet have passed.
Why did we detect a feeding preference for diseased conspecifics only in male house finches? Our results indicate that this difference was not driven by focal bird behaviour: focal males and females responded similarly to differences in aggression, since the interaction between sex and differences in wins on feeding preference was non-significant. Therefore, in mixed-sex flocks, which are the rule during non-breeding in house finches, we expect females to be just as likely as males to prefer feeding in the proximity of infected, non-aggressive males. Instead, the detected sex-specific feeding preference resulted from behavioural differences in the conspecific house finches: infected male conspecifics were significantly less likely to win aggressive interactions than healthy male conspecifics, driving male focal birds to prefer feeding in their vicinity. Female conspecifics, however, showed no change in the potential to win with MG infection. It remains unknown why the potential to win interactions differed between healthy and diseased males and females in our study. Previous studies suggest that the suppression of sickness behaviours depends on life-history trade-offs and can, for instance, vary with body condition (Owen-Ashley et al. 2006). Since female house finches tend to be dominant in aggressive interactions (Brown & Brown 1988), the motivation for infected females to maintain social status may be higher than for infected males. Alternatively, the physiological costs of infection and/or social defeat may be higher for male house finches.
Infection-induced behavioural changes are prevalent among host–pathogen systems (Moore 2002) and can alter the ability of a disease to invade and persist in a population (Lloyd-Smith et al. 2004). The population-level consequences for the observed behavioural changes towards infected house finches are likely to be critical for MG dynamics. Transmission experiments indicate that MG deposited on a feeder is only infectious for short (12 h or less) timescales (Dhondt et al. 2007). Therefore, house finches that associate in close proximity with infectious individuals while feeding may be most likely to contact viable MG and contribute to the continued persistence of MG epidemics in wild populations. The extent to which infection-induced behavioural changes such as those observed here drive transmission dynamics and epidemic persistence in host–pathogen systems is an area ripe for further exploration.
Acknowledgements
All housing protocols and experimental procedures were approved by Virginia Tech's Institutional Animal Care and Use Committee prior to the commencement of research.
We thank Tamara Fetters and Grace Martin for help analysing video observations, Laila Kirkpatrick and Bambi Jarrett for help with bird care and Cas Eikenaar, Erik Osnas and three anonymous referees for useful feedback on the manuscript. This work was supported by NSF-EF 0622705 under the NSF-NIH Ecology of Infectious Diseases programme.
References
- Altizer S., Hochachka W. M., Dhondt A. A.2004Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J. Anim. Ecol. 73, 309–322 (doi:10.1111/j.0021-8790.2004.00807.x) [Google Scholar]
- Behringer D. C., Butler M. J., Shields J. D.2006Avoidance of disease by social lobsters. Nature 441, 421–421 (doi:10.1038/441421a) [DOI] [PubMed] [Google Scholar]
- Brown M. B., Brown C. R.1988Access to winter food resources by bright-colored versus dull-colored house finches. Condor 90, 729–731 (doi:10.2307/1368370) [Google Scholar]
- Dhondt A. A., Dhondt K. V., Hawley D. M., Jennelle C. S.2007Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathol. 36, 205–208 (doi:10.1080/03079450701286277) [DOI] [PubMed] [Google Scholar]
- Faustino C. R., Jennelle C. S., Connolly V., Davis A. K., Swarthout E. C., Dhondt A. A., Cooch E. G.2004Mycoplasma gallisepticum infection dynamics in a house finch population: seasonal variation in survival, encounter and transmission rate. J. Anim. Ecol. 73, 651–669 (doi:10.1111/j.0021-8790.2004.00840.x) [Google Scholar]
- Hagelin J. C., Jones I. L.2007Bird odors and other chemical substances: a defense mechanism or overlooked mode of intraspecific communication? Auk 124, 741–761 (doi:10.1642/0004-8038(2007)124[741:BOAOCS]2.0.CO;2) [Google Scholar]
- Hart B. L.1988Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137 (doi:10.1016/S0149-7634(88)80004-6) [DOI] [PubMed] [Google Scholar]
- Hawley D. M.2006Asymmetric effects of experimental manipulations of social status on individual immune response. Anim. Behav. 71, 1431–1438 (doi:10.1016/j.anbehav.2005.12.004) [Google Scholar]
- Hochachka W. M., Dhondt A. A.2000Density-dependent decline of host abundance resulting from a new infectious disease. Proc. Natl Acad. Sci. USA 97, 5303–5306 (doi:10.1073/pnas.080551197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini P. R., Dhondt A. A., Dobson A.2004Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc. R. Soc. Lond. B 271, 2569–2577 (doi:10.1098/rspb.2004.2938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesecker J. M., Skelly D. K., Beard K. H., Preisser E.1999Behavioral reduction of infection risk. Proc. Natl Acad. Sci. USA 96, 9165–9168 (doi:10.1073/pnas.96.16.9165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G. V., Sydenstricker K. V., Kollias H. W., Ley D. H., Hosseini P. R., Connolly V., Dhondt A. A.2004Experimental infection of house finches with Mycoplasma gallisepticum. J. Wildl. Dis. 40, 79–86 [DOI] [PubMed] [Google Scholar]
- Ley D. H., Berkhoff J. E., McLaren J. M.1996Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis. 40, 480–483 (doi:10.2307/1592250) [PubMed] [Google Scholar]
- Lloyd-Smith J. O., Getz W. M., Westerhoff H. V.2004Frequency-dependent incidence in models of sexually transmitted diseases: portrayal of pair-based transmission and effects of illness on contact behaviour. Proc. R. Soc. Lond. B 271, 625–634 (doi:10.1098/rspb.2003.2632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehle C.1995Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335 (doi:10.2307/1941192) [Google Scholar]
- Møller A. P., Dufva R., Allander K.1993Parasites and the evolution of host social-behavior. San Diego, CA: Academic Press Inc [Google Scholar]
- Moore J.2002Parasites and the behavior of animals. New York, NY: Oxford University Press [Google Scholar]
- Owen-Ashley N. T., Turner M., Hahn T. P., Wingfield J. C.2006Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii). Horm. Behav. 49, 15–29 [DOI] [PubMed] [Google Scholar]
- Rosenqvist G., Johansson K.1995Male avoidance of parasitized females explained by direct benefits in a pipefish. Anim. Behav. 49, 1039–1045 (doi:10.1006/anbe.1995.0133) [Google Scholar]
- Schlaepfer M. A., Runge M. C., Sherman P. W.2002Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480 (doi:10.1016/S0169-5347(02)02580-6) [Google Scholar]