Abstract
COG1410, a small, novel ApoE-mimetic peptide derived from the receptor binding region of Apolipoprotein E (ApoE), has been classified as anti-inflammatory in nature and improves motor, sensorimotor, and cognitive dysfunction following cortical contusion injury (CCI). In order to further examine COG1410’s preclinical efficacy on cognitive recovery, the present study evaluated COG1410 following moderate fluid percussion injury (FPI). Animals were prepared with a moderate, unilateral FPI over the hippocampus. Following FPI, animals received a regimen of five doses of COG1410 or vehicle at 2 and 4 hrs (1.0 mg/kg, i.v.) followed by additional doses administered 24, 48, and 72 hrs (1.0 mg/kg, i.p.). Prior to injury, animals were trained for four days (4 trials/day) in the Morris water maze (MWM) and then tested for retrograde amnesia on post-FPI day 11 and then on a working memory task on day 18. Testing for motor dysfunction on the tapered balanced beam began on day 2 post-FPI. Administration of this regimen of COG1410 significantly improved retention of memory in the retrograde amnesia test compared to vehicle post-FPI. However, COG1410 did not significantly improve acquisition of working memory in the MWM. Motor dysfunction on the tapered beam post-FPI was improved in the COG1410-treated group compared to vehicle treatment. Cortical lesion analysis revealed that the COG1410-treated animals demonstrated significantly less tissue loss compared to vehicle-treated animals. The results of this study suggest that COG1410 significantly limited the behavioral dysfunction and tissue loss associated with FPI and demonstrated continued preclinical efficacy for TBI.
Keywords: Traumatic brain injury, therapy, recovery of function, amnesia, treatment, FPI
Introduction
Traumatic Brain Injury (TBI) is an extremely serious public health issue within the United States. Conservative estimates put the incidence rate for closed head injuries at 200 per 100,000, yielding 500,000 cases in the United States [35]. Penetrating head wounds in the United States occur at a rate of 12 per 100,000 people [35], but this number is compounded by the recent military operations in Iraq and Afghanistan. One military study of a mechanized battalion of marines in Iraq reported 70% of all injuries being to the upper extremity and head [9]. Another largely debilitating aspect of TBI is that no proven pharmacological treatment exists and all promising candidates for treatment have failed during Phase II and III clinical trials [35, 45].
In recent years, most TBI treatments have mainly focused on preventing or reducing the cascade of excitotoxicity following TBI [3]. However, a vast literature exists detailing the role of inflammation following TBI, but there are no current effective treatments targeting the inflammatory process [29, 45]. Apolipoprotein E (ApoE: protein, APOE: gene) is the most common apolipoprotein synthesized in the brain in response to injury. This 199 residue, 34 kDa protein is well known for its anti-inflammatory effects following brain injury, as well as its ability to reduce oxidation and excitotoxicity, and also appears to exhibit many neurotrophic properties [1, 5, 19–20, 26-27, 36].
COG1410 has demonstrated many neuroprotective properties in recent animal studies. It has been demonstrated that, in a murine model of closed head TBI (using the pneumatically induced skull impact model), a single dose of COG1410 administered 2 hrs post-TBI significantly improved vestibulomotor function on the Rotorod test, improved learning and memory in the Morris water maze (MWM), and demonstrated hippocampal neuronal sparing and microglial activity suppression [22]. It has also been shown that a single dose of 0.8 mg/kg COG1410 administered 30 min post-CCI significantly improved sensorimotor and somatosensory function, but did not improve vestibulomotor function [13]. This dose also aided in significant cortical tissue sparing as well as reducing astrocytic activation post-TBI. In a more recent study, a bilateral frontal CCI model was employed where animals received a 2-dose regimen (30 min and 24 hrs post-CCI) of 0.8 mg/kg COG1410. This regimen significantly improved memory retention in both a reference and working memory task in the MWM. COG1410 appeared to improve somatosensory performance on the tactile adhesive removal task and also demonstrated significant cortical neuronal and tissue sparing [16]. However, COG1410 has never been tested in the fluid percussion injury (FPI) model of TBI, which models more diffuse injury than the CCI model and may replicate aspects of blast TBI (bTBI).
The purpose of the present study was to examine the preclinical efficacy of a new dosing regimen of COG1410 in the FPI model. Current thinking in translational TBI research suggests testing novel therapies in at least two different rodent models of TBI [39]. Following unilateral FPI over the hippocampus, animals were administered a 1.0 mg/kg dose of COG1410 at 2 and 4 hrs (i.v.) post-FPI with additional doses administered at 24, 48, and 72 hrs (i.p.) post-FPI. Rats were tested on a battery of behavioral tests and their brains were harvested for histological analyses.
Methods and Methods
Subjects
Thirty-six, ~4 month old, male Long-Evans rats (mean weight, 481g) were used in this experiment. This study included 4 groups of animals (FPI-COG1410, FPI-Vehicle, Sham-COG1410, Sham-Vehicle). One animal from each group (FPI-COG1410, FPI-Vehicle, Sham-Vehicle) was removed from the study post-FPI due to respiratory failure and were not replaced. The experimental procedures conducted within this study were reviewed and approved by the Institutional Animal Care and Use Committee and the study was conducted in a facility certified by the American Association for the Accreditation of Laboratory Animal Care. Rats were maintained on a standard 12 hr light/dark cycle with food and water available ad libitum.
Surgery
Aseptic procedures and conditions were maintained for all animals during the surgical procedure in accordance with previously detailed methods [12, 41]. Brain injury was induced by FPI (VCU Biomedical Engineering, Richmond VA) using methods established by McIntosh et al. [32]. Each rat was anesthetized using a mixture of Isoflurane (2–4%), nitrous oxide (0.2 L/min), and oxygen (0.8 L/min) without intubation. When the rat reached stage III level of anesthesia (i.e., nonresponsive, exhibiting no hindlimb withdrawal reflex), its head was shaved and was placed into a stereotaxic device (Stoelting Instruments, Wood Dale, IL). Normal body temperature (37°C) was maintained throughout surgery with the use of a rectal probe feedback device attached to a heating pad. Betadine was applied to the rat’s head and a midline incision was made in the skin and underlying fascia. A unilateral, circular 4.0 mm craniotomy was made over the left hemisphere, centered at 4.4 mm posterior to bregma and 2.4 mm lateral to the midline. The dura remained intact. A plastic female Luer coupling was attached to the dry skull over the craniotomy using Temp Bond (Kerr Manufacturing Co, Romulus MI). Dental acrylic was used to secure the Luer coupling, which was then filled with sterile 0.9% saline. The animal was attached to the FPI device and a moderate fluid pressure pulse (mean = 1.80 atm; SD = 0.09) was delivered as soon as the hindlimb withdrawal reflex was elicited at a stage III-1 anesthesia level to ensure that all rats were injured at the same surgical level of anesthesia. The duration of the ensuing apnea (defined as the amount of time to regain spontaneous breathing) and loss of consciousness (defined as the amount of time until a positive hindlimb withdrawal reflex was displayed following injury) were recorded. Following the return of the hindlimb withdrawal reflex, the animal was placed back into the stereotaxic device, brought back to a stage III level of anesthesia, and the incision was sutured. Sham animals underwent the same procedures, including the craniotomy, but did not receive an FPI.
Synthesis of Peptides
Peptides were synthesized by NeoMPS (San Diego, CA) to a purity of 95%. COG1410 is Ac-AS(Aib)LRKL(Aib)KRLL-amide, which is derived from apoE residues 138–149 with Aib substitutions at positions 140 and 145. The peptides were reconstituted in sterile, 0.9% saline solution at a dose of 1.0 mg/kg prior to administration [13].
Drug Administration
Rats were randomly assigned to either the COG1410 (1.0 mg/kg) or vehicle (0.9% saline) group. Preliminary data on the pharmacokinetics of COG1410 indicated a half-life of 13 ±5 mins. Rats received a five-dose regimen of either COG1410 or vehicle administered intravenously by tail vein infusions at 2 hrs and 4 hrs post-FPI under light-isoflurane anesthesia. Additional injections were administered at 24 hrs, 48 hrs, and 72 hrs post-FPI. All testing and analyses were conducted without knowledge of drug assignment.
Cognitive Assessment
The MWM has been widely utilized to assess cognitive performance following brain injury. A blue fiberglass tank, with a diameter of 1.5 m and a height of 76 cm, was filled with water to a depth of 32 cm and maintained at 24°C. A 10×10 cm Plexiglas platform was submerged 1 cm below the surface of the water. Video tracking system and SMART tracking software (San Diego Instruments, San Diego, CA) were used to record the animal’s path length and latency. All animals were assessed for retention of memory on the retrograde amnesia task in accordance to previously detailed methods [38, 42] with alterations in the number of testing days and trials per day. Beginning four days prior to FPI, animals were trained for four consecutive days in a reference memory paradigm of the Morris water maze. Each rat was given 4 trials per day, starting from one of four release points in random order. On post-FPI day 11, animals were tested for retrograde amnesia and were given four trials. For both training and testing, a trial was terminated when the animal reached the submerged platform located in the center of the northeast quadrant, or when 90 sec had elapsed. Each animal was allowed to remain on the platform for 30 sec and was then placed in a warm holding cage for 15 min before the next trial.
A working memory performance trial was conducted on day 18 post-FPI using previously established methods [10–12, 16, 24]. The platform was submerged at the center of a new randomly chosen quadrant (southwest). Each animal was given 4 trials, starting from one of four randomly chosen release points (ITI = 15 min). The first trial was considered an information trial and was not included in subsequent analyses. Each trial ended when the animal located the platform, or when 90 sec had elapsed.
Tapered Beam Walk Test
Prior to FPI, all animals were trained (2 trials per day for 3 days) on this test of vestibulomotor function and motor coordination. Animals were trained to traverse a 165 cm long elevated, tapering beam with a 2 cm ledge in order to escape into their home cage [40]. The analysis reported in this study included the percent fault (calculated by dividing the number of foot faults by the total number of steps and multiplying by 100). Criterion performance was assessed as the ability to traverse the beam with no more than 2 foot slips on a single trial for 2 consecutive days. Following surgery, each animal was tested for 5 trials per day on days 2, 4, 6, 8, 10, 12, and 14 post-FPI. Post-injury performance was rated on the number of full slips with the contralateral hindlimb (paw was completely on the ledge).
Histology
On day 22 post-FPI, animals were anesthetized with urethane (3.0 g/kg, i.p.) and transcardially perfused with 0.9% phosphate buffered saline (PBS) followed by 10% phosphate buffered formalin (PBF). Brains were then post-fixed for 2 days in PBF following removal from the cranium. Brains were cryopreserved in a 30% sucrose solution 3 days prior to frozen sectioning. Serial sections (40 μm) were sliced using a sliding microtome with an electronic freezing stage and collected into PBS.
Lesion Analysis
A series of coronal sections were mounted on gelatin-subbed microscope slides, stained with cresyl violet, dehydrated and cover slipped. The extent of the lesion was analyzed with an Olympus microscope (BX-51) and DP-70 camera. Throughout the extent of the injury, images were captured using the digital capturing system and area measurements of the lesioned tissue were determined using ImageTool software. The Calvalieri method was used to calculate the volume of the remaining ipsilateral and contralateral cortex [4]. The number of sections and section thickness (40 μm) were multiplied by the mean area of the lesion cavity (calculated at five stereotaxic coordinates surrounding the lesion: −3.80, −4.30, −4.80, −5.30, and −5.80 relative to bregma) [37]. The extent of cortical injury was calculated using the formula [100-(ipsilateral cortex volume/contralateral cortex volume)*100] [12–16].
Statistical Analysis
Analysis of Variance (ANOVA) tests were performed using procedures for general linear models (SPSS 15.0 for Windows) with operations for repeated measures, where appropriate, for all behavioral measures. Initial analyses were performed between the 2 sham groups (sham-vehicle (n = 9) and sham-COG1410 (n = 6)) on all of the behavioral tests. These analyses revealed no significant differences between the sham groups, thus both groups were combined into one sham group for all subsequent analyses. The between group factor was group (1.0 mg/kg COG1410 (n = 9), vehicle (n = 9), and sham (n = 15)) and the within group factor was day of testing. Huynh-Feldt probabilities (HFP) and Fisher’s least significant difference tests (LSD) were used to correct for Type-1 error associated with repeated measures and post hoc means comparisons, respectively. Anatomical data were analyzed with one-way ANOVA procedures and Fisher’s LSD. A significance level of p < 0.05 was used for all statistical analyses.
Results
Severity of Injury
In order to determine if there was a significant difference in the severity of the injury between groups, duration of unconsciousness and apnea scores, recorded for each injured rat, were analyzed in a one-way ANOVA. There were no significant differences reported between the groups of injured rats on duration of unconsciousness [F(1,16) = 0.03, p = 0.87] or apnea [F(1,16) = 0.49, p = 0.49]. Duration of unconsciousness was equal across groups: COG1410 (mean = 165.1, SEM = 12.8) and saline (mean = 160.6, SEM = 23.1). Duration of apnea was also equal across groups: COG1410 (mean = 14.6, SEM = 1.3) and saline (mean = 16.4, SEM = 2.2). Hence, the level of injury sustained by the animals in each treatment group was equally matched.
Sham Analysis
To determine the effect of administration of COG1410 on normal behavior on the retrograde amnesia test in the MWM, a one-way ANOVA, with group (sham-vehicle, sham-COG1410) was performed for each data set. The analysis showed no significant difference in sham performance on the learning trials, the main effect of group was not significant [F(1,13) = 2.78, p = 0.12].
The effect of administration of COG1410 on normal behavior on the working memory test in the MWM was analyzed with a one-way ANOVA using group (sham-vehicle, sham-COG1410) as the factor in the analysis. The analysis showed no significant difference in performance, the main effect of group was not significant [F(2,14) = 1.03, p = 0.59].
The effect of administration of COG1410 on normal behavior on the tapered beam test was analyzed with a repeated measure ANOVA, with group (sham-vehicle, sham-COG1410) and day (2, 4, 6, 8, 10, 12, and 14) as the factors in the analysis. The analysis showed no significant difference in performance, the main effect of group was not significant [F(1,13) = 2.34, p = 0.15]; nor was the day x group interaction [F(6,78) = 1.87, p = 0.10]. There was also a non-significant main effect for day [F(6,78) = 1.10, p = 0.38].
MWM: Retrograde Amnesia
The pre-injury acquisition learning trials in the MWM (16 trials) was analyzed with a one-way repeated measures ANOVA, with group (COG1410, vehicle, and shams) and trial (1–16) as the factors in the analysis. There were no significant differences between the groups in the latency to locate the platform across any pre-injury training trials prior to CCI, [F(2,30) = .56, p = 0.58] (see Fig. 1A).
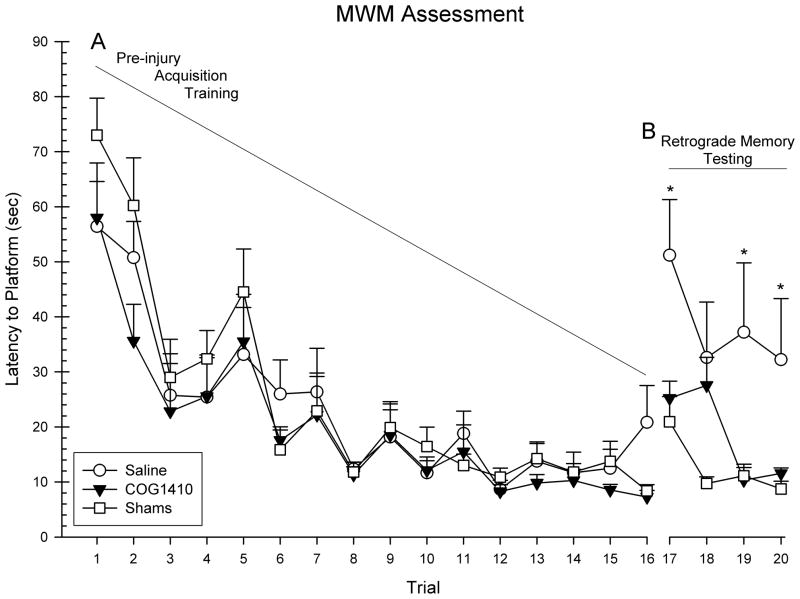
Figure 1.
Administration of COG1410 (1.0 mg/kg) following unilateral FPI significantly reduced retrograde amnesia following injury. Shown are the mean (+SEM) latencies to locate the escape platform during pre-injury acquisition training period (A). Treatment with COG1410 significantly reduced the latency to locate the escape platform on 3 of the 4 post-FPI trials compared to vehicle treatment (* = p < 0.05) (B).
Post-FPI performance on retention of memory in the retrograde amnesia task was evaluated with a repeated measure ANOVA, with group (COG1410, vehicle, and shams) and trial (17–20) as the factors in the analysis. This analysis revealed that the between group effect was significant [F(2,30) = 7.84, p = 0.002], as was the day effect [F(3,90) = 7.39, p = 0.001]. However, the interaction was not significant [F(6,90) = 1.54, p = 0.18]. Planned comparison post hoc analysis of the significant main effect of group on each memory trial revealed that the COG1410 group showed improved memory retention compared to the vehicle group on trials 17, 19 and 20 [LSD(16) = 26.00, p = 0.01]; [LSD(16) = 26.89, p = 0.01]; [LSD(16) = 20.67, p = 0.02], respectively (See Figure 1B). Post hoc analysis also revealed that the COG1410 group was not significantly different across trials compared to the sham group on trials 17, 19, and 20 (p > 0.05).
MWM: Working Memory Performance
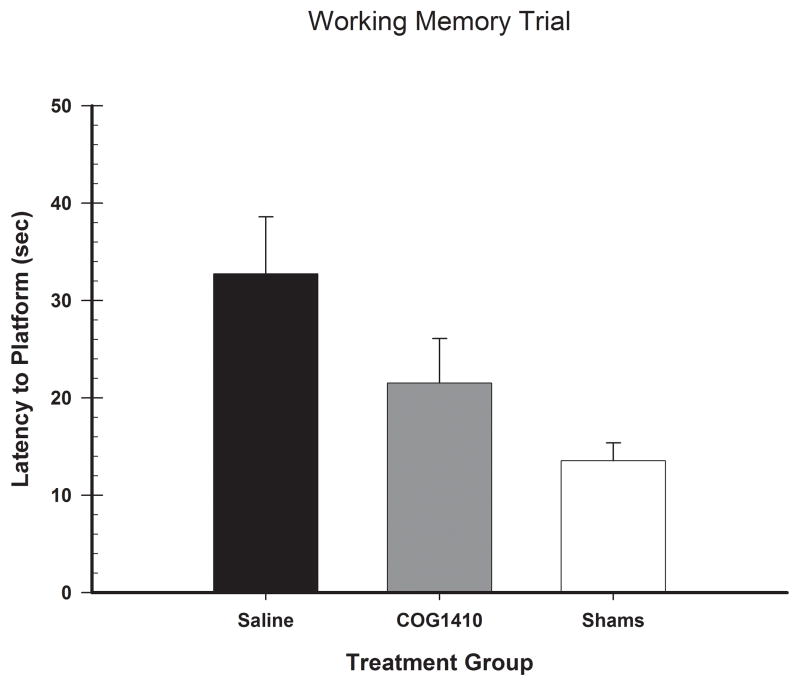
Post-FPI performance on the working memory test was assessed with a one-way ANOVA, with group (COG1410, vehicle, and shams) as the factor in the analysis. There was a significant difference between treatment, the main effect for group was significant [F(2,30) = 6.68, p = 0.004] (See Figure 2). As evidenced in Figure 2, there is an improvement in performance by the COG1410 treatment; however, the post hoc analysis of the significant main effect of treatment revealed that the COG1410 group just failed to meet significance compared to the vehicle group [LSD(16) = 11.22, p = 0.07].
Figure 2.
Administration of COG1410 (1.0 mg/kg) following unilateral FPI lessened the working memory deficit. The graph shows the plotted mean (+SEM) latency to locate the escape platform of the working memory trial. Treatment with COG1410 did not significantly improve performance in working memory compare to vehicle. However, behavioral improvement was observed in the treated group.
Tapered Beam Walk Test
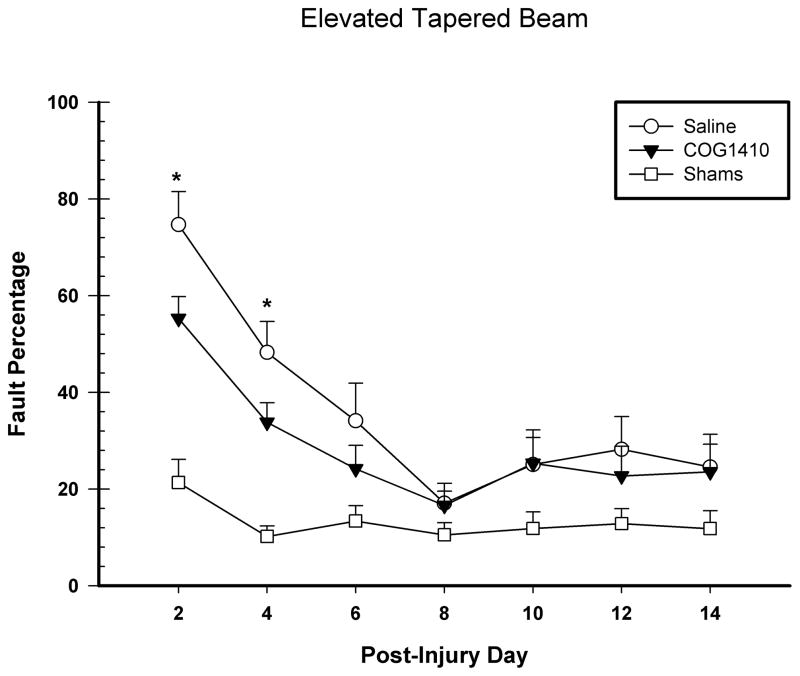
Locomotor performance on the tapered beam walk task was analyzed with a repeated measure ANOVA with group (COG1410, vehicle, and shams) and day (2, 4, 6, 8, 10, 12, and 14) as the factors in the analysis. The animals’ ability to traverse the beam improved with time following injury; the main effect for day was significant [F(6,180) = 54.72, p = 0.001]. The main effect for group was also significant [F(2,30) = 9.31, p = 0.001] as well as the day x group interaction [F(12,180) = 10.56, p = 0.001] (See Figure 3). Post hoc analysis of the significant interaction revealed that the COG1410-treated animals showed significant improvement on day 2 [LSD(16) = 19.39, p = 0.03] and day 4 [LSD(16) = 14.44, p = 0.03] post-FPI compared to vehicle-treated animals.
Figure 3.
Administration of COG1410 (1.0 mg/kg) following unilateral FPI significantly improved vestibulomotor behaivor following injury. The graph shows the plotted mean (+SEM) fault percentages of impairments with the contralateral hindlimb on the beam. Treatment with COG1410 significantly improved vestibulomotor performance on days 2 and 4 post-FPI compared to vehicle treatment (* = p < 0.05).
Lesion Analysis
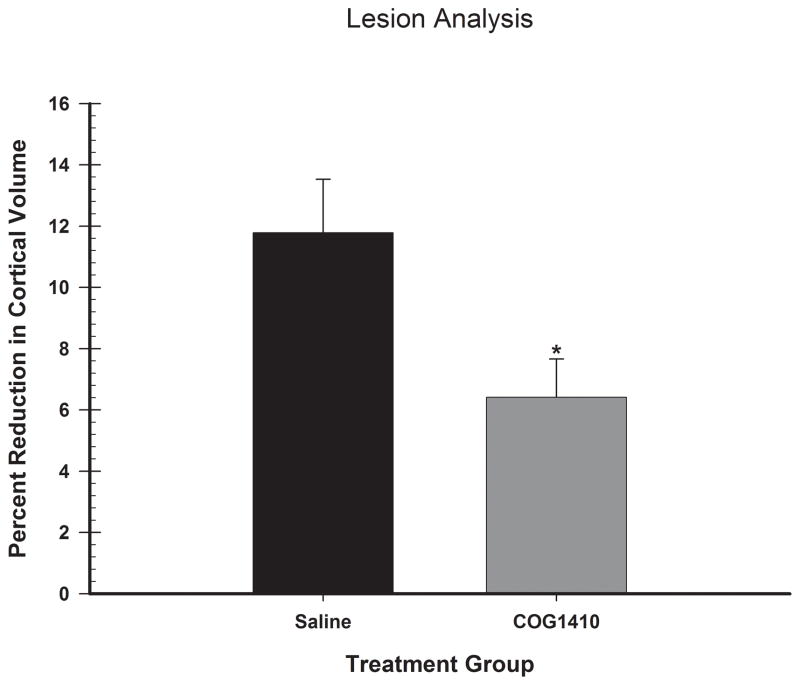
The reduction in cortical tissue volume throughout the injury site was analyzed with a one-way ANOVA, with group (COG1410 and vehicle) as the factor in the analysis. A significant difference in injury volume between groups was found [F(1,16) = 6.26, p = 0.02] (See Figure 4). Representative histology is shown in Figure 5 and indicates the COG1410 administration significantly reduced tissue loss post-FPI.
Figure 4.
Administration of COG1410 reduced tissue loss following FPI. Analysis of the percent reduction in injured cortical volume compared to contralateral cortex is shown. Plotted are the mean (+SEM) percent reductions in cortical volume for each group. COG1410 treatment (1.0 mg/kg) significantly reduced the amount of injury-induced tissue reduction compared to vehicle treatment following unilateral FPI (* = p<0.05).
Figure 5.
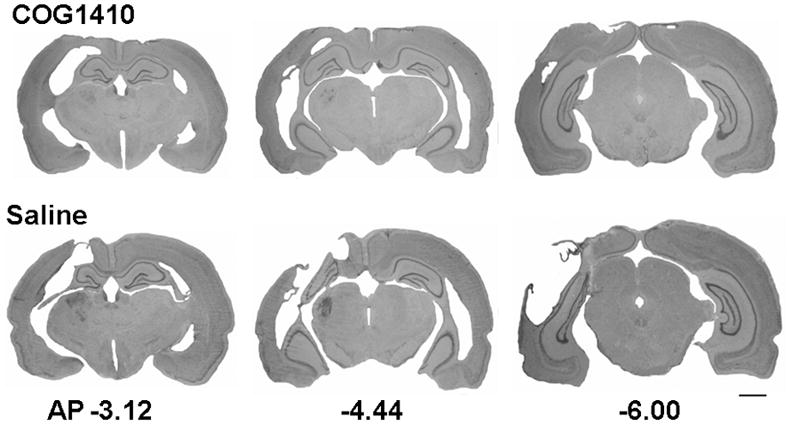
Representative histology showing the tissue protection provided my COG1410 treatment post-FPI. Shown are representative images of the effect of COG1410 (1.0 mg/kg) and vehicle treatment through the extent of the injury (0.44x). The FPI produced marked cortical cavitation that was significantly reduced by COG1410 treatment. Measurement bar = 2.0 mm.
Discussion
In the current study, FPI was used to model moderate TBI and to test the preclinical efficacy of COG1410. The FPI model has never been used to screen COG1410 and this model is thought to produce greater diffuse brain injury and may replicate aspects of blast injury that are observed in military TBI. FPI produced a strong retrograde amnesia for platform location in the MWM. Few studies have examined this form of a memory deficit following FPI. Previously, it has been shown that FPI produced a retrograde memory deficit following FPI, tested 42 hours after injury [43]. In the current study, we demonstrated a significant memory deficit at 11 days post-FPI, suggesting that it is an enduring deficit. In contrast, on day 18 there was a strong deficit in working memory (the ability to learn a new platform location in the MWM). FPI also produced a behavioral deficit on the elevated tapered beam; however, the behavior on the test quickly recovered in the injured rats. It is likely that the amount of pre-FPI beam training that was performed, in addition to being tested 5 times per day on each of the 7 post-FPI test days, resulted in the quick recovery on this behavioral test, and reduced its preclinical testing efficacy.
The treatment results from the present study suggest that the small ApoE-derived peptide, COG1410, exhibited neuroprotective benefit when administered in a five dose regimen following moderate, diffuse FPI. The behavioral data represents significant effects on recovery of function, specifically in the retrograde amnesia task within the MWM. For this task, it was observed that COG1410 administration improved memory for the previously learned escape platform location in the MWM. With the exception on 1 of 4 days, the COG1410 animals were not significantly different from the non-injured sham group in terms of their memory of the escape location. However, further assessment in the MWM created mixed results. Working memory performance in the MWM showed that the COG1410-treated animals were not statistically different from the vehicle-treated animals on any day of testing. The COG1410 group (mean = 21.5) did have a greater initial recovery on day 18 compared to vehicle-treated (mean = 32.7) animals; however, it was not statistically significant. A possible explanation for this is the amount of testing that the animals had previously received in the MWM. All animals received 4 days of training (16 trials) prior to injury and another day of testing (4 trials) for the retrograde amnesia task post-TBI, so the animals were already well adapted to the task itself. We have seen a similar result in a different study with the same testing paradigm [38].
Vestibulomotor dysfunction was evaluated on the elevated tapered beam. COG1410-treated animals exhibited a significant improvement in performance compared to vehicle-treated animals on days 2 and 4 post-FPI. Also, COG1410-treated animals were statistically not different than the sham controls on days 6 and 10, suggesting an abatement of the injury induced deficit. Furthermore, the Vehicle-treated group was significantly different than the sham controls on days 2, 4, 10, 12, and 14. Thus, treatment with COG1410 altered the typical recovery pattern on this behavioral test. These data support previous findings that COG1410 improves vestibulomotor performance post-TBI [22]. As well as, a previous finding that COG1410 improved sensorimotor recovery, on a variety of tests, following cortical contusion injury (CCI) [13, 16].
Additionally, the anatomical data from this present study showed a significant neuroprotective effects. A cortical lesion analysis was conducted and the percent reduction in brain volume was calculated. Based on the results, the COG1410-treated group had significantly less reduction in brain volume (mean = 6.42%) compared to the vehicle-treated group (mean = 11.79%). This effect is similar to what we have shown in two separate models of CCI following treatment with COG1410 [13, 16]. Thus, it appears that the ability of COG1410 to offset cortical neuropathology is relatively stable and strong.
In the present study, the data suggests that treatment with this five dose regimen of COG1410 significantly improved cognitive and vestibulomotor performance and spared cortical neuronal tissue following TBI, without showing any detrimental side-effects. These finding are consistent with previous studies where COG1410 was administered following TBI at slightly lower concentrations and shorter durations (2 doses, over 48 hrs). It has previously been shown that treatment with COG1410 can improve cognitive performance [16, 23]. Specifically, administration of a 2 dose regimen of COG1410 improved cognitive performance in the MWM in both a reference and working memory task [16]. In a reference memory task, somewhat similar to the current retrograde amnesia task, it was shown that a single injection of COG1410 improved memory retention following TBI [22]. Likewise, it has also been shown that treatment with COG1410 can significantly improve vestibulomotor performance post-TBI [22]. Thus, it appears that the current 5 day regimen (the longest treatment period to date) of COG1410 may provide the highest degree of preclinical efficacy compared to the previously used dosage regimens.
Apolipoprotein E exists in three different isoforms within the human: the E2, E3, and E4 alleles, respectively. These isoforms vary by only a single interchange of either cysteine or arginine residues at locations 112 and 158 of apoE [47]. In recent years, the therapeutic effects of apoE have been found to be isoform specific. For instance, the presence of the apoE4 allele has been shown to lead to poorer outcome in many neurological disorders including traumatic brain injury [8, 44] and has also been shown to increase the expression of inflammatory cytokines, such as TNFα and IL-6, upon stimulation with lipopolysaccharide [28]. In contrast, the apoE3 allele demonstrates a down regulation of these inflammatory cytokines compared to apoE4 [20, 28] and apoE3 is more effective at suppressing the activation of microglia in paradigms of brain inflammation [2, 20]. Because of the large size of apoE (34 kDa), it is too large to readily cross the blood brain barrier (BBB) which limits its usefulness as a novel therapeutic for TBI [25]. This results in the creation of a first generation apoE-mimetic peptide created from the receptor binding region of apoE called COG133 (apoE residues 133–149) [20] which retains the neuroprotective properties of the apoE holoprotein [1, 20, 28–30, 33]. However, COG133 was found to have limited therapeutic use because the window of administration appeared to be only limited to 30 min post-TBI [23, 29]. In turn, COG1410, a shorter analog to COG133 which encompasses apoE residues 138–149 with aminoisobutyric acid (Aib) substitutions at locations 140 and 145, was created. COG1410 retains many of the neuroprotective properties of its predecessor, but has been shown to exhibit superior efficacy and potency in vitro [21]. It has also been demonstrated that the window of administration is much larger for COG1410 (2 hrs) compared to that of COG133 (30 mins) [23].
The examination of allele specific antioxidant activities has been performed in vitro. Cells lines secreting APOE isoforms of E2, E3, and E4 have been screened for antioxidant capacity [34]. It was found that the antioxidant capacity was greater in the E2 isoform and the least in the E4 isoform. Several recent papers have examined the pattern of gene expression following CCI in APOE2, APOE3 and APOE4 transgenic mice on the expression of anti-oxidant genes as a function of APOE genotype [6–7]. Differences between the genotypes were found suggesting that APOE3 (from the APOE3/3 mice) plus CCI appeared to induce higher levels of anti-oxidant enzymes than was observed in APOE4 (from APOE4/4) mice plus CCI. There were some limited differences on differentially regulated genes (with respect to APOE genotype) that relate to the 1 day or the 1 month time points post-CCI that were evaluated in the study. No behavioral measures were examined so it is unclear if these findings would result in functional differences. APOE4 has also been shown to be associated with poor functional outcome and increased cerebral edema in a murine model of intracerebral hemorrhage [18]; as well as, being a significant predictor of poor functional outcome in human intracerebral hemorrhage [17]. A murine model of sepsis demonstrated that expression of human APOE4 resulted in increased mortality and that treatment with COG1410 reduced this mortality and reduced TNF alpha, interleukin-1beta, interleukin-6, and interleukin-12 levels [46]. However, APOE4 has also not been shown to be associated with cognitive decline following major noncardiac surgery [31].
Although, the pharmacokinetics for COG1410 has not been fully established, some preliminary work has begun. Initial pharmacokinetic studies on intravenous administration of COG1410 have shown that the half-life of COG1410 is 13 ± 5 minutes in blood plasma (M. Vitek, unpublished data). This means that less than 1% of the original dose of COG1410 remains in the blood plasma 2 hrs following IV administration. It is possible that binding of COG1410 to cell surface receptors like the apoE receptors found on endothelial cells and numerous cells in the blood reduce the availability of COG1410. Thus, the preliminary pharmacokinetic data supports the timing of the dosing in the current study. The common observation from all of our behavioral studies is that the protective effects of single or multiple doses of COG1410 appears to be long lived representing an early event where COG1410 rapidly protects/repairs damage from a TBI [13, 16]. While the exact mechanism is a matter of active research, the desired behavioral endpoint is the same, significant improvements in motor and behavioral performance even when COG1410 is administered 2 hrs post-injury.
In summary, the results from the present study support and extend previous findings that COG1410 demonstrated preclinical efficacy as a therapy following moderate TBI. Continued preclinical testing is needed because a variety of variables have yet to be investigated (e.g., gender effects of treatment, window of opportunity, and age effects). However, it appears that COG1410 may have substantial preclinical efficacy for the treatment of TBI.
Acknowledgments
Funding provided by Cognosci Inc. grants R44NS048689 and R44AG020473 from the NIH. Special thanks to Andrea Quigley, Keith Gregory, and Sarah Heck for technical assistance. Cognosci, Inc. has filed for patent protection for COG1410.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aono M, Bennett ER, Kim KS, Lynch JR, Myers J, Pearlstein RD, Warner DS, Laskowitz DT. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–45. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- 2.Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–81. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- 3.Clausen T, Bullock R. Medical treatment and neuroprotection in traumatic brain injury. Curr Pharm Des. 2001;7:1517–32. doi: 10.2174/1381612013397267. [DOI] [PubMed] [Google Scholar]
- 4.Coggeshall RE. A consideration of neural counting methods. TINS. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- 5.Colton CA, Brown CM, Cook D, Needham LK, Xu Q, Czapiga M, Saunders AM, Schmechel DE, Rasheed K, et al. APOE and the regulation of microglial nitric oxide production: a link between genetic risk and oxidative stress. Neurobiol Aging. 2002;23:777–85. doi: 10.1016/s0197-4580(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 6.Crawford F, Wood M, Ferguson S, Mathura V, Gupta P, Humphrey J, Mouzon B, Laporte V, Margenthaler E, et al. Apolipoprotein E-genotype dependent hippocampal and cortical responses to traumatic brain injury. Neuroscience. 2009;159:1349–62. doi: 10.1016/j.neuroscience.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson S, Mouzon B, Kayihan G, Wood M, Poon F, Doore S, Mathura V, Humphrey J, O’Steen B, et al. Apolipoprotein E genotype and oxidative stress response to traumatic brain injury. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.01.031. in press. [DOI] [PubMed] [Google Scholar]
- 8.Friedman G, Froom P, Sazbon L, Grinblatt I, Shochina M, Tsenter J, Babaey S, Yehuda B, Groswasser Z. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–8. doi: 10.1212/wnl.52.2.244. [DOI] [PubMed] [Google Scholar]
- 9.Gondusky JS, Reiter MP. Protecting military convoys in Iraq: an examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Mil Med. 2005;170:546–9. doi: 10.7205/milmed.170.6.546. [DOI] [PubMed] [Google Scholar]
- 10.Hoane M, Wolyniak J, Akstulewicz S. Administration of riboflavin improves behavioral outcome and reduces edema formation and GFAP expression following traumatic brain injury. J Neurotrauma. 2005:1112–22. doi: 10.1089/neu.2005.22.1112. [DOI] [PubMed] [Google Scholar]
- 11.Hoane MR, Akstulewicz SL, Toppen J. Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in the rat. J Neurotrauma. 2003;20:1189–98. doi: 10.1089/089771503770802871. [DOI] [PubMed] [Google Scholar]
- 12.Hoane MR, Tan AA, Pierce JL, Anderson GD, Smith DC. Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J Neurotrauma. 2006;23:1535–48. doi: 10.1089/neu.2006.23.1535. [DOI] [PubMed] [Google Scholar]
- 13.Hoane MR, Pierce JL, Holland MA, Birky ND, Dang T, Vitek MP, McKenna SE. The novel apolipoprotein E-based peptide COG1410 improves sensorimotor performance and reduces injury magnitude following cortical contusion injury. J Neurotrauma. 2007;24:1108–18. doi: 10.1089/neu.2006.0254. [DOI] [PubMed] [Google Scholar]
- 14.Hoane MR, Pierce JL, Holland MA, Anderson GD. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008;154:861–8. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoane MR, Pierce JL, Kaufman NA, Beare JE. Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxid Med Cell Longev. 2008;1:46–53. doi: 10.4161/oxim.1.1.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoane MR, Kaufman NA, Vitek MP, McKenna SE. COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J Neurotrauma. 2009;26:1–10. doi: 10.1089/neu.2008.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James ML, Blessing R, Bennett E, Laskowitz DT. Apolipoprotein E modifies neurological outcome by affecting cerebral edema but not hematoma size after intracerebral hemorrhage in humans. J Stroke Cerebrovasc Dis. 2009;18:144–9. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James ML, Sullivan P, Lascola C, Vitek MP, Laskowitz DT. Pharacogenomic effects of apolipoprotein E on intracerebral hemorrhage. Stroke. 2009;40:632–9. doi: 10.1161/STROKEAHA.108.530402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76:70–4. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 20.Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, Bennett ER. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- 21.Laskowitz DT, Fillit H, Yeung N, Toku K, Vitek MP. Apolipoprotein E-derived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta Neurol Scand Suppl. 2006;185:15–20. doi: 10.1111/j.1600-0404.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 22.Laskowitz DT, McKenna SE, Song P, Wang H, Durham L, Yeung N, Christensen D, Vitek MP. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J Neurotrauma. 2007;24:1093–107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- 23.Laskowitz DT, Vitek MP. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–69. doi: 10.2217/14622416.8.8.959. [DOI] [PubMed] [Google Scholar]
- 24.Lindner MD, Plone MA, Cain CK, Frydel BR, Francis JM, Emerich DF, Sutton RL. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J Neurotrauma. 1998;15:199–216. doi: 10.1089/neu.1998.15.199. [DOI] [PubMed] [Google Scholar]
- 25.Linton MF, Gish R, Hubl ST, Bèutler E, Esquivel C, Bry WI, Boyles JK, Wardell MR, Young SG. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. The Journal of clinical investigation. 1991;88:270–81. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lomnitski L, Chapman S, Hochman A, Kohen R, Shohami E, Chen Y, Trembovler V, Michaelson DM. Antioxidant mechanisms in apolipoprotein E deficient mice prior to and following closed head injury. Biochim Biophys Acta. 1999;1453:359–68. doi: 10.1016/s0925-4439(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 27.Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–13. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 28.Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–33. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- 29.Lynch JR, Wang H, Mace B, Leinenweber S, Warner DS, Bennett ER, Vitek MP, McKenna S, Laskowitz DT. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exp Neurol. 2005;192:109–16. doi: 10.1016/j.expneurol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 30.McAdoo JD, Warner DS, Goldberg RN, Vitek MP, Pearlstein R, Laskowitz DT. Intrathecal administration of a novel apoE-derived therapeutic peptide improves outcome following perinatal hypoxic-ischemic injury. Neurosci Lett. 2005;381:305–18. doi: 10.1016/j.neulet.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 31.McDonagh D, Mathew J, White W, Phillips-Bute B, Laskowitz DT, Podgoreanu M, Newman M, Group NOR. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomakers of brain injury. Anesthesiology. 2010;112:852–9. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–44. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 33.Misra UK, Adlakha CL, Gawdi G, McMillian MK, Pizzo SV, Laskowitz DT. Apolipoprotein E and mimetic peptide initiate a calcium-dependent signaling response in macrophages. J Leukocyte Biol. 2001;70:677–83. [PubMed] [Google Scholar]
- 34.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 35.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–57. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science (New York, NY) 1994;264:850–2. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. New York: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 38.Quigley A, Tan AA, Hoane MR. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res. 2009;1304:138–48. doi: 10.1016/j.brainres.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schallert T, Woodlee MT. Orienting and Placing. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat. New York: Oxford University Press; 2005. pp. 129–40. [Google Scholar]
- 41.Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, Clough RW. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–69. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 43.Smith DH, Okiyama K, Gennarelli TA, McIntosh TK. Magnesium and ketamine attenuate cognitive dysfunction following experimental brain injury. Neurosci Lett. 1993;157:211–4. doi: 10.1016/0304-3940(93)90739-8. [DOI] [PubMed] [Google Scholar]
- 44.Sorbi S, Nacmias B, Piacentini S, Repice A, Latorraca S, Forleo P, Amaducci L. ApoE as a prognostic factor for post-traumatic coma. Nat Med. 1995;1:852. doi: 10.1038/nm0995-852. [DOI] [PubMed] [Google Scholar]
- 45.Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–9. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Christensen D, Vitek MP, Sullivan P, Laskowitz DT. APOE genotype affects outcome in a murine model of sepsis: Implications for a new treatment strategy. Anaesth Intensive Care. 2009;37:38–45. doi: 10.1177/0310057X0903700111. [DOI] [PubMed] [Google Scholar]
- 47.Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]