Abstract
Background
Excessive alcohol intake produces structural and functional deficits in corticolimbic pathways that are thought to underlie the cognitive deficits in the alcohol use disorders. Animal models of binge alcohol administration support the direct link of high levels of alcohol consumption and neurotoxicity in the hippocampus and surrounding cortex. In contrast, voluntary wheel running enhances hippocampal neurogenesis and generally promotes the health of neurons.
Methods
We investigated whether voluntary exercise prior to binge alcohol exposure could protect against alcohol-induced cell loss. Female Long-Evans rats exercised voluntarily for 14 days before undergoing 4 days of binge alcohol consumption. Brains were harvested immediately after the last dose of alcohol and examined for various histological markers of neurodegeneration, including both cell death (FluoroJade B) and cell birth (Ki67) markers.
Results
Rats that exercised prior to binge exposure were significantly less behaviorally intoxicated, which was not a result of enhanced hepatic metabolism. Rats that exercised prior to binge alcohol consumption had reduced loss of dentate gyrus granule cells and fewer FluoroJade B positive cells in the dentate gyrus and associated entorhinal-perirhinal cortex compared to non-exercisers. However, exercise did not protect against cell death in the piriform cortex nor protect against alcohol-induced decreases in cell proliferation, evidenced by a similar alcohol-induced reduction in Ki67 labeled cells between exercise and sedentary rats.
Conclusions
We conclude that exercise can reduce behavioral sensitivity to ethanol intoxication and protect vulnerable brain areas from alcohol-induced cell death. Exercise neuroprotection of alcohol-induced brain damage has important implications in understanding the neurobiology of the AUDs as well as in developing novel treatment strategies.
Keywords: alcoholism, neurodegeneration, dentate gyrus, adult neurogenesis, ethanol
INTRODUCTION
In their lifetimes, over 30% of Americans will meet the diagnostic criteria for an alcohol use disorder (AUD), commonly referred to as alcoholism (Hasin et al., 2007). Excessive intake, the hallmark of an AUD, has been linked to deficits in brain structure and function according to MRI analyses of brain volume to cell counts in postmortem brain tissue from alcoholics (Harper, 1985; Pfefferbaum et al., 1992; Sullivan and Pfefferbaum, 2005). Animal models have provided a direct link between excessive alcohol intake and neurodegeneration and further, have begun to elucidate the various mechanisms that result in a net loss of cells (Collins et al., 1996; Crews et al., 2000; Crews and Nixon, 2009; Obernier et al., 2002b; Walker et al., 1980). Several different lines of evidence, such as lifetime consumption not correlating with neurodegeneration (Tivis et al., 1995) and that even adolescent abusers demonstrate neuropathology (De Bellis et al., 2000), supports the fact that pattern of intake, namely binge drinking, is more predictive of neurodegeneration rather than lifetime duration of intake (Hunt, 1993). Some hypothesize that binge drinking, and specifically brain damage from binge drinking, is one of the critical links between alcohol experimentation and developing an AUD (Crews, 1999). Epidemiological studies indicate that binge alcohol consumption is on the rise in young people (Courtney and Polich, 2009), and particularly college students as a university environment appears to foster binge drinking (Hartzler and Fromme, 2003; Neal and Fromme, 2007). Indeed, 18–25 year olds drink more alcohol that all other age groups and have the highest rates of AUDs (Hasin et al., 2007; Johnston et al., 2007). Although variables such as gender and ethnicity have been studied in relationship to alcohol consumption in young adults (Corbin et al., 2008), the influence of lifestyle factors such as exercise has received little attention.
Exercise induces many beneficial physiological changes, including reduced body fat (Alessio et al., 2005; Lambert and Jonsdottir, 1998; Moraska et al., 2000; Skalicky and Viidik, 1999), increased expression of cardioprotective proteins (Noble et al., 1999), enhanced muscle mitochondrial biogenesis (Moraska et al., 2000), increased circulation of trophic factors (Asano et al., 1998; Carro, 2000) and decreased blood pressure (Pescatello et al., 2004). Exercise also benefits the brain, by inducing angiogenesis (Black et al., 1990; Swain et al., 2003) and bolstering antioxidant activity (Alessio et al., 2005; Somani et al., 1995). In particular, exercise promotes the health of the hippocampus, where it increases the proliferation, survival and neuronal differentiation of progenitor cells (van Praag et al., 1999b) enhances LTP (Bengochea and Gonzalo, 1990; Farmer et al., 2004; van Praag et al., 1999a) and is correlated with improvements in learning and memory (Cotman and Berchtold, 2002; van Praag et al., 1999a).
In contrast, excessive alcohol consumption causes degeneration of the hippocampus in humans and animal models of AUDs (Agartz et al., 1999; Bengochea and Gonzalo, 1990; Cadete-Leite et al., 1988; Obernier et al., 2002a; Sullivan et al., 1995; Walker et al., 1980). Binge alcohol administration in rats has been shown to produce neurodegeneration by inducing necrosis in corticolimbic regions including the hippocampus as well as by inhibiting adult neurogenesis (Crews and Nixon, 2009; Nixon and Crews, 2002; Obernier et al., 2002a). Because exercise promotes the health of the hippocampus, we investigated the possibility that it could protect the brain against binge alcohol-induced damage. Further, because binge drinking is increasing in young women (Keyes et al., 2008), and women are more likely to binge drink in college (Reifman and Watson, 2003), the present study investigated the neuroprotective potential of exercise against future binge alcohol consumption in young female rats.
MATERIALS AND METHODS
Animals and Housing Conditions
All experimental procedures were conducted in accordance with the National Institutes of Health (NIH) Public Health Service Policy on Humane Care and Use of Laboratory Animals (NIH, 2002), and were approved by the Institutional Animal Care and Use Committee of the University of Houston. Upon arrival, rats were group-housed in clear Plexiglas cages and given 1 week to acclimate to vivarium conditions, which included ad libitum rat chow and water, and a reversed light/dark cycle (lights off 10:00 / on 22:00). Thirty-seven female Long-Evans rats (Harlan), weighing 190 – 220 grams at the beginning of the experiment were divided into four groups in a 2×2 design comparing ethanol versus control diet and exercise versus sedentary: sedentary control (SC; n = 10) which were sedentary and received isocaloric control diet; sedentary ethanol (SE; n = 7) which were sedentary and received ethanol diet, exercise control diet (EC; n = 10) which exercised daily and received isocaloric control diet; and exercise ethanol (EE; n = 10) which had daily exercise and the ethanol diet. Prior to beginning the experiments, all rats were tamed by gentle handling, to acclimate them to the daily handling of the experiment and make them amenable to gavage. Female rats were chosen because of their consistent running behavior and they have not been studied in this model of an AUD.
Exercise
Rats were given voluntary access to exercise wheels for a total of 14 days. In order to precisely monitor distance traveled, rats were removed from their home cages and placed into individual running wheels equipped with counters, as previously described (Leasure and Decker, 2009; Stranahan et al., 2006). Exercise began at 1030 daily (the beginning of the dark cycle) and stopped at 1600, when the animals were removed from the exercise wheels and returned to the home cages. Thus, although rats in the exercise groups were socially isolated while exercising, this occurred for only 5.5 hours daily, leaving these animals group-housed the majority of the time.
Binge consumption paradigm
Immediately after the exercise period, rats were subjected to a binge model of alcohol exposure. The four-day binge model is a well-accepted model of alcohol abuse with a characteristic and consistent pattern of cell death in male rats (Collins et al., 1996; Crews et al., 2000). As the majority of those diagnosed with an AUD drink in a binge pattern and because a binge pattern of drinking is predictive of neuropathology (Hunt, 1993), this model was chosen to best mimic the human condition. Food was removed during the period of alcohol administration, but water was always available. Ethanol was administered via gavage according to a paradigm modified from Majchrowicz (1975), which maintains consistent intoxication while avoiding mortality (Knapp and Crews, 1999; Nixon and Crews, 2004). Briefly, rats were gavaged with ethanol diet (25% ethanol w/v in vanilla Ensure™, Abbott Laboratories, Columbus, OH) or isocaloric control diet every 8 hours for 4 days. The initial dose for each animal was 5 g/kg; thereafter each dose was determined based on a six-point behavioral intoxication scale that corresponded to an accompanying dose of ethanol (see Table 1). Blood ethanol concentration (BEC) was determined from tail blood samples taken 90 minutes after the morning dose on day 3. Samples were centrifuged, then stored at −20°C until further analysis. Serum was extracted and BEC determined using a GM7 Analyser based on external standards (Analox, Waltham, MA).
Table 1.
Modified Majchrowicz scale
| Intoxication Score |
Indications | Dose (g/kg) |
|---|---|---|
| 0 | Normal rat | 5 |
| 1 | Hypoactive, mild ataxia | 4 |
| 2 | Ataxic, abdomen elevated | 3 |
| 3 | Delayed righting reflex, Ataxic with no abdominal elevation |
2 |
| 4 | Loss of righting reflex, Retains eye blink reflex |
1 |
| 5 | Loss of righting reflex Loss of eye blink reflex |
0 |
Acute Alcohol Administration
A separate experiment investigated the effect of exercise on ethanol pharmacokinetics. Two groups of rats were allowed to exercise voluntarily for 2 weeks as described above (n=6) or remained sedentary (n=6). Rats were then food deprived for 8 hours before being administered a single 5g/kg dose of ethanol via gavage the day following the last day of exercise. Tail blood samples were taken at 30 minutes, 60 minutes, 90 minutes, 3 hours, 5 hours and 9 hours. Blood was collected and analyzed by a GM7 Analyser as reported above.
Histological procedures
Immediately after the last dose of alcohol, each rat was given an overdose of anesthetic and intracardially perfused with cold saline, followed by 4% paraformaldehyde until the upper body was stiff. Brains were removed, post-fixed overnight and then refrigerated in 30% sucrose. Brains were cut in 40 µm coronal sections on a Vibratome from a random start point at the level of the caudate putamen through the approximately Bregma −6.6. Each section was stored in cryoprotectant in 24-well microtiter plates at −20°C.
Quantification of Dentate Gyrus Granule Cells
Every 12th section from the earliest emergence of the inferior blade of the dentate gyrus (Bregma −1.72) through Bregma −5.88 was mounted to gelatin coated slides and processed for cresyl violet counterstain. This resulted in six to nine sections per subject quantified for the dentate gyrus. The number of granule cells in the dentate gyrus was determined using the optical fractionator method, applied via the automated stereology system, StereoInvestigator (MicroBrightField, Williston, VT) similar to published methods (West, 1991) on a Nikon Eclipse 80i upright microscope. For every section, the dentate gyrus was traced at 10X in StereoInvestigator. The optical fractionator was then applied, and cells counted at 100X using an oil-immersion objective, with counting frames of 40 µm × 40 µm, and a 200 µm grid. For every counting frame, the top of the section was set, and then a top guard zone of 4 µm and an optical dissector height of 12 µm applied. Because the average mounted section thickness was approximately 20 µm, these parameters resulted in all granule cells present in the middle 12 µm of each section being counted, thereby eliminating the problems of uneven section surface and cell stripping. The number of granule cell profiles was totaled and used to estimate the total number of granule cells per dentate gyrus ± SEM.
FluoroJade B labeling
FluoroJade B staining of degenerating cells was conducted following published methods (Morris et al., 2009; Schmued and Hopkins, 2000). Briefly, every 12th section was mounted to Superfrost Plus® slides (Fisher Scientific, Waltham, MA) and dried overnight. Slides were processed through graded alcohols, rinsed in dH20 then incubated in 0.06% KMnO4 for 10 minutes. Following a wash in dH20, sections were then incubated in FJB (Chemicon, Temecula CA) in the dark. After additional washes in dH20, sections were dried then coverslipped in Cytoseal® (Stephens Scientific, Wayne, NJ). Quantification of FJB-positive (FJB+) cells was conducted on an Olympus BX-51 microscope fitted with epifluorescence including a 488λ cube for blue light excitation. A profile counting methodology was used to quantify all cells visualized within the region of interest. This method was chosen over unbiased stereological techniques because of difficulty in accurately detecting section thickness in sections with very low background staining as our FJB method produces and to compare across regions where stereology is not appropriate. Further, we have shown previously that profile methodologies result in identical percent change between control and experimental groups (Crews et al., 2004), and relative difference is the question of interest in this study. FJB+ cells were counted in brain regions that consistently show alcohol-induced cell death in this model: the hippocampal dentate gyrus (granular cell layer and subgranular zone combined), the entorhinal and perirhinal cortices combined and the piriform cortex (Obernier et al., 2002a; Obernier et al., 2002b). Specifically, in the dentate gyrus, each blade was counted and averaged separately and the mean number of FJB+ cells per dentate gyrus section was calculated by adding the averages from the superior and inferior blades together. FJB+ cells are reported as mean number of cells per section ± SEM.
Ki67 Immunohistochemistry
Immunohistochemistry for the endogenous cell proliferation marker, Ki67 was performed on every 12th section of the dentate gyrus as defined above and followed previously reported methods (Morris et al., 2009). Ki67 is expressed in cells that are actively proliferating, in G1 through M phase of the cell cycle (Scholzen and Gerdes, 2000). Ki67 was chosen to assess proliferating cells because the use of exogenous markers such as Bromo-deoxy-Uridine have been criticized for potential experimental differences in bioavailability and these experiments investigate two events (namely, exercise and alcohol administration) that could, in theory, alter the bioavailability of Bromo-deoxy-Uridine. Briefly, free-floating sections were washed in tris-buffered saline (TBS) followed by treatment with 0.3% hydrogen peroxide to exhaust endogenous peroxidases. Following washes, sections were blocked in TBS+ (TBS/0.1% Triton-X/3% horse serum) and then incubated in anti-Ki67 (VectorLabs, Burlingame, CA; 1:200) for 48 hours at 4°C. After washes in TBS+, sections were incubated with biotinylated secondary antibody (horse anti-mouse, Vector Laboratories, Burlingame, CA; 1:200) rinsed again and then incubated with avidin-biotin complex (ABC, Vector Labs, Burlingame, CA). Finally, diaminobenzidine (DAB) was used as the chromagen with 0.0006% hydrogen peroxide and Nickel enhancement. Sections were mounted onto slides, counterstained in Neutral Red and coverslipped with Cytoseal®.
The number of Ki67-positive (Ki67+) cells in the dentate gyrus was estimated using the optical fractionator method applied via an automated stereology system, (newCAST™, Visiopharm, Denmark) on a BX-51 Olympus Microscope (Olympus, Center Valley, PA) coupled to a Prior stage and digital camera (DP70; Olympus, Center Valley, PA). All Ki67+ cells were counted in the dentate gyrus and subgranular zone (i.e. 100% area sampling fraction) in every 12th section (480 µm apart) of the dentate gyrus. Cells were counted at 1200X using a 60X oil-immersion objective lens with an optical dissector height of 15µm. The total number of immunopositive profiles was then used to estimate the total number of Ki67+ per dentate gyrus ± SEM. Coefficients of error (CE) were calculated using the method of Gunderson and were less than 0.05 for all groups (Gunderson et al., 1999).
Statistical Analyses
All values presented are expressed as mean ± standard error of the mean. Running distance, body weight, behavioral intoxication and ethanol dose data were analyzed using repeated measures ANOVA. All other data were analyzed with two-way ANOVA using the variables Activity, Diet and the Activity by Diet interaction. Planned post hoc t-test comparisons were conducted when necessary. When applicable, significance of correlations was determined using the critical value table for Pearson’s Correlation Coefficient. For all statistical analyses, a p value of less than 0.05 was deemed significant.
RESULTS
Body Weights & Distance Run
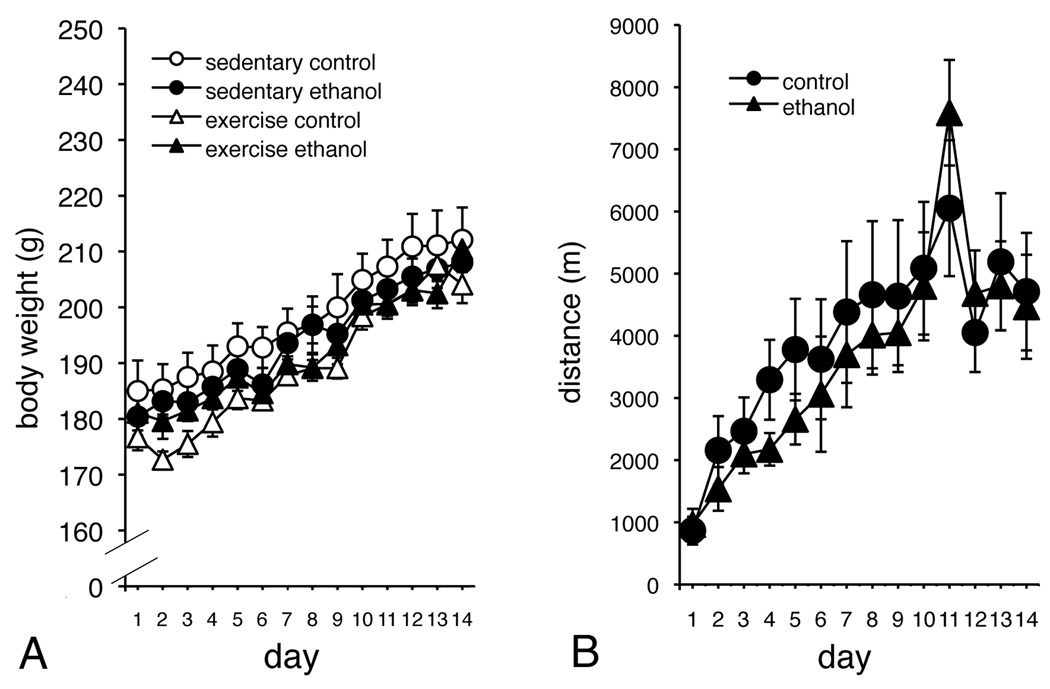
As shown in Figure 1A, all rats were similar in weight and gained similar amounts of weight. There was no main effect of Group for body weight (F(3,33) = 2.105, p > 0.05), and no significant Group × Time interaction (F(39,429) = 0.814, p > 0.05). However, all animals gained a significant amount of weight over Time (F(13,429) = 112.709, p < 0.05). For rats in the exercise groups, there was no main effect of Group for distance run (F(1,18) = 0.121, p > 0.05), and no significant Group × Time interaction (F(13,234) = 0.922, p > 0.05). There was a significant effect of Time, however, indicating that exercising animals increased the distance covered during the course of the exercise period (F(13,234) = 16.789, p < 0.05; see Figure 1B), consistent with what we have previously observed (Leasure and Decker, 2009; Leasure and Jones, 2008).
Figure 1.
There was no difference between the groups for body weight, although all rats gained weight during the course of the experiment (A). There was no difference for distance traveled between exercising rats that received ethanol, and those that received isocaloric control diet (B).
Behavioral Response to Binge Alcohol Administration
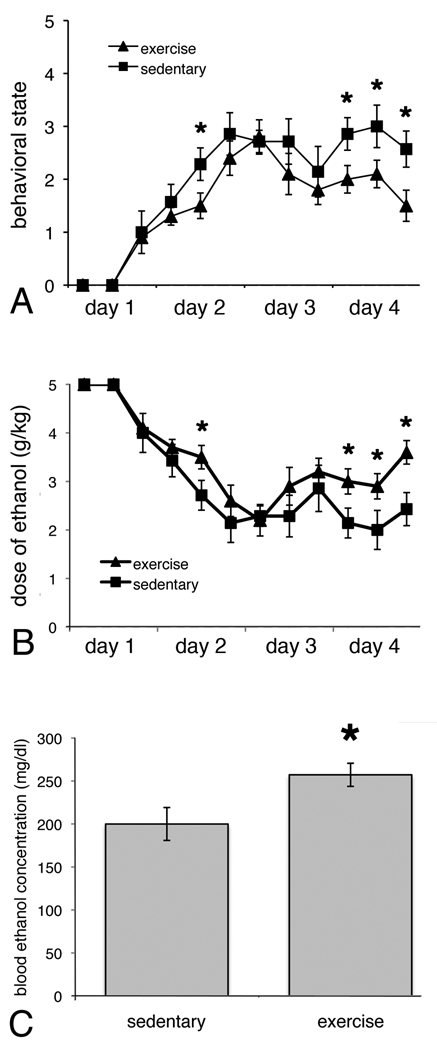
Rats that exercised for two weeks prior to binge ethanol consumption (EE) behaved significantly less intoxicated compared to rats that were sedentary (F(1,15) = 11.34, p < 0.05; see Figure 2A). A significant effect of Time (F(11,165) = 27.49, p > 0.05) indicates that all rats given alcohol acted progressively more intoxicated (sedated or motorically impaired) during the course of the 4-day binge. The Group × Time interaction was not significant (F(11,165) = 1.17, p > 0.05). Because dose of ethanol administered was dependent on behavioral intoxication (with more alcohol given to those rats that acted less drunk), rats that had exercised actually received more alcohol than sedentary rats (F(1,15) = 12.11, p < 0.05; see Figure 2B). Furthermore, there was a significant main effect of Time (F(11,165) = 27.95, p < 0.05), indicating that dose of alcohol initially decreased during the 4-day binge, corresponding to increased intoxication, then increased as tolerance developed which is characteristic of this model. There was no significant Group × Time interaction (F(11,165) = 1.17, p > 0.05). Not surprisingly, exercised rats had significantly higher blood ethanol levels (F(1,14) = 5.337, p < 0.05; Figure 2C) when assessed on the third day of the binge, a point at which they had received slightly higher alcohol doses (p=0.06, t-test).
Figure 2.
Rats that exercised prior to binge ethanol administration acted significantly less intoxicated than sedentary rats (n=7; A), despite having received more ethanol (B). Arrow in B shows the point at which tail bloods were taken. The BECs of the exercising rats (n=10) were significantly higher than sedentary rats (C). * p < 0.05, significantly different from sedentary.
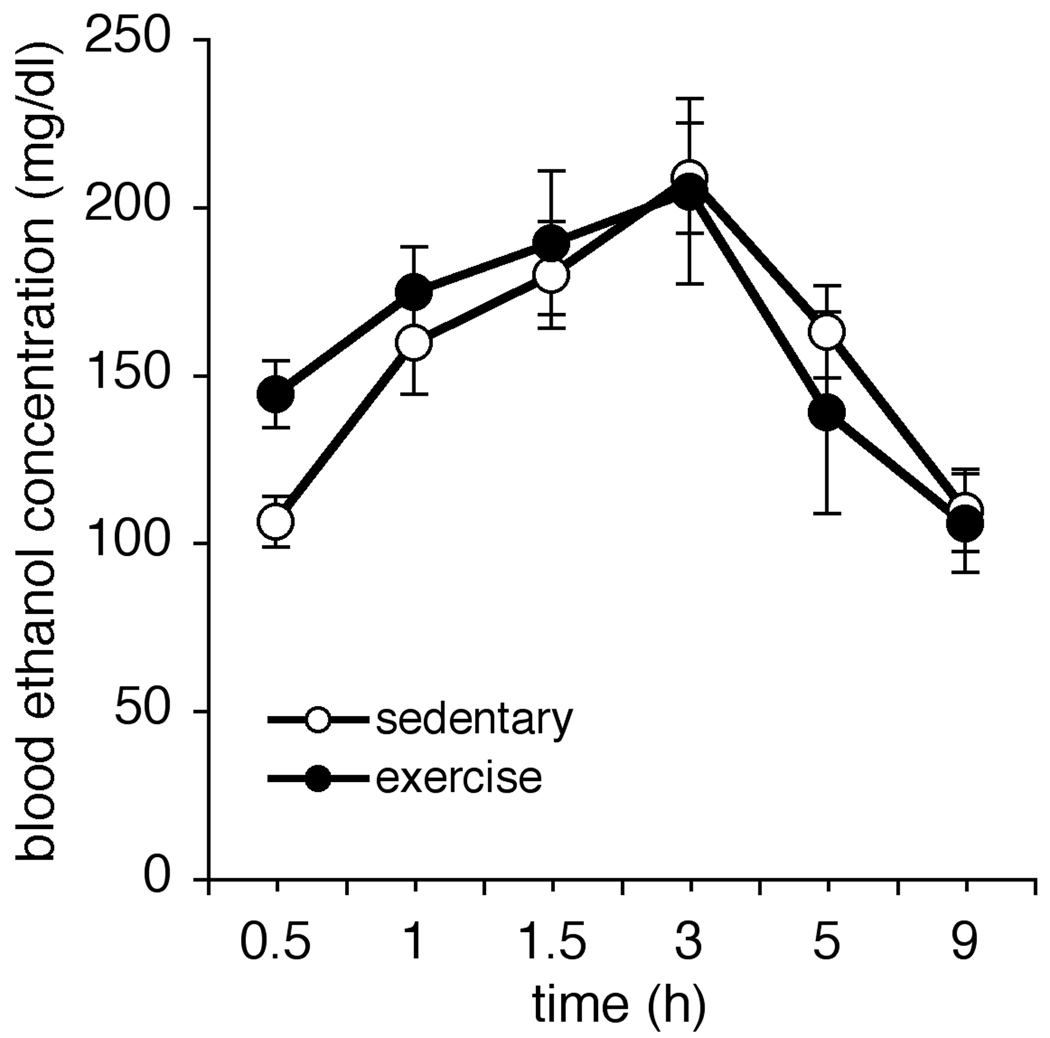
In order to determine whether rats that exercised before binge alcohol acted less intoxicated because physical activity had altered ethanol metabolism, we conducted a control experiment. Six additional female Long-Evans rats exercised for two weeks, and six remained sedentary. The next morning, all twelve were given a single 5g/kg dose of ethanol, and tail blood samples were taken 30 minutes, 60 minutes, 90 minutes, 3 hours, 5 hours and 9 hours thereafter (see Figure 3). There was no significant effect of Group on BEC (F(1,10) = 0.057, p > 0.05) and no significant Group × Time interaction (F(5,50) = 2.14, p > 0.05). The main effect of Time was significant (F(5,50) = 26.48, p < 0.05). Taken together these analyses show that blood alcohol levels change over time but change similarly between exercise and sedentary groups over time, which suggests that there is no difference in ethanol metabolism between exercising and sedentary rats.
Figure 3.
The decreased intoxication scores in rats that exercised were not due to enhanced metabolism of alcohol. There were no significant differences in BECs between sedentary rats (n=6) and rats that exercised (n=6) prior to a single 5 g/kg dose of ethanol.
Number of Granule Cells in the Dentate Gyrus
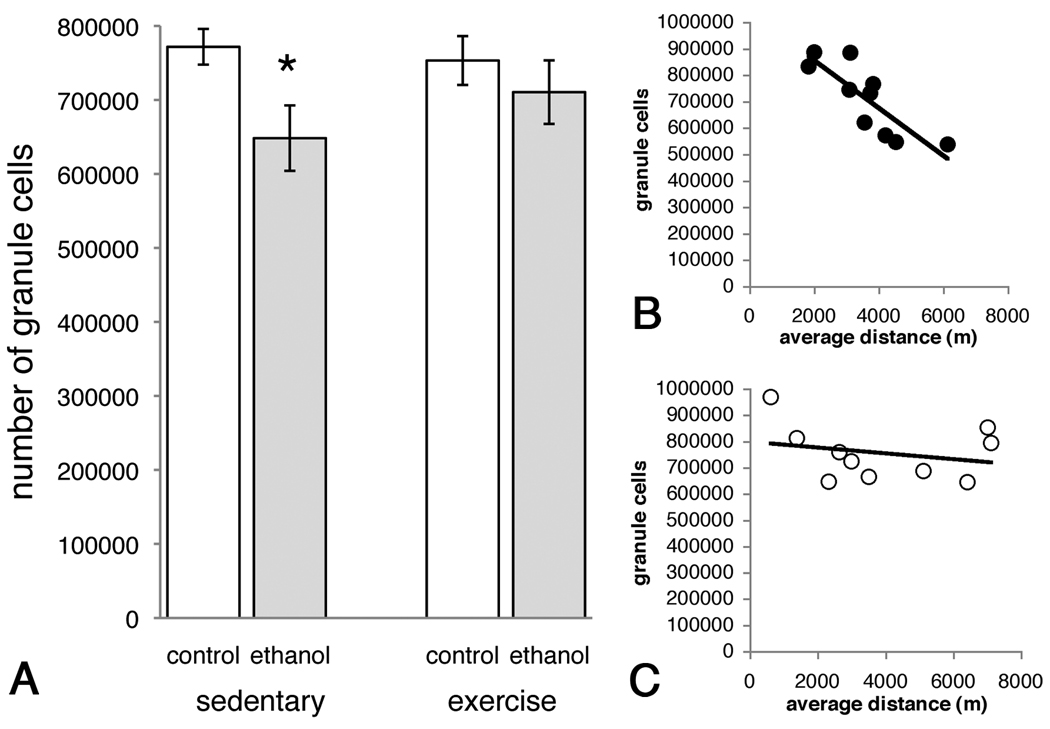
This model of binge alcohol consumption caused a significant loss of granule cells in the dentate gyrus, as determined by stereological methods. Specifically, Two-way ANOVA revealed a significant main effect of Diet (F(1,33) = 5.167, p < 0.05) but there was no significant main affect of Activity (F(1,33) = 0.357, p > 0.05) and no significant Activity × Diet interaction (F(1,33) = 1.219, p > 0.05; Figure 4A). Posthoc comparison showed that ethanol reduced the number of granule cells by 16% in SE rats compared to SC, a reduction that was only 8% in EE versus EC rats. Coefficients of error ranged from 0.03 to 0.04 and were calculated according to the method of Gunderson (Gunderson, 1999). Among exercise groups, however, there was a negative correlation between increased average running distance and decreased number of granule cells in rats in the EE group (Figure 4, r2 = 0.687, p < 0.05) but not the EC group (Figure 4C, r2 = 0.061, p > 0.05). However, there was no correlation between average dose per day and average running distance (data not shown, r2 = −0.134, p > 0.05).
Figure 4.
Binge alcohol consumption caused significant loss of granule cells in the dentate gyrus (A). Exercise was partially protective against this loss, although the degree of neuroprotection was dependent on the average distance run; longer distances correlated with fewer remaining granule cells (B). In control animals, there was no correlation between average distance and number of remaining granule cells (C). * p<0.05 posthoc comparison to SE.
Cell Death in Hippocampus and Associated Cortical Regions
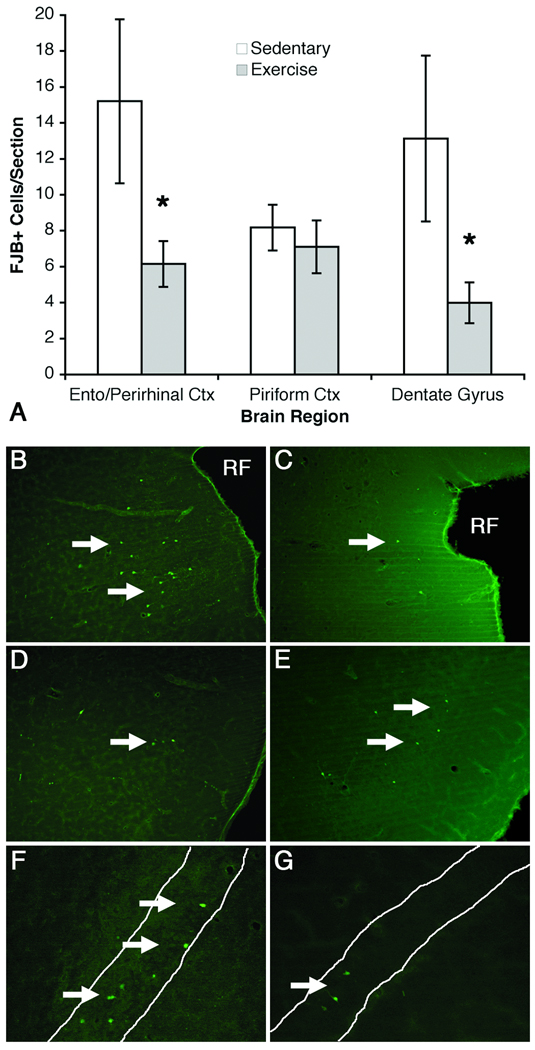
As cell death has been consistently observed in this model in male rats, FJB labeling was examined in hippocampus and associated cortical regions. Similar to past reports in males, binge ethanol exposure resulted in FJB+ cells in expected cortical and hippocampal locations. Similar to past reports, little to no FJB+ cells were observed in any regions of control animals (Crews et al., 2006b). As can be seen in Figure 5, all ethanol-exposed rats showed some FJB+ cells in each region, which is supported by main effects of Diet in all regions examined (detailed below). However, two-way ANOVA revealed that exercise affected regions differently. In the entorhinal-perirhinal cortices exercise prevented alcohol-induced increase in FJB+ cells as revealed by significant main effects of Diet (F(1,32) = 32.12, p < 0.0001) and Activity (F(1,32) = 5.70, p < 0.05) as well as a significant interaction of Diet and Activity (F(1,32) = 5.95, p < 0.05). A similar pattern was observed for the dentate gyrus, ANOVA revealed significant main effects of Diet (F(1,32) = 20.21, p < 0.0001) and Activity (F(1,32) = 5.78, p < 0.05) as well as a significant interaction of Diet and Activity (F(1,32) = 5.91, p < 0.05). In the piriform cortex, although ethanol increased the number of FJB+ cells over controls reflected by the main effect of Diet (F(1,30) = 69.99, p < 0.0001), there was no difference in the number of FJB+ cells between exercise (EE) and sedentary ethanol (SE) animals. Accordingly there was no main effect of Activity (F(1,30) = 0.33, p > 0.05) nor any interaction (F(1,30) = 0.35, p > 0.05). Thus, exercise had regionally specific effects on alcohol-induced FJB labeling of degenerating cells.
Figure 5.
Exercise had regionally-specific effects on alcohol-induced cell death, evidenced by FJB labeling (A). As all control groups averaged 0.2 cells per section or less, only ethanol groups are graphed in A. Exercise reduced the number of degenerating cells (FJB+ cells) in the dentate gyrus and combined entorhinal-perirhinal cortices, but not in the piriform cortex. Representative photomicrographs are shown for SE rats for the entorhinal -perirhinal cortex (B), piriform cortex (D) and dentate gyrus (F) and for EE rats for the entorhinal perirhinal (C) piriform cortex (E) and dentate gyrus (G). Arrows highlight FJB+ cells and * indicates cells shown in the inset. * p < 0.05 posthoc comparison to SE.
Proliferation of Progenitor Cells in the Dentate Gyrus
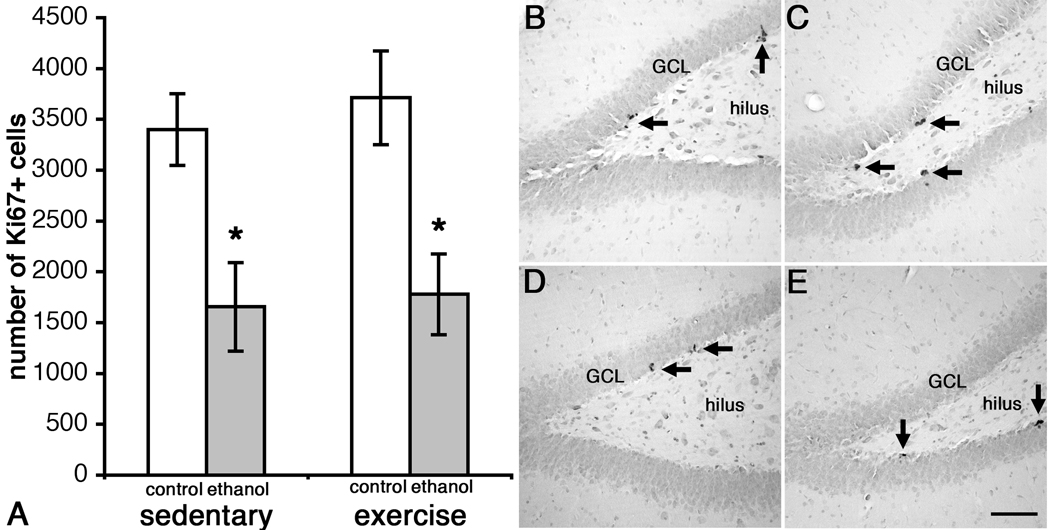
As expected, Ki67-positive (Ki67+) cells were observed along the subgranular zone (SGZ) in clusters (see Figure 6). The total number of Ki67+ cells was estimated by stereology in six to nine sections per brain. Coefficients of error ranged from 0.01 to 0.04 and averaged 0.02 in all groups except SE, which averaged 0.03. As can be seen in Figure 6a, ANOVA revealed only a main effect of Diet (F(1,14) 19.76, p < 0.001) and not Activity (F(1,14) = 0.05, p > 0.05) nor an interaction of Diet and Activity (F(1,14) = 0.28, p > 0.05). Thus, binge ethanol exposure reduced the number of proliferating cells in female rats, but prior exercise did not reverse or alter this effect.
Figure 6.
Binge ethanol administration decreases the number of Ki67+ cells in the dentate gyrus of adult female rats (A). Representative photomicrographs show clusters of Ki67+ cells along the subgranular zone of the dentate gyrus in SC (n=6, B), EC (n=4, C), SE (n=4, D) and EE rats (n=4, E). Scale bar = 100µm. * p < 0.05 ethanol-exposed versus controls.
DISCUSSION
The present study yielded several novel findings. First, we show that 4 days of binge alcohol consumption causes detectable loss of granule cells in the dentate gyrus. While neurodegeneration was assumed to occur from alcohol-induced cell death in the hippocampus and surrounding cortex in this model of binge consumption (Obernier et al., 2002a), a significant loss of fully differentiated dentate gyrus granule cells has not previously been reported. Second, we show that moderate voluntary exercise prior to binge alcohol consumption modulates cellular degeneration in the hippocampus and associated regions. Third, we show that the neuroanatomical sparing is accompanied by behavioral changes. Rats that exercised prior to binge alcohol consumption acted significantly less intoxicated than those that did not. Finally, this is the first report of binge-induced brain damage in female rats in this model of an AUD.
Previously, it was thought that weeks of ethanol consumption, characteristic of chronic ethanol models, were required to produce the loss of granule cells in the hippocampus (Walker et al., 1980). Here, we show that only 4 days of binge ethanol administration produced a significant, 16% loss of granule cells in the dentate gyrus of the hippocampus in SE versus SC rats. In support of this observation of neurodegeneration, binge ethanol exposure also increased the number of FJB+ cells in both the dentate and surrounding cortex (entorhinal/perirhinal cortices, piriform cortex). The observation of increased FJB+ cells is consistent with past reports of other indicators of cell death in various models of AUDs, such as amino cupric silver stain, necrosis, and pyknosis (Collins et al., 1996; He et al., 2005; Herrera et al., 2003; Obernier et al., 2002a; Obernier et al., 2002b). Although FJB detects both apoptotic and necrotic forms of cell death, the Majchrowicz model of binge exposure results in a primarily necrotic form of cell death (Obernier et al., 2002a). Furthermore, binge ethanol exposure inhibited the number of proliferating progenitor cells (Ki67+ cells) in the dentate gyrus, an effect that was not reversed by prior exercise. These effects are consistent with recent work that suggests that ethanol affects both cell death and cell birth mechanisms when producing its detrimental effects on hippocampal integrity (Crews and Nixon, 2009; Morris et al., 2009). Notably, the observed reduction in the number of granule cells occurred with only four days of exposure, essentially the equivalent of one “bender” in a bingeing alcoholic. And though this single 4-day binge has been shown to result in behavioral deficits (e.g. Obernier et al., 2002b), the neuroanatomical or behavioral effects of repeated bingeing within the context of this model is not known. As alcoholics do not just binge once, the long-term effect of repeated binges warrants investigation.
This study focused on the effects of prior exercise on alcohol intoxication and its effects on neurodegeneration. The specific focus of the current study was due to the peak of cell death occurring on the fourth day of exposure in this model (Crews et al., 2000) as well as the very different events that occur as alcohol dependence develops versus events in withdrawal and abstinence. For example, binge alcohol exposure inhibits cell proliferation during alcohol intoxication, however, during withdrawal and abstinence binge alcohol results in highly dynamic effects on cell proliferation (e.g. Nixon and Crews, 2004; Nixon et al., 2008). Because these cell proliferation events may result from alcohol-induced cell death, it was necessary to investigate the effects on cell death first. Further, this study did not investigate the other mechanisms involved in adult neurogenesis, many of which may contribute to long term effects of binge alcohol exposure. These questions are outside of the scope of a single study therefore multiple future studies should to examine both the long-term effects of exercise on alcohol-induced neurodegeneration and the role of neurogenesis or gliogenesis in this effect.
Exercise has been shown to offer significant neuroprotection in many neurodegenerative conditions. Exercise is neuroprotective when it occurs prior to various forms of brain injury such as stroke (Briones, 2006; Briones et al., 2005; Hayes et al., 2008; Stummer et al., 1994), and can delay symptom onset in rodent models of neurodegenerative disease (Carro et al., 2001; Faherty et al., 2005; Kaspar et al., 2005). In a rodent model of fetal alcohol syndrome, exercise rehabilitates various neurological outcomes (Christie et al., 2005; Redila et al., 2006; Thomas et al., 2008). Furthermore, in mice that had access to a running wheel coincident with alcohol self-administration, exercise counteracted the deleterious effect of alcohol on neural stem cell proliferation (Crews et al., 2004). In the present study, we found that 2 weeks of voluntary exercise decreased alcohol-induced cell death in the dentate gyrus and surrounding cortex, as assessed by FJB. Prior exercise also partially protected the dentate gyrus against binge-induced loss of granule cells, although this effect was dependent on distance run. Rats that ran further had fewer remaining granule cells. Too much or too vigorous exercise may be counterproductive to brain health (Leasure and Jones, 2008), particularly following injury (Griesbach et al., 2004). Our results suggest that mild exercise, rather than intense exertion, may best fortify the brain against future insults, such as binge alcohol consumption.
We have previously shown that exercise increases the volume of the hippocampal dentate gyrus (Leasure and Decker, 2009). In the present study, we did not find that exercise alone increased the number of granule cells in the hippocampus, suggesting that exercise-induced volume increases may result from increased cell size or dendritic complexity, versus an increase in total cell number (Eadie et al., 2005; Redila and Christie, 2006). Nonetheless, exercise protected existing dentate gyrus granule cells from binge alcohol consumption, likely because of its ability to attenuate oxidative damage (Radak et al., 2001) and/or its ability to increase expression of neurotrophins and their receptors (Farmer et al., 2004; Gomez-Pinilla et al., 1997; Li et al., 2008; Liu et al., 2008; Neeper, 1995; Oliff, 1998; Russo-Neustadt, 2000; Widenfalk et al., 1999). Indeed, alcoholic neuropathology is hypothesized to be due to effects on these exact mechanisms. Alcohol is thought to promote oxidative damage but also has vast effects on neurotrophic factor signaling. Brain-derived-neurotrophic-factor (BDNF), in particular, has been hypothesized to have roles in everything from promoting alcohol dependence to alcohol-induced brain damage (Pandey et al., 2005a; Pandey et al., 2005b). Generally, in models of AUDs, BDNF mRNA is decreased after long-term or chronic alcohol exposure, an effect that corresponds to observations of alcohol-induced neurodegeneration (reviewed in Davis, 2008). In addition, alcohol-induced decreases in BDNF have been correlated to alcohol-induced effects on new granule cell survival (e.g. (Herrera et al., 2003; Nixon and Crews, 2002). Exercise enhancement or reversal of alcohol-induced effects on BDNF could explain the mechanism of exercise neuroprotection in this model of alcohol-induced brain damage. Obviously, future studies should address the contribution of BDNF, as well as the trkB receptor, in exercise neuroprotection of alcohol-induced brain damage.
Alcoholic neurodegeneration is thought to be due to an imbalance of pro-survival to pro-inflammatory/death events. As such, the effect of exercise on neurotrophic factor signaling is only part of the story. Alcohol-induced loss of pro-cell survival signals, such as trophic factor expression is thought to couple with alcohol induced oxidative stress to result in alcoholic neurodegeneration (Crews and Nixon, 2009). Oxidative stress describes disrupted homeostasis of intracellular redox due to an imbalance in reactive oxygen species/free radical production and endogenous antioxidant depletion. Oxidative stress is a key factor in alcohol-induced tissue injury in several organ systems including the brain (Calabrese et al., 2002; Gonenc et al., 2005; Montoliu et al., 1994). Strikingly, the hippocampus may be particularly vulnerable to alcohol-induced oxidative stress injury (Renis et al., 1996). Indeed, several markers of oxidative stress have been reported in the serum of alcoholics (Thome et al., 1997a; Thome et al., 1997b; Thome et al., 1997c). Besides generating reactive oxygen species, alcohol exposure may also inhibit the activity of endogenous antioxidants such as glutathione and superoxide dismutase (Johnsen-Soriano et al., 2007; Ku et al., 2006; Siler-Marsiglio et al., 2005; Siler-Marsiglio et al., 2004). Intriguingly, only those antioxidants that stimulate endogenous antioxidant production are neuroprotective in binge-induced neurodegeneration (Crews et al., 2006a). Thus, exercise action on redox homeostasis is also consistent with its role in neuroprotection of alcohol-induced neurodegeneration.
Prior exercise, however, did not protect the dentate against the ethanol-induced decrease in progenitor cell proliferation. Furthermore, we did not find that prior exercise alone increased proliferation of progenitor cells in the dentate, which is consistent with past findings that the neurogenic effects of exercise are acute (Berchtold et al., 2005; Leasure and Jones, 2008; Naylor et al., 2005; Stranahan et al., 2006; van Praag et al., 1999b). Although we have shown previously that exercise increased cell proliferation in alcohol-drinking mice, cell proliferation was examined in currently exercising animals (Crews et al., 2004). In this study, exercise had ceased 4 days before Ki67 was assessed and exercise-induced increases in cell proliferation return to non-exercise levels within 24 hours (Berchtold et al., 2005). In other words, exercise does not have long-term effects on cell proliferation. It is also possible (but not likely) that the brief social isolation or daily removal from the running wheel that occurred during exercise counteracted its neurogenic effect. Another possibility is that gonadal hormones, which are known to regulate neurogenesis (Galea, 2008; Tanapat et al., 1999), played a role. The number of newborn cells is transiently increased during proestrus (Tanapat et al., 1999). Although we did not monitor the estrus cycle in our animals, it is unlikely that none of our exercising controls were in proestrus when they were sacrificed (which could cause a lack of increase in Ki67+ cells). Thus, our results lend support to previous findings that suggest that exercise does not have long-term effects on the cell proliferation aspect that underlies hippocampal integrity.
One intriguing behavioral effect observed in this study was that exercisers behaved significantly less intoxicated. This does not appear to be due to enhanced hepatic metabolism of alcohol in exercising rats, because in our control experiment, the BECs of exercisers and sedentary rats were not different. Although one group has reported decreased ethanol clearance after several weeks of exercise training (Ardies et al., 1989), others have not (Canzanelli, 1934; Carpenter, 1933). Our control study clearly does not support any differences in ethanol elimination, as the descending curves are identical. If anything, the slight difference at the 30 min timepoint might indicate that either first pass metabolism of ethanol is altered or ethanol is absorbed differently in exercisers versus controls. The latter is most plausible as others have shown no effect of exercise on alcohol dehydrogenase activity (Ardies et al., 1989). Furthermore, ethanol absorption and distribution is affected by differences in body fat (lower fat equals lower BEC) and it is highly likely that exercising animals have lower body fat than their sedentary counterparts (Alessio et al., 2005; Leasure and Jones, 2008). Thus, lower fat would result in lower BEC, which is reflected as reduced intoxication behavior. However, this model can overcome pharmacokinetic differences with its variable dosing. The reduced intoxication behavior resulted in rats receiving higher doses of alcohol overall and after three days of significantly higher doses, blood ethanol levels were slightly higher in exercise rats when measured on day 3. Regardless of any metabolic differences, exercise was neuroprotective against alcohol-induced damage despite EE rats receiving higher doses of ethanol and having higher BECs.
Finally, to our knowledge, this is the first time the Majchrowicz binge model has been conducted on female rats. Examination of alcoholic neuropathology has been overlooked in women due to their lower rates of alcohol dependence; however these rates are unfortunately on the rise (Keyes et al., 2008). Females may be more susceptible to alcohol-induced neurodegeneration than men (Hommer et al., 2001; Prendergast, 2004) as both structural and cognitive impairments emerge in women much faster in the course of the disease than in men (Mann et al., 1992; Nixon et al., 1995). Although no direct comparisons are made, it is important to note that alcohol-induced neurodegeneration via cell death and inhibition of cell birth were observed and are similar to prior reports in male rats (Crews et al., 2006a; Obernier et al., 2002a). Indeed, the number of FJB+ cells in SE entorhinal-perirhinal and piriform cortices is nearly identical to that observed in males (Kelso et al., in preparation; unpublished data). However, these data only reflect a single timepoint and binge-induced cell death begins during the second day of binge exposure and degenerating cells are visible throughout the first week in males (Crews et al., 2000; Obernier et al., 2002b). Furthermore, female mice show increased cell death versus males after alcohol withdrawal (Hashimoto and Wiren, 2008). Thus, future studies should investigate the extent and timecourse of alcohol-induced damage in females as well as examine whether exercise is neuroprotective in males rats exposed to binge ethanol.
We conclude that exercise can reduce behavioral sensitivity to ethanol intoxication and protect vulnerable brain areas from alcohol-induced cell death. Exercise neuroprotection in AUDs could impact multiple aspects of addiction. First, as the hippocampus is linked to learning, memory and mood, and all of these are disrupted in AUDs, exercise neuroprotection of hippocampal integrity could prevent or restore the cognitive and mood deficits reported in alcoholism. Depression and anxiety are highly comorbid with AUDs and may be causal in excessive alcohol drinking (drinking to escape or the self-medication hypothesis) as well as in relapse to alcohol dependence (Koob and Le Moal, 1997; Schuckit and Monteiro, 1988). Depression and anxiety are related to deficits in hippocampal integrity (Fuchs et al., 2004; Revest et al., 2009) and exercise neuroprotection implies that it could improve negative mood states (Ernst et al., 2006) which could, in theory, reduce alcohol consumption. Indeed, exercise incorporated into alcoholism treatment programs reduces several addiction outcomes including alcohol consumption (e.g. Brown et al., 2009). Exercise may impact more than just neurodegeneration-related behaviors as others have shown that it reduces alcohol urges (Ussher et al., 2004) or provides an alternative reward state (Thoren et al., 1990). In summary, these data have important implications for the development of novel treatment strategies and understanding the role of hippocampal integrity in the AUDs.
Acknowledgements
The authors gratefully acknowledge the technical support of Aleksander Smith (UK), M. Ayumi Deeny (UK), Abhilasha Khattri (UH) and Darby Hawley (UH).
This work was supported by the National Institute on Alcohol Abuse and Alcoholism AA016959 (KN), and start-up funding from the University of Houston (JLL) and the University of Kentucky (KN).
REFERENCES
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Alessio HM, Hagerman AE, Nagy S, Philip B, Byrnes RN, Woodward JL, Callahan P, Wiley RL. Exercise improves biomarkers of health and stress in animals fed ad libitum. Physiol Behav. 2005;84(1):65–72. doi: 10.1016/j.physbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Ardies CM, Morris GS, Erickson CK, Farrar RP. Both acute and chronic exercise enhance in vivo ethanol clearance in rats. J Appl Physiol. 1989;66(2):555–560. doi: 10.1152/jappl.1989.66.2.555. [DOI] [PubMed] [Google Scholar]
- Asano M, Kaneoka K, Nomura T, Asano K, Sone H, Tsurumaru K, Yamashita K, Matsuo K, Suzuki H, Okuda Y. Increase in serum vascular endothelial growth factor levels during altitude training. Acta Physiol Scand. 1998;162(4):455–459. doi: 10.1046/j.1365-201X.1998.0318e.x. [DOI] [PubMed] [Google Scholar]
- Bengochea O, Gonzalo LM. Effect of chronic alcoholism on the human hippocampus. Histol Histopathol. 1990;5(3):349–357. [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87(14):5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones T. Environment, physical activity and neurogenesis: Implications for prevention and treatment of Alzheimer's Disease. Current Alzheimer Research. 2006;3(1):49–54. doi: 10.2174/156720506775697197. [DOI] [PubMed] [Google Scholar]
- Briones TL, Suh E, Hattar H, Wadowska M. Dentate gyrus neurogenesis after cerebral ischemia and behavioral training. Biol Res Nurs. 2005;6(3):167–179. doi: 10.1177/1099800404271328. [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart G, Dubreuil ME, Gordon AA. Aerobic exercise for alcohol recovery: rationale, program description, and preliminary findings. Behav Modif. 2009;33(2):220–249. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Uylings HBM, Paula-Barbosa MM. Granule cell loss and dendritic regrowth in the hippocampal dentate gyrus of the rat after chronic alcohol consumption. Brain Research. 1988;473:1–14. doi: 10.1016/0006-8993(88)90309-5. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Latteri S, Colombrita C, Ravagna A, Catalano C, Pennisi G, Calvani M, Butterfield DA. Long-term ethanol administration enhances age-dependent modulation of redox state in different brain regions in the rat: protection by acetyl carnitine. Int J Tissue React. 2002;24(3):97–104. [PubMed] [Google Scholar]
- Canzanelli A, Guild R, Rapport D. The use of ethyl alcohol as a fuel in muscular exercise. Am J Physiol. 1934;110:416–421. [Google Scholar]
- Carpenter T, Lee RC, Burdett M. The effect of muscular exercise on the disappearance of ethyl alcohol in man. Am J Physiol. 1933;105:17. [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the effects of exercise on the brain. J Neurosci. 2000;20(8):2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21(15):5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21(6):1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corse TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic "binge" intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20(2):284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Corbin WR, Vaughan EL, Fromme K. Ethnic differences and the closing of the sex gap in alcohol use among college-bound students. Psychol Addict Behav. 2008;22(2):240–248. doi: 10.1037/0893-164X.22.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135(1):142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006a;30(11):1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and neurodegeneration. CNS Drug Reviews. 1999;5(4):379–394. [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24(11):1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006b;137(2):437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44(2):115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33(1):63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Davis MI. Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther. 2008;118(1):36–57. doi: 10.1016/j.pharmthera.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JP, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31(2):84–92. [PMC free article] [PubMed] [Google Scholar]
- Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res Mol Brain Res. 2005;134(1):170–179. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14 Suppl 5:S481–S490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57(2):332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764(1–2):1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- Gonenc S, Uysal N, Acikgoz O, Kayatekin BM, Sonmez A, Kiray M, Aksu I, Gulecer B, Topcu A, Semin I. Effects of melatonin on oxidative stress and spatial memory impairment induced by acute ethanol treatment in rats. Physiol Res. 2005;54(3):341–348. [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016(2):154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Harper C. Brain atrophy in chronic alcoholic patients: a quantitative pathological study. Journal of Neurology, Neurosurgery & Psychiatry. 1985;48(3):211–217. doi: 10.1136/jnnp.48.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Fromme K. Heavy episodic drinking and college entrance. J Drug Educ. 2003;33(3):259–274. doi: 10.2190/2L2X-F8E1-32T9-UDMU. [DOI] [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology. 2008;33(5):1084–1096. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115(3):289–296. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21(10):2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci U S A. 2003;100(13):7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158(2):198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10(6):559–561. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Johnsen-Soriano S, Bosch-Morell F, Miranda M, Asensio S, Barcia JM, Roma J, Monfort P, Felipo V, Romero FJ. Ebselen prevents chronic alcohol-induced rat hippocampal stress and functional impairment. Alcohol Clin Exp Res. 2007;31(3):486–492. doi: 10.1111/j.1530-0277.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2006: Volume I, Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2007. [Google Scholar]
- Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57(5):649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93(1–2):21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23(4):633–643. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Ku BM, Joo Y, Mun J, Roh GS, Kang SS, Cho GJ, Choi WS, Kim HJ. Heme oxygenase protects hippocampal neurons from ethanol-induced neurotoxicity. Neurosci Lett. 2006;405(3):168–171. doi: 10.1016/j.neulet.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Jonsdottir IH. Influence of voluntary exercise on hypothalamic norepinephrine. J Appl Physiol. 1998;85(3):962–966. doi: 10.1152/jappl.1998.85.3.962. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Decker L. Social Isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus in press. 2009 doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Chen HI, Yu L, Kuo YM, Wu FS, Chuang JI, Liao PC, Jen CJ. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol Learn Mem. 2008;90(1):81–89. doi: 10.1016/j.nlm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Mann K, Batra A, Gunthner A, Schroth G. Do women develop alcoholic brain damage more readily than men? Alcohol Clin Exp Res. 1992;16(6):1052–1056. doi: 10.1111/j.1530-0277.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Valles S, Renau-Piqueras J, Guerri C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: Effect of chronic alcohol consumption. J Neurochem. 1994;63(5):1855–1862. doi: 10.1046/j.1471-4159.1994.63051855.x. [DOI] [PubMed] [Google Scholar]
- Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1321–R1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- Morris SA, Smith AR, Eaves DW, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus in press. 2009 doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AS, Persson AI, Eriksson PS, Jonsdottir IH, Thorlin T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol. 2005;93(5):2406–2414. doi: 10.1152/jn.01085.2004. [DOI] [PubMed] [Google Scholar]
- Neal DJ, Fromme K. Hook 'em horns and heavy drinking: alcohol use and collegiate sports. Addict Behav. 2007;32(11):2681–2693. doi: 10.1016/j.addbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83(5):1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24(43):9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31(2):218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Parsons OA. Behavioral dysfunction and cognitive efficiency in male and female alcoholics. Alcohol Clin Exp Res. 1995;19(3):577–581. doi: 10.1111/j.1530-0277.1995.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Noble EG, Moraska A, Mazzeo RS, Roth DA, Olsson MC, Moore RL, Fleshner M. Differential expression of stress proteins in rat myocardium after free wheel or treadmill run training. J Appl Physiol. 1999;86(5):1696–1701. doi: 10.1152/jappl.1999.86.5.1696. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002a;26(4):547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002b;72(3):521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcriipts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Chandler LJ, Nixon K, Crews FT, Hensler JG, Ukai W, Saito T. Neurotrophic Factor Signaling in Alcoholism. Alcohol Clin Exp Res. 2005a;29(6):1098–1105. [Google Scholar]
- Pandey SC, Chartoff EH, Carlezon WA, Jr, Zou J, Zhang H, Kreibich AS, Blendy JA, Crews FT. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin Exp Res. 2005b;29(2):176–184. doi: 10.1097/01.alc.0000153550.31168.1d. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36(3):533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Kim KO, Zipursky RB, Mathalon DH, Lane B, Ha CN, Rosenbloom MJ, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Prendergast MA. Do women possess a unique susceptibility to the neurotoxic effects of alcohol? J Am Med Womens Assoc. 2004;59(3):225–227. [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, Nyakas C, Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38(1):17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137(4):1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16(3):305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Reifman A, Watson WK. Binge drinking during the first semester of college: continuation and desistance from high school patterns. J Am Coll Health. 2003;52(2):73–81. doi: 10.1080/07448480309595727. [DOI] [PubMed] [Google Scholar]
- Renis M, Calabrese V, Russo A, Calderone A, Barcellona ML, Rizza V. Nuclear DNA strand breaks during ethanol-induced oxidative stress in rat brain. FEBS Lett. 1996;390(2):153–156. doi: 10.1016/0014-5793(96)00647-3. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry in press. 2009 doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the exression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101(2):305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Monteiro MG. Alcoholism, anxiety and depression. Br J Addict. 1988;83(12):1373–1380. doi: 10.1111/j.1360-0443.1988.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Khurana NC, Heaton MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005;1052(2):202–211. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Shaw G, Heaton MB. Pycnogenol and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J Neurobiol. 2004;59(3):261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- Skalicky M, Viidik A. Comparison between continuous and intermittent physical exercise on aging rats: changes in patterns of spontaneous activity and connective tissue stability. Aging (Milano) 1999;11(4):227–234. doi: 10.1007/BF03339663. [DOI] [PubMed] [Google Scholar]
- Somani SM, Ravi R, Rybak LP. Effect of exercise training on antioxidant system in brain regions of rat. Pharmacol Biochem Behav. 1995;50(4):635–639. doi: 10.1016/0091-3057(94)00357-2. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9(4):526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W, Weber K, Tranmer B, Baethmann A, Kempski O. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke. 1994;25(9):1862–1869. doi: 10.1161/01.str.25.9.1862. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19(1):110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. 2008;122(6):1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J, Foley P, Gsell W, Davids E, Wodarz N, Wiesbeck GA, Boning J, Riederer P. Increased concentrations of manganese superoxide dismutase in serum of alcohol-dependent patients. Alcohol Alcohol. 1997a;32(1):65–69. doi: 10.1093/oxfordjournals.alcalc.a008235. [DOI] [PubMed] [Google Scholar]
- Thome J, Nara K, Foley P, Gsell W, Wiesbeck GA, Boning J, Riederer P. Time course of manganese superoxide dismutase concentrations in serum of alcohol-dependent patients during abstinence. Drug Alcohol Depend. 1997b;44(2–3):151–155. doi: 10.1016/s0376-8716(96)01330-0. [DOI] [PubMed] [Google Scholar]
- Thome J, Zhang J, Davids E, Foley P, Weijers HG, Wiesbeck GA, Boning J, Riederer P, Gerlach M. Evidence for increased oxidative stress in alcohol-dependent patients provided by quantification of in vivo salicylate hydroxylation products. Alcohol Clin Exp Res. 1997c;21(1):82–85. [PubMed] [Google Scholar]
- Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc. 1990;22(4):417–428. [PubMed] [Google Scholar]
- Tivis R, Beatty WW, Nixon SJ, Parsons OA. Patterns of cognitive impairment among alcoholics: Are there subtypes? Alcohol Clin Exp Res. 1995;19:496–500. doi: 10.1111/j.1530-0277.1995.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Addiction. 2004;99(12):1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209(4457):711–713. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- West MJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res. 1999;34(3):125–132. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]