Abstract
The goal of this study was to develop a polymeric carrier for delivery of anti-tumor drugs and sustained release of these agents in order to optimize anti-tumor activity while minimizing systemic effects. We used oligo(poly(ethylene glycol) fumarate) (OPF) hydrogels modified with small negatively charged molecules, sodium methacrylate (SMA), for delivery of doxorubicin (DOX). SMA at different concentrations was incorporated into the OPF hydrogel with a photo-crosslinking method. The resulting hydrogels exhibited sensitivity to the pH and ionic strength of the surrounding environment. Our results revealed that DOX was bound to the negatively charged hydrogel through electrostatic interaction and was released in a timely fashion with an ion exchange mechanism. Release kinetics of DOX was directly correlated to the concentration of SMA in the hydrogel formulations. Anti-tumor activity of the released DOX was assessed using a human osteosarcoma cell line. Our data revealed that DOX released from the modified, charged hydrogels remained biologically active and had the capability to kill cancer cells. In contrast, control groups of unmodified OPF hydrogels with or without DOX did not exhibit any cytotoxicity. This study demonstrates the feasibility of using SMA-modified OPF hydrogels as a potential carrier for chemotherapeutic drugs for cancer treatments.
1. Introduction
Hydrogels have been extensively investigated as drug delivery carriers in biomedical applications [1]. They are relatively inexpensive and well suited to deliver drugs in a minimally invasive manner. Hydrogels can be modified to reversibly bind multiple molecules of drug per molecule of polymer [2]. These polymer-drug conjugates can be administered as prodrugs to give a sustained release of the active drug over time [3, 4]. Advantages thereof include decreased toxicity effects of free drug, economizing of the amount of drug needed due to increased circulation time, and facilitating solubilization of hydrophobic drugs [5]. The particular polymer and molecular weight thereof can be selected to suit the specific application, of which chemotherapy applications are of special interest.
The stimuli-responsive (polyelectrolytes) hydrogels are generally created when an ionic monomer is incorporated into the hydrogel network, in which the charged group is formed and called fixed charge since its mobility is much less than that of freely mobile ions within the interstitial fluid [6-8]. Therefore, the responsive hydrogel is the multiphase mixture consisting of polymeric network matrix solid phase, interstitial fluid phase and ion phase including mobile ionic species and fixed charge. These materials offer the ability to precisely control the concentration of incorporated drugs. A variety of proteins and DNA molecules incorporated into polyelectrolytes were found to retain their bioactivity upon release [9].
Coupling anticancer drugs to synthetic polymers is a promising approach to improve the efficacy and reduce the side effects of these drugs [2, 5]. Recently, several biodegradable polymer systems have been developed with the goal of achieving controlled release of anticancer drugs [10]. This strategy proves advantageous because therapeutic levels of the desired anticancer agent are maintained for prolonged periods while reducing systemic side effects [11, 12]. Glial wafers, which are carmustine (1,3 bis(2-chloroethyl)-1-nitrosourea) containing polymer implants, were approved by the FDA in 1996 for the treatment of glioblastoma multiforme [13].
Doxorubicin (DOX) is a chemotherapeutic agent commonly used in the treatment of a wide range of cancers. The use of DOX and its derivatives causes dilated cardiomyopathy and congestive heart failure due to the accumulation of the drug and DOX-induced cardiotoxicity [14, 15]. Sulfonate-modified polyvinyl alcohol (PVA) microspheres have been previously applied as embolization agents for treatment of hypervascular tumors [16, 17]. The beads have been shown to sequester oppositely charged drugs such as DOX by an ion exchange mechanism [18].
In this study, we have copolymerized oligo (polyethylene glycol) fumarate (OPF) with sodium methacrylate (SMA) to produce negatively charged hydrogels for DOX delivery. We hypothesized that the negatively charged hydrogels can be effectively used for positively charged doxorubicin hydrochloride loading through ionic bonding to the drug. We investigate the effect of SMA incorporation on the equilibrium swelling of the hydrogels in aqueous solutions of varying pH and ionic strength. Additionally, this work evaluates the effect of SMA incorporation on the loading efficiency and release kinetics of DOX, with the objective that SMA-modified hydrogels ultimately can be used in vivo for local delivery of chemotherapeutic drugs to minimize their side effects. The anti-tumor effect of released DOX has been investigated in vitro using human osteosarcoma cells.
2. Materials and Methods
2.1. Synthesis
OPF was synthesized using poly(ethylene glycol) (PEG) with an Mn of 10,000, according to a previously described method [19]. Briefly, 50 g PEG was azeotropically distilled in toluene to remove residual water and then dissolved in 500 ml distilled methylene chloride. The resulting PEG was placed in an ice bath and purged with nitrogen for 10 min. Then, 0.9 mol triethylamine (TEA; Aldrich, Milwaukee, WI) per mol PEG and 1.8 mol distilled fumaryl chloride (Acros, Pittsburgh, PA) per mol PEG were added dropwise. The reaction vessel was then removed from the ice bath and stirred at room temperature for 48 hours. For purification, methylene chloride was removed by rotary evaporation. The resulting OPF was dissolved in ethyl acetate and filtered to remove the salt from the reaction of TEA and chloride. OPF was recrystallized in ethyl acetate and vacuum dried overnight.
2.2. Hydrogel Fabrication
Hydrogels were made by dissolving 1g OPF macromer in deionized water containing 0.15% (w/w) of a photoinitiator (Irgacure 2959, donated by Ciba-Specialty Chemicals) and 0.3 g N-vinyl pyrrolidinone (NVP; Aldrich, Milwaukee, WI). Negative charge was incorporated into OPF by addition of SMA (Aldrich, Milwaukee, WI), a bifunctional molecule containing both an anionic head and a reactive methacryl group that copolymerizes with the fumarate group of OPF (Fig. 1). A series of negatively charged hydrogels was produced by adding SMA at different concentrations (Table 1).
Fig. 1.
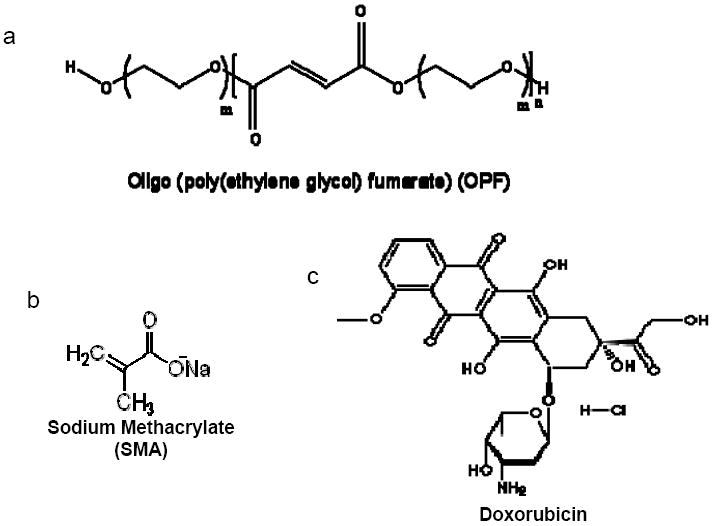
Chemical structures of oligo(polyethylene glycol) fumarate (OPF), sodium methacrylate (SMA), and doxorubicin HCL (DOX). The OPF (a), composed of repeating PEG and fumarate chains, was crosslinked with SMA (b) in the presence of the photoinitiator Irgacure 2959 and ultraviolet light to form negatively charged hydrogels. DOX (c) was loaded into negatively charged hydrogels containing SMA by electrostatic interaction.
Table 1.
SMA-modified hydrogel characteristics:
| Hydrogel | OPF:SMA ratio | Sol fraction |
|---|---|---|
| w/w | (%) | |
| HG-0 | 1:0 | 27.4±1.4 |
| HG-SMA50 | 1:0.05 | 26.5±0.4 |
| HG- SMA100 | 1:0.1 | 23.8±1.4 |
| HG-SMA200 | 1:0.2 | 18.7±2.7 |
| HG-SMA300 | 1:03 | 11.6±1.3 |
To prepare hydrogel films, the OPF/SMA mixture was pipetted between two glass slides with a 1-mm spacer and polymerized by exposure to UV light (365 nm) at an intensity of 8 mW/cm2 (Black-Ray Model 100AP) for 30 min. Hydrogel disks were vacuum dried after fabrication, weighed (Wi, initial weight) and swollen to equilibrium for 24 hours at 37°C in phosphate-buffered saline (PBS), deionized water, or NaCl solutions of varying concentrations and pHs. Swollen samples were blotted dry and weighed (Ws, swollen weight), then dried in reduced pressure and weighed again (Wd, final dry weight). The swelling ratio and sol fraction of the hydrogels were calculated using the following equations:
2.3. Attenuated Total Reflectance (ATR) Fourier Transform Infrared (FTIR) Spectroscopy
The surfaces of OPF hydrogels with and without modification were characterized using a micro ATR-FTIR spectrophotometer (Nicolet 8700), coupled to a Continuum microscope (Thermo Electron Corp., Madison, WI). The microscope used an ATR slide on a germanium crystal and spectra were collected at a resolution of 4 cm-1 for 128 scans with a sampling area of 150×150 μm. Multiple spectra were collected from each hydrogel surface. The differences in surface composition among the various hydrogel formulations were quantified by measuring the ratios of the characteristic peaks.
2.4. Compression Testing
Hydrogel films were cut into disks of 7 mm diameter with a cork borer and swollen in PBS (pH 7.4) overnight. Compressive moduli of the swollen hydrogels were determined using a dynamic mechanical analyzer (DMA-2980, TA Instruments, New Castle, DE) at a controlled force mode with a loading rate of 4 N/min. The moduli were calculated from the slope of the stress versus strain curves at low strains (< 20%).
2.5. DOX loading
Hydrogel disks were incubated in 1 ml aqueous DOX loading solutions of varying concentration at 37°C. The diffusion rates of DOX were determined by measuring the change in DOX concentration of the loading solution every hour up to 5 hours and after 24 hours using a plate reader at 490 nm. Disks were removed from the solution after 24 hours and placed in either PBS or NaCl solutions of different concentrations for release measurements.
2.6. In vitro release of DOX
To measure the release of absorbed DOX, hydrogel disks were separately placed in a fresh tissue culture plate containing 1 ml media (NaCl 0.1M, 1M or PBS) and incubated at 37°C. Media was collected every day up to 7 days and every 3 days thereafter. The release of DOX was quantified using the plate reader at 490 nm. All release data were normalized to the concentration of DOX initially absorbed by the disks.
2.7. Antitumor effect of released DOX
MG63 human osteosarcoma cells (CRL-1427™; ATCC, Manassas, VA) were plated at 15,000 cells/cm2 in 24 well plates containing 1 ml media. The culture medium used was Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham media (1 ml; DMEM-F12, D2906 Sigma) supplemented with 10% charcoal stripped fetal calf serum (Thermo Fisher Scientific, Logan, UT), 0.9 M sodium bicarbonate, 10 U/ml penicillin G, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Cells were maintained at 37°C and 5% CO2 for 24 hours. To determine the biological activity of DOX released by the hydrogels, transwells (Corning, Corning, NY) containing charged hydrogels disks loaded with DOX were submersed in cell culture media. The MTS Assay (Promega Corporation, Madison, WI), a measure of survival by metabolic activity, was used to assess cell survival.
2.8. Statistical Analysis
The data for hydrogel characterization are reported as means ± standard deviations (SD) for triplicate samples, unless otherwise described in the experimental section. Single factor analysis of variance (ANOVA) was performed (StatView, version 5.0.1.0, SAS Institute, Inc, Cary, NC) to assess the statistical significance of the results. When the global F-test was positive at the 0.05 level, Bonferroni’s method was used for multiple comparison tests to determine differences among the experimental groups.
3. Results
3.1. Hydrogel fabrication and characterization
Different concentrations of negatively charged monomer (SMA) were added to the OPF solution and crosslinked in the presence of UV light (Figure 1). As shown in Table 1, five groups of hydrogels were fabricated by varying the ratio of OPF/SMA in the formulations. Sol fraction decreased with increasing SMA concentration in the hydrogel formulations indicating an increase in crosslinking of SMA-modified constructs (Table 1).
The ATR-FTIR spectra of unmodified, lyophilized OPF hydrogels and OPF hydrogels incorporating SMA (HG-SMA300) are compared in Fig. 2a. As shown, after copolymerization of OPF with SMA, a new peak emerged at 1550 cm-1 that is characteristic of the carboxylic acid from SMA. Bands at 1725 and 1045 cm-1 are assigned to the carbonyl and carbon-oxygen bonds (C-O-C) of OPF, respectively. Most of the bands either broadened or disappeared after hydration in deionized water. There were only two major bands at 1650 and 1085 cm-1 that are assigned to carbonyl and C-O bonds, respectively, and a broad band at 3370 cm-1 corresponding to the hydroxyl group (OH) (Fig. 2b). To quantify the dependence of surface composition on swelling behavior in the solvent, the ratio of the C-O peak at 1085 cm-1 to the carbonyl peak at 1650 cm-1 was calculated for hydrogels of all formulations (Fig. 2c). After hydration in deionized water, the 1085/1650 peak ratio decreased with increasing SMA concentration.
Fig. 2.
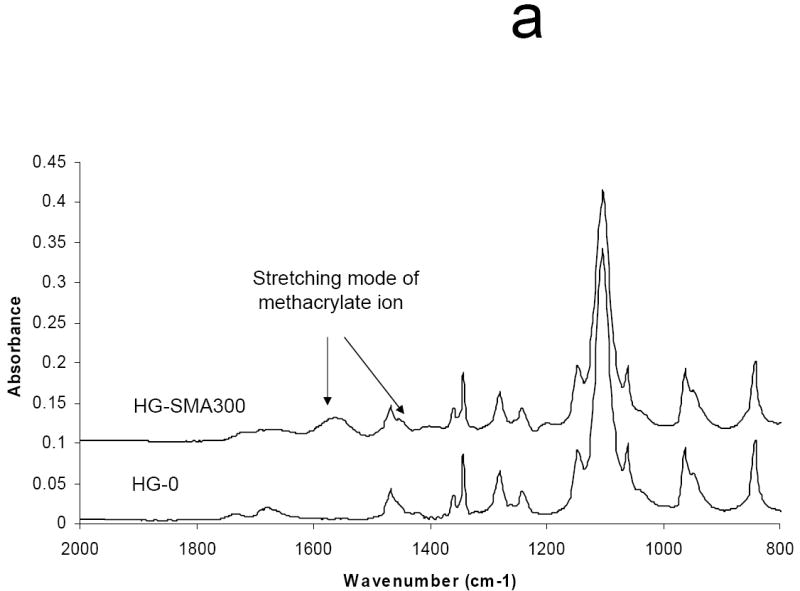
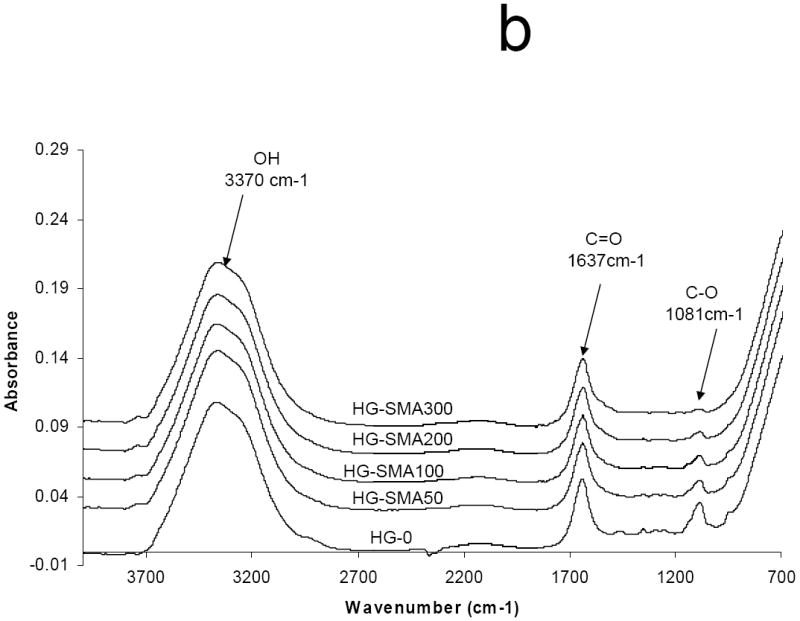
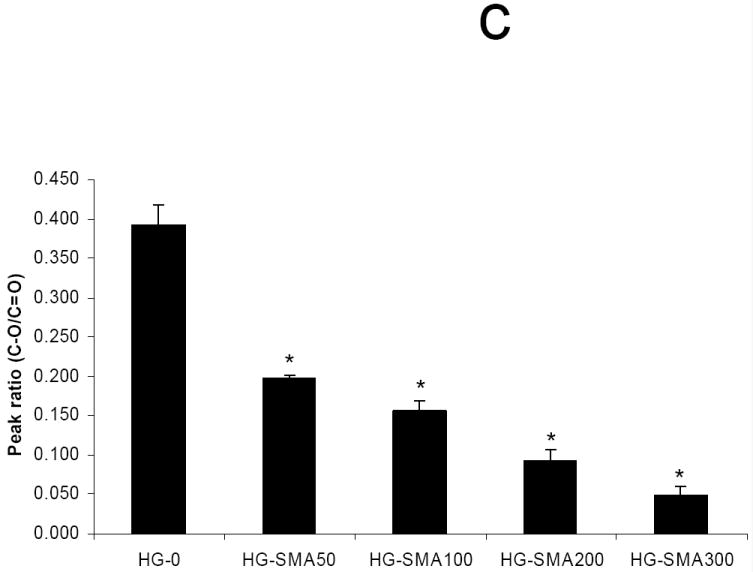
Micro-ATR-FTIR of unmodified SMA-modified hydrogels after crosslinking and lyophilization. The new peaks at 1550 and 1730 cm-1 are assigned to carboxylate group incorporated into OPF hydrogel (a). Hydrogel spectra with different SMA concentrations after hydration in deionized water (b). Comparisons of C-O/C=O peak ratio on the hydrogel surfaces as a function of SMA concentration after hydration in deionized water (c). The decrease in C-O peak intensity (1085 cm-1) correlates with an increase in SMA concentration when the hydrogels are swollen in water. (*) p<0.005 as compared to HG-0.
Polyelectrolyte properties of negatively charged hydrogels were assessed by measuring their swelling ratios in deionized water and PBS as well as solutions of varying pH and ionic strength (Figures 3a, b and c). The swelling ratios in deionized water increased as the concentration of SMA increased. For example, the swelling ratio for HG-0 (no SMA) was 9.9±0.1, lower than that of all the SMA containing hydrogel formulations which had swelling ratios ranging from 16.3 to 29.6 (p<0.05). In PBS, the unmodified OPF hydrogel had a swelling ratio of 8.9±0.3, and it increased to 10.1±0.1 with the initial addition of SMA to the formulation (p<0.05). Increasing the SMA concentration above that initial addition did not further change the swelling ratio (Figure 3a). Swelling ratio appeared to be a function of ionic strength in NaCl solutions with concentrations ranging from 0.00001-1M (Figure 3b). As shown, at higher ionic strength (1M NaCl), swelling ratios of all hydrogels with different charge densities significantly decreased. The hydrogels with higher charge density (HG-SMA300) were more responsive to the ionic strength of the solution. Figure 3c reveals the effect of pH on swelling ratio of SMA-modified hydrogels. Swelling ratios of charged hydrogels increased with increasing pH of the solution; for example, the swelling ratio of HG-SMA300 was about 3 at pH<2, and it increased to about 10 when pH increased from 2 to 3. Swelling ratios of SMA-modified hydrogels did not change significantly with increase in pH from 3 to 12.
Fig. 3.
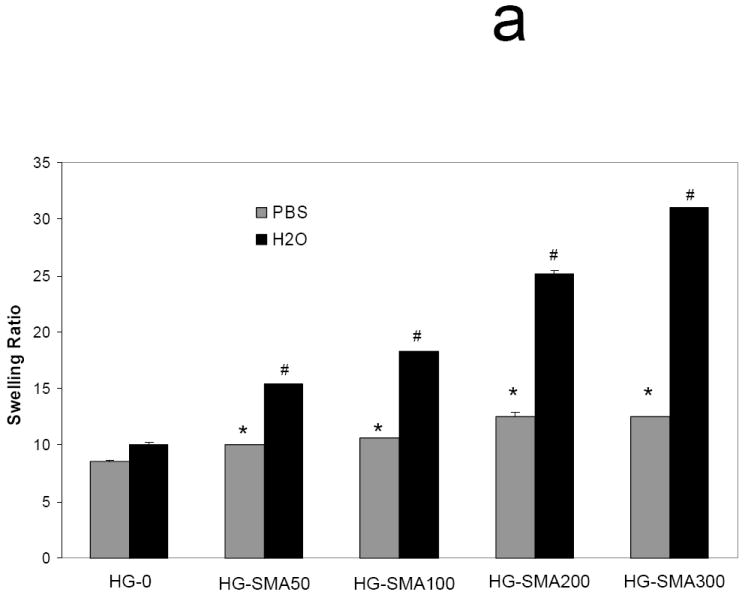
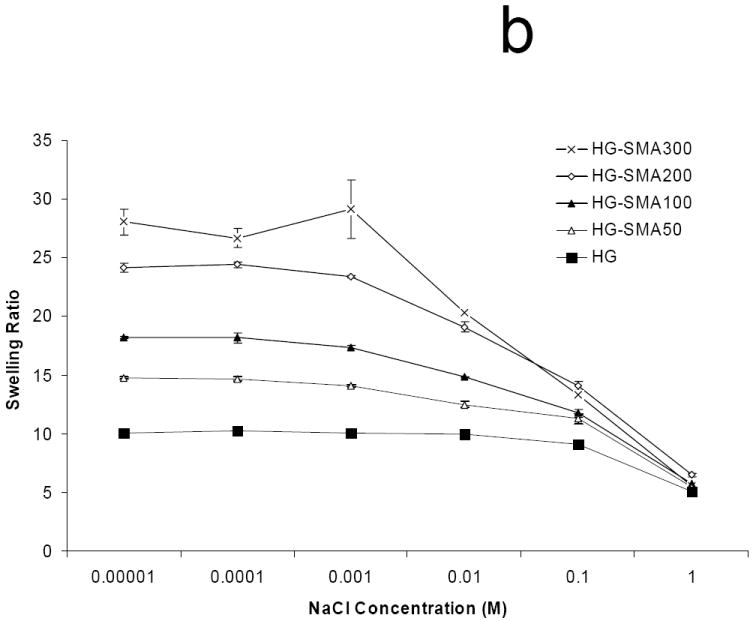
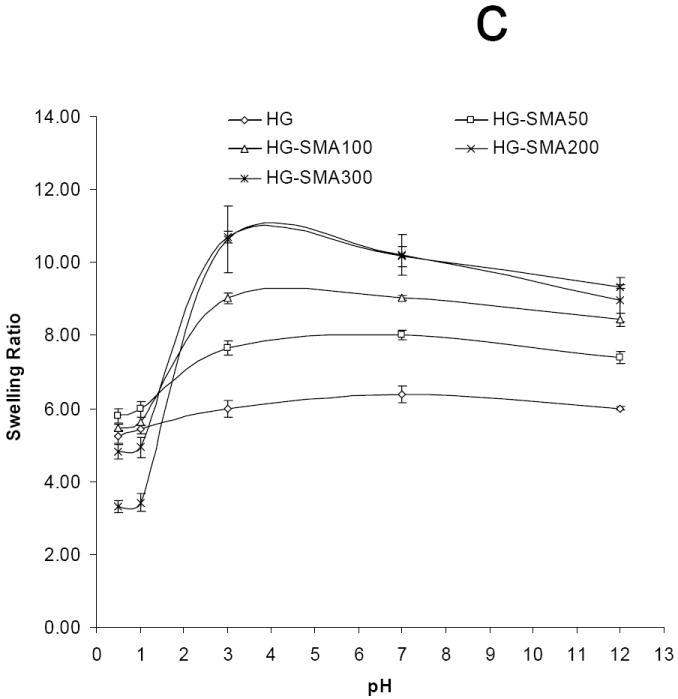
Equilibrium swelling ratio of hydrogels in deionized water and PBS (a). Swelling ratio in deionized water increased with increase in concentration of SMA in hydrogel formulation. The change in swelling ratio occurs in a lesser degree in PBS. Swelling ratios of SMA-modified hydrogels are dependent upon ionic concentration of NaCl solutions (b). Swelling ratios of SMA-modified hydrogel increase with increase in pH (c). Hydrogels with higher concentration of SMA exhibit more sensitivity to the pH and ionic strength of environment. (*) p<0.005 as compared to HG-0 in PBS. (#) p<0.005 as compared to HG-0 in water.
Figure 4 shows that the compressive modulus of the OPF hydrogel did not change with addition of SMA at 1:0.05 OPF/SMA weight ratios. However, it decreased significantly with increase in SMA concentration in hydrogel formulation (p<0.01). Unmodified hydrogel had a modulus of 260±8 kPa, and it decreased to 202±10 kPa in HG-SMA300.
Fig. 4.
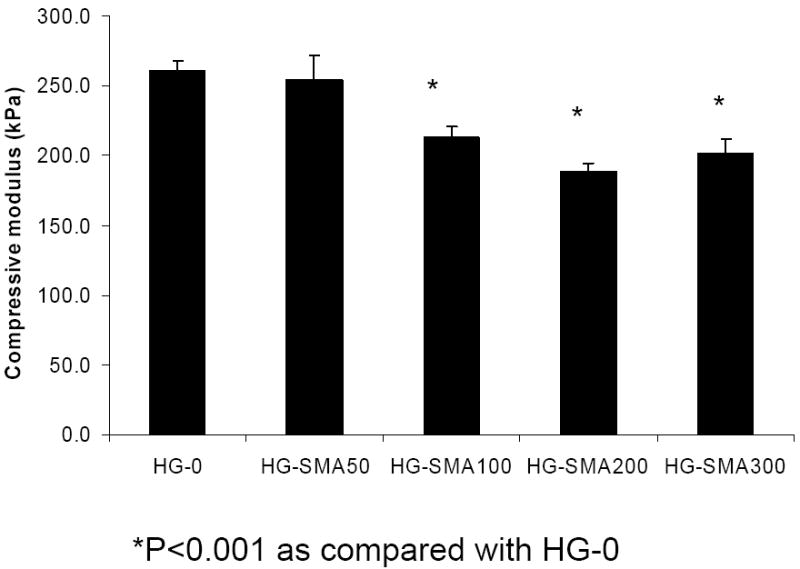
Compressive modulus of modified hydrogels as a function of SMA concentration in hydrogel formulations. (*) p<0.001 as compared to HG-0.
3.2. DOX loading efficiency
In order to assess the loading efficiency of DOX in charged hydrogels, an aqueous solution of DOX (50, 200 and 400 μg/ml) was added to the hydrogel disks. Measurement of the drug concentration at different time points showed that charged hydrogels absorbed DOX within a few hours in a highly efficient manner (Fig. 5a). A gross picture of hydrogel disks in DOX solution shows that the drug solutions became depleted in color and the disks turned red; however, the drug solutions containing hydrogels without charge were still red after 24 hours (Fig. 5b). Negatively charged hydrogels with different formulations absorbed >99% of DOX from loading solutions with concentrations of 50 and 200 μg/ml. However, loading efficiency slightly decreased with an increase in DOX concentration in loading solutions of 400 μg/ml. Unlike charged hydrogels, the loading efficiency of hydrogels without charge (HG-0) significantly decreased with an increase in DOX concentration in loading solution from 44.8% to 21.4% (Fig 5c). All SMA-modified hydrogels including HG-SMA50, HG-SMA100 and HG-SMA200 were loaded with almost 22 mg/g hydrogel when DOX concentration in loading was 400 μg/ml. However, HG-SMA300 absorbed 18 mg/g hydrogel from this loading solution (Fig. 5d). Hydrogels without charge (HG-0) absorbed about five times less DOX than other hydrogel formulations with charge (Fig 5d). We found that a higher amount of DOX can be loaded into charged hydrogels by increasing the concentration of DOX in loading solution (data not shown).
Fig. 5.
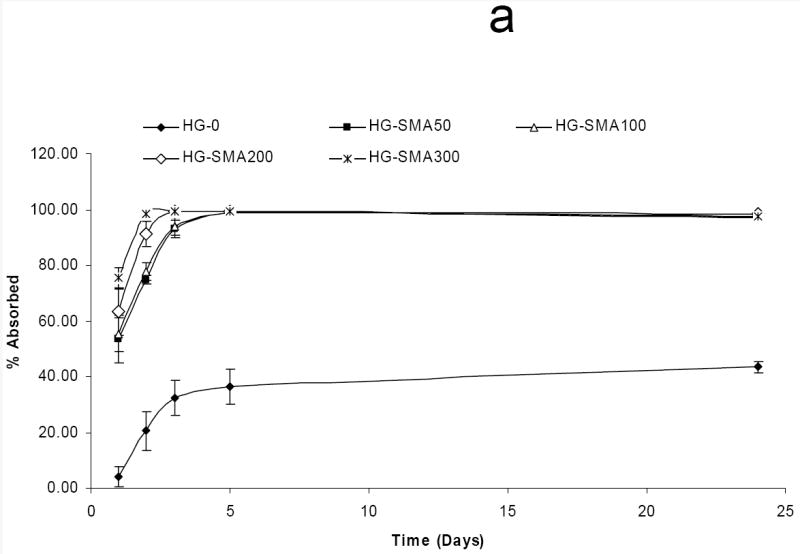
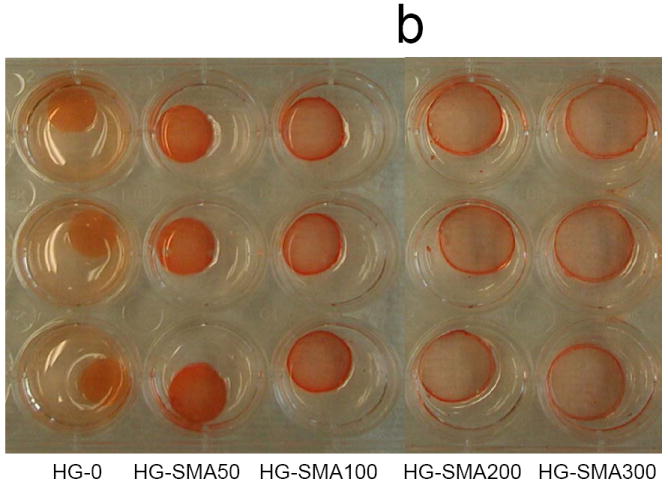
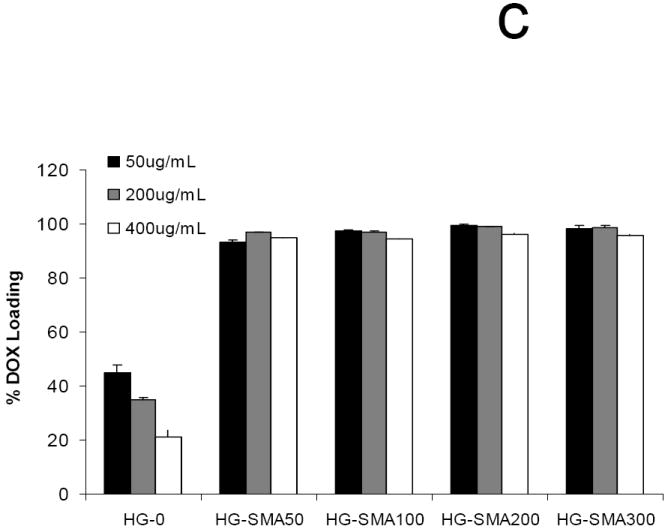
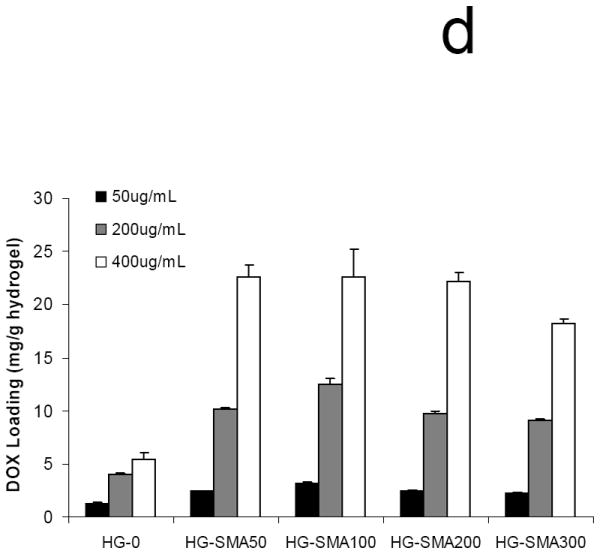
Percent DOX absorbed to the hydrogels of varying formulations vs. time (a). In a few hours, most of the DOX in loading solution (50 μg/ml) was absorbed to the charged hydrogels. Gross picture of DOX absorbed to the hydrogels of varying formulations (b). Percent DOX loaded to the hydrogels from 1 ml loading solutions of varying concentrations (c). Amount of DOX loaded from 1 ml loading solution of varying concentrations normalized to the weight of hydrogel disks (d).
3.3. DOX release
After loading with DOX in either 50 or 200 μg/ml loading solution, hydrogel disks were placed in 1 ml PBS and release of DOX was measured at 37°C. Figure 6a shows the release profile of DOX from hydrogels loaded at 50 μg/ml after 15 days in PBS. HG-0 exhibited a burst release of about 53% on the first day, while DOX release from SMA-modified hydrogels on the first day was significantly less than HG-0 (p<0.05). There was a decreasing trend for DOX release on the first day with increasing SMA concentration in hydrogel formulations as follows: 5.9%<9.3%<17.8%<24.3%<53.4%. Both unmodified and SMA modified hydrogels exhibited controlled release profiles over the 15 day time period. The same trend was observed for unmodified and SMA modified hydrogels loaded with a higher amount of DOX (200 μg/ml) in Figure 6b. Interestingly, HG-0 loaded in 200 μg/ml DOX solution had a burst release of 53.9%, which was very close to the burst release of this hydrogel at lower loading dose (50 μg/ml). A decreasing trend was also observed for DOX release from the hydrogels with increased SMA level in their formulations. It appears that an increase in loading dose did not affect the release profile from SMA-modified hydrogels. Figure 6c reveals the total release of DOX from hydrogels of different formulations. The total DOX release from hydrogels decreased with an increase in SMA concentration in the hydrogel formulations. After 15 days, 100% of DOX initially absorbed to the HG-0 hydrogel was released. However, with incorporation of SMA into the hydrogel formulations, the total release decreased to 57% for HG-SMA50, and 20% for HG-SMA300.
Fig. 6.
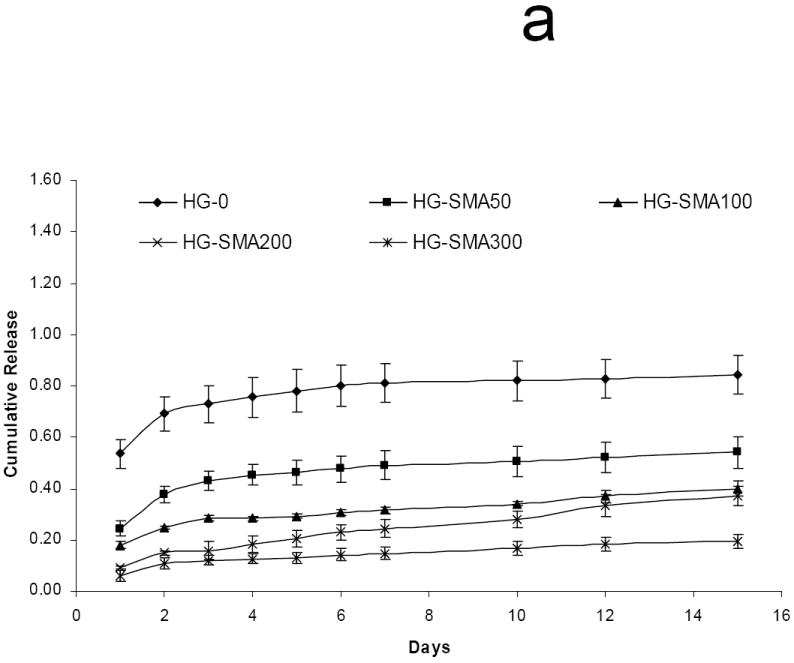
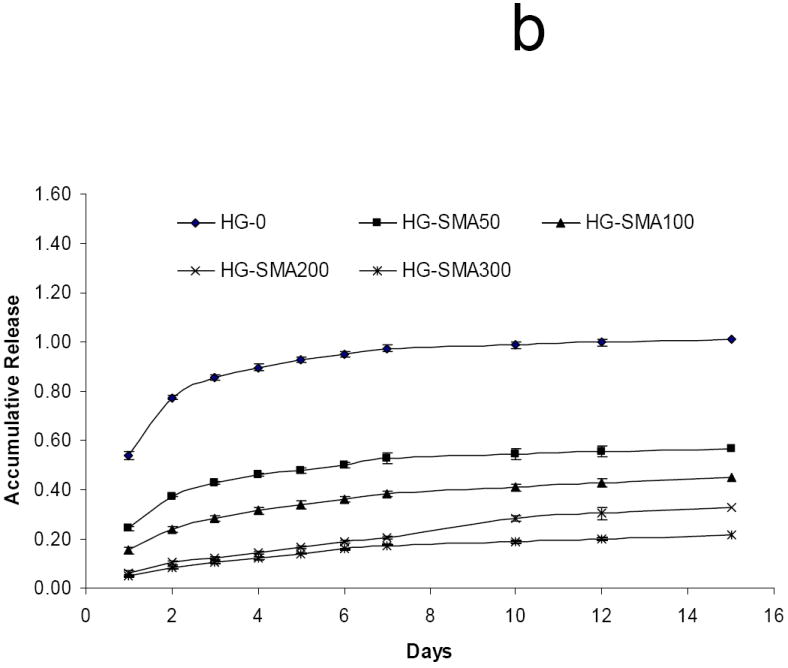
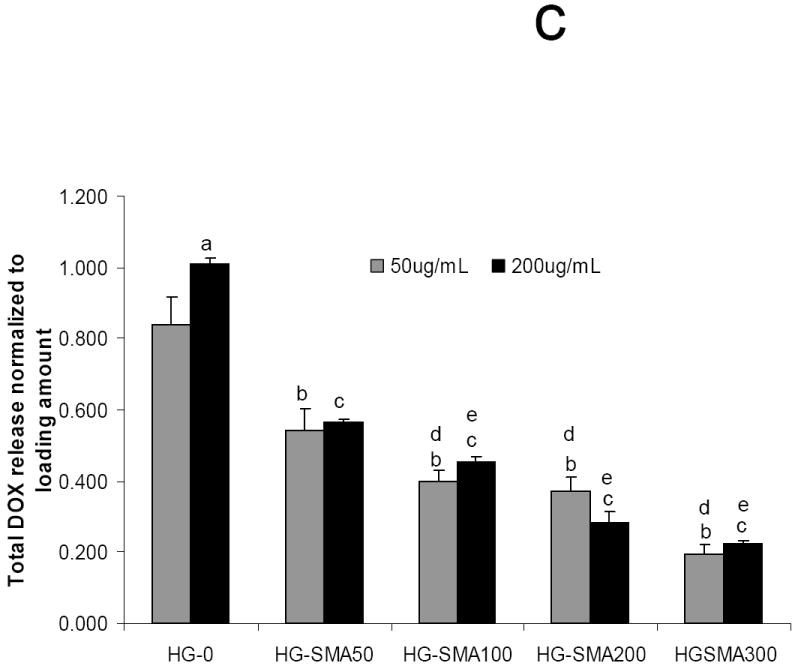
Release of DOX from hydrogels loaded at 50 μg/ml (a) and 200 μg/ml (b) loading solutions in PBS. Total amount of DOX released from each hydrogel formulation over 15 days (c). The amounts of DOX released from hydrogels are normalized to initial amounts of DOX loaded to each hydrogel formulation. (a) p<0.001 as compared to total release at 50 μg/m loading, (b) p<0.001 as compared to HG-0 at 50 μg/m loading, (c) p<0.001 as compared to HG-0 at 200 μg/m loading, (d) p<0.01 as compared to HG-5 at 50 μg/m loading, (e) p<0.01 as compared to HG-5 at 200 μg/m loading.
To characterize the effect of ionic strength on the release, DOX loaded hydrogel disks were placed in 1 ml of aqueous NaCl solutions of different concentrations (0.01, 0.1 and 1.0 M) and the release profiles were measured at 37°C for 15 days. Figures 7a and 7b show a direct relationship between ion concentration and DOX release from the hydrogels. Hydrogels without SMA released DOX at the same rate in all NaCl solutions; in contrast, the release profiles of SMA modified hydrogels were different in different salt solutions. At low salt concentrations (0.01M) only a small amount of DOX was released from SMA-modified hydrogels, and the effect of charge density on the release profiles of the hydrogels in this solution was lessened. Conversely, at higher salt concentrations (0.1M) the release profiles were influenced by the charge density of the hydrogels, and DOX released from the SMA-modified hydrogels increased in this solution. Figure 7c shows that in 1M NaCl solution the release of DOX from charged hydrogels somewhat decreased when compared to the release profile in 0.1M NaCl solution. This could be due to decreased solubility of DOX in concentrated NaCl solution, as seen in Figure 7d where the total release of DOX first increased with an increase in salt solution then decreased.
Fig. 7.
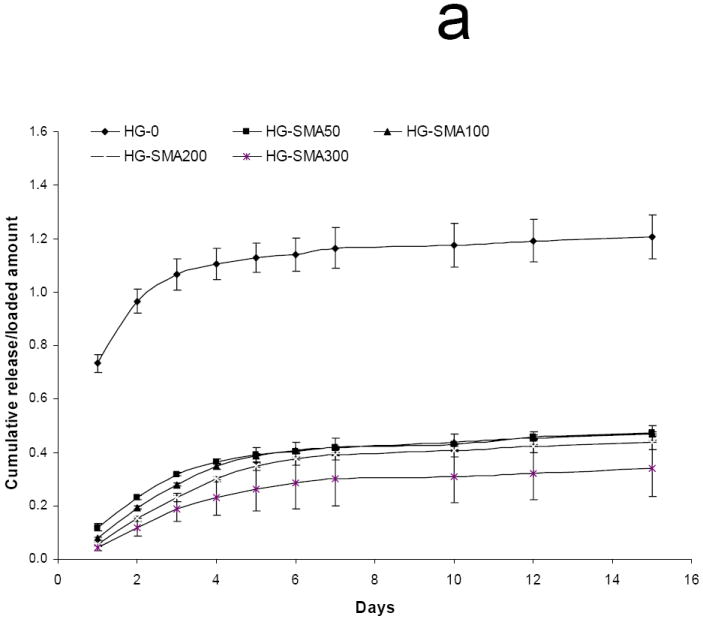
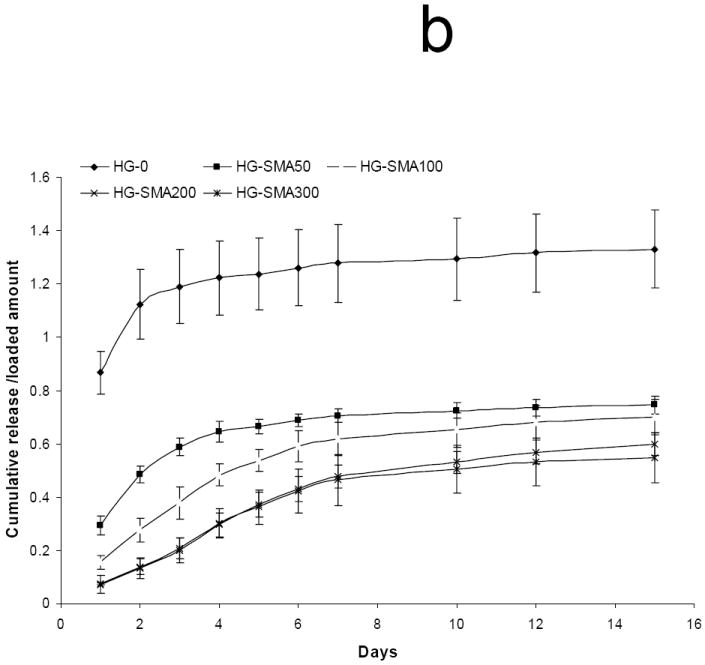
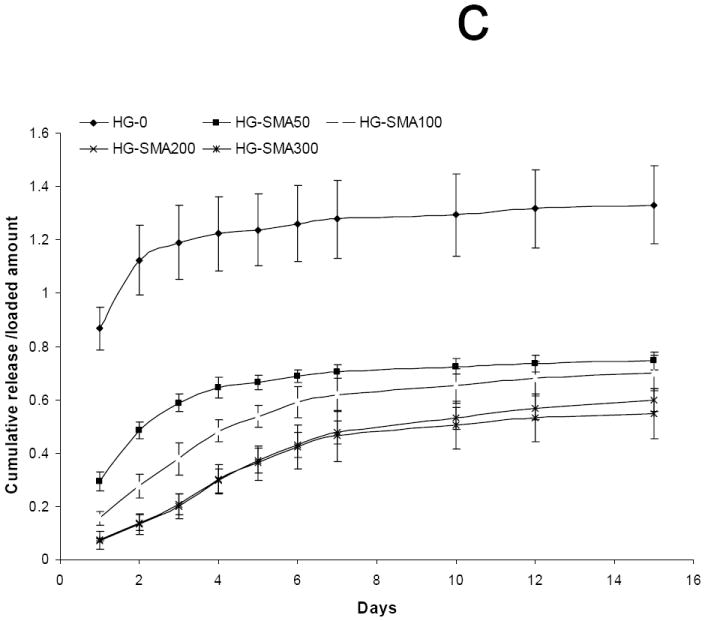
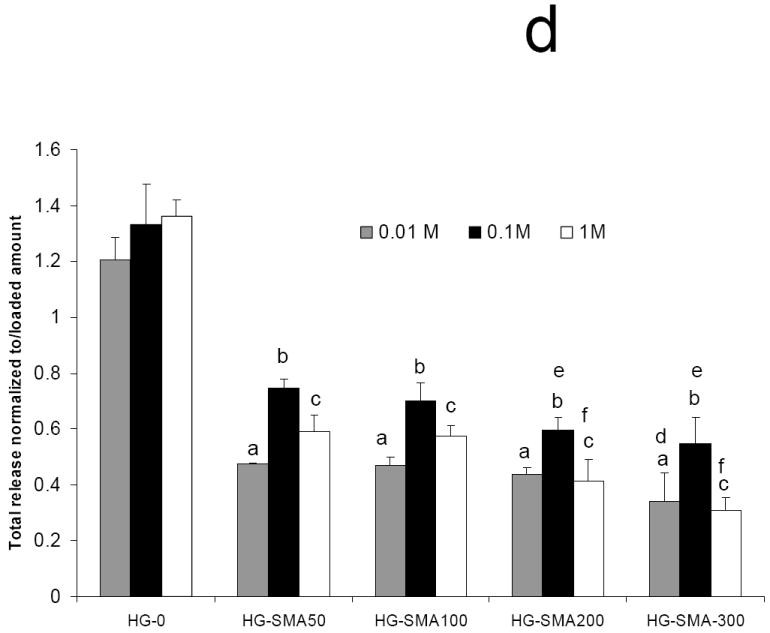
Release of DOX from hydrogels loaded at 50 μg/ml, in 0.01M (a), 0.1M (b), and 1M (c) NaCl solutions. Total amount of DOX released from each hydrogel formulation in NaCl of different concentrations over 15 days (d). The amounts of DOX released from hydrogels are normalized to initial amounts of DOX loaded to each hydrogel formulation. (a) p<0.001 as compared to HG-0 in 0.01M NaCl solution, (b) p<0.001 as compared to HG-0 in 0. 1M NaCl solution, (c) p<0.001 as compared to HG-0 in 1M NaCl solution, (d) p<0.05 as compared to HG-5 in 0.01M NaCl solution, (e) p<0.05 as compared to HG-5 in 0.1M NaCl solution, (f) p<0.05 as compared to HG-5 in 1M NaCl solution.
3.4. DOX biological activity
Figure 8a shows the dose-dependent effects of DOX on the viability of osteosarcoma MG63 cells. The maximum cell killing effect of DOX was seen at concentrations of 1 μg/ml and greater. Figure 8b shows the antitumor effect of DOX after release from the hydrogels. As seen in this figure, MG63 cells cultured in the presence of hydrogel disks without DOX maintained their viability, indicating that neither the OPF hydrogel itself nor any potentially leached materials from the hydrogels had any effect of toxicity. However, MG63 cell survival was minimal when they were exposed to the DOX released from hydrogels over three days.
Fig. 8.
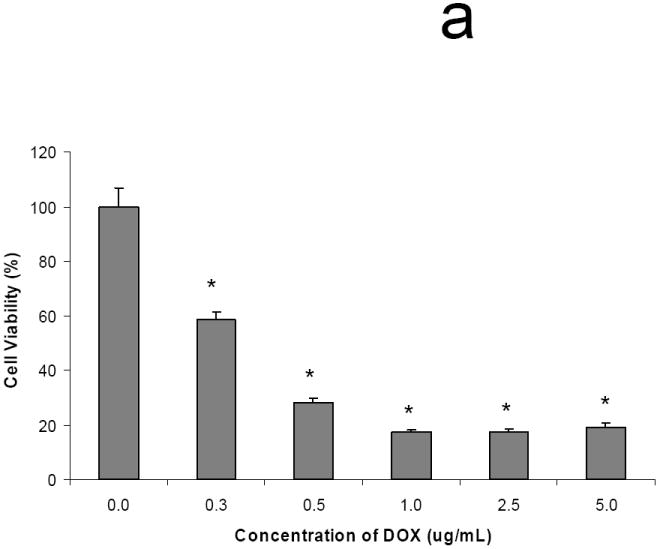
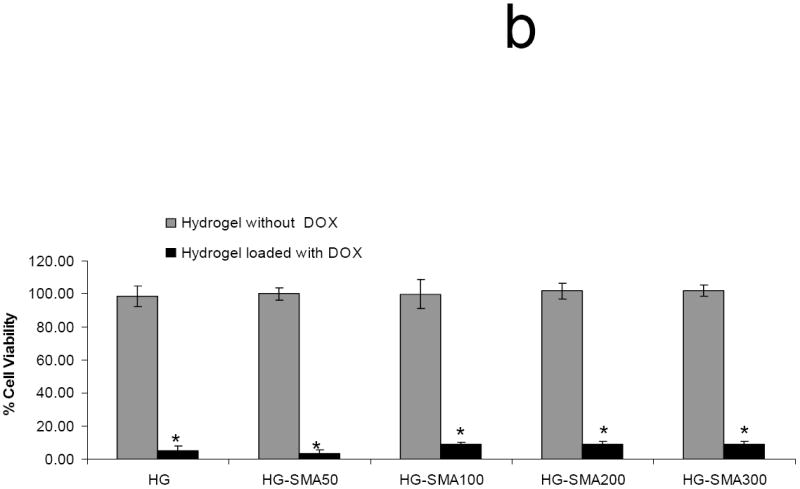
Dose-dependent cell killing effect of DOX (a). Maximum cell killing effect was observed at concentrations of 1 μg/ml and greater. (*) p<0.001 as compared to control. DOX released from hydrogels maintained its cell killing effects after 3 days in culture, while hydrogels without DOX showed no toxicity associated with release of leaching materials (b). (*) p<0.001 as compared to the hydrogels without DOX.
4. Discussion
The major goal of this study was to develop polyelectrolyte negatively charged hydrogels with properties that could be varied in a controlled manner, and to provide a controlled delivery system for DOX. OPF is an injectable and biodegradable material previously used in our group for cartilage tissue engineering and nerve regeneration [20, 21]. We have reported that the physical and chemical properties of OPF hydrogels can be modified with incorporation of a positively charged monomer 2-(methacryloyloxy) ethyl]-trimethylammonium chloride (MAETAC), and positively charged OPF promoted neurite outgrowth from primary neuronal cells[20]. In this study, the OPF macromer, copolymerized with varying amounts of a pH-dependent ionic monomer (SMA), becomes a hydrogel that contains fixed charge. Zeta-potential and conductivity measurements confirmed the electrically charged nature of SMA-modified hydrogels and that the amount of charge was dependent upon the SMA concentration in the polymerization formulation (data not shown). Incorporation of the unsaturated SMA monomer increased the frequency of crosslinks between OPF polymer chains, as demonstrated by a decreased sol fraction of charge-modified OPF hydrogels compared to unmodified hydrogels. This decrease in the sol fraction is indicative of a highly crosslinked network.
Attenuated total reflectance FTIR data demonstrated that SMA was successfully incorporated into the OPF hydrogel. After hydration in deionized water, the carbon-oxygen (C-O) peak intensity decreased on hydrogel surfaces as the concentration of SMA increased. The change in C-O peak intensity was inversely related to the swelling ratio of hydrogels. When the C-O peak intensity decreased, the swelling ratio of SMA-modified hydrogels increased. Such correlation suggests a conformational change in the SMA-modified hydrogel backbone, which is involved in ion and solvent transport, in different solvents. This results in SMA-modified hydrogels sensitivity to ionic strength and pH of the solvents. The swelling ratio of charged hydrogels in deionized water was shown to be a function of SMA concentration in hydrogel precursor solution. However, in PBS, swelling ratios of SMA-modified hydrogels were unchanged. We demonstrated that swelling ratios of SMA hydrogels changed with the change in ionic strength of NaCl solutions as well. With an increase in the ionic strength of the solution, the swelling ratio of charged hydrogels decreased. Hydrogels became more sensitive to the salt concentration with an increase in SMA concentration in hydrogel formulations. In contrast, there was little to no response to the ionic strength of the solution from hydrogels without charge (HG-0). When the polymeric network contains charged moieties such as carboxylic acids, the swelling becomes more complex. The swelling pressure may be greatly enhanced as a result of the localization of charged moieties, setting up an electrostatic repulsion between charged polymers. Since soluble polymers in a crosslinked network are interconnected covalently, the mobility of fixed charge moieties on the polymer backbone is hindered and they cannot diffuse freely into the outer solution. Hence, counter ions (original bounded cations) released from the polymer chains are confined inside the gel due to an electroneutrality effect. Consequently, the total mobile ion concentration inside the gel will exceed that in the external solution, leading to an osmotic pressure difference which tends to drive solvent into the gel from the less concentrated external solution.
Furthermore, we demonstrated that SMA-modified hydrogels are responsive to the pH of the solution. SMA-modified hydrogels had low swelling ratios at pH<3, however, it significantly increased at pH=3 and greater. Again, the hydrogels containing higher amounts of SMA appeared to exhibit more pH sensitivity. The swelling ratio of HG-SMA300 at pH=3 was about three times higher that that at pH=1. However, it did not change significantly with an increase in pH from 3 to 12. As expected for charged hydrogels, compressive modulus decreased with increasing swelling ratio of SMA-modified hydrogels. The compressive modulus of these hydrogels decreased with increasing SMA concentration in their formulations.
SMA-modified hydrogels could be effectively loaded with DOX due to the electrostatic interactions between negatively charged hydrogels and the protonated primary amine group on DOX. Uptake of DOX by SMA-modified hydrogels was shown to be an active process. These hydrogels had a high loading efficiency and absorbed about 99% of the drug from the loading solution within a few hours. Loading kinetics were shown to be a function of SMA concentration; for example 99% of the DOX in loading solution was taken up by HG-SMA300 hydrogel after 2h, while 95%, 80% and 60% was absorbed by HG-SMA200, HG-SMA100 and HG-SMA50, respectively. After 4 h, most of the drug was absorbed by SMA-modified hydrogels. This is favorable for clinical use of this material as the drug can be loaded in a short time period prior to administration. For comparisons, we measured the equilibrium loading efficiency of hydrogels after 24 hours. The loading efficiency of unmodified hydrogels decreased from 43% to about 25% as the concentration of DOX increased in loading solution. Unlike unmodified hydrogels, the loading efficiency of SMA-modified hydrogels did not change significantly with an increase in concentration of DOX in loading solution. SMA-modified hydrogels exhibited a high capacity for DOX uptake due to the electrostatic interactions between the drug and charged functional groups within the hydrogels. Our data revealed that SMA-modified hydrogels were capable of absorbing about 25mg of DOX per gram of hydrogel from loading solution at a concentration of 400 μg/ml. With an increase in concentration of DOX in loading solution (1mg/ml), charged hydrogels were still able to uptake about 99% of DOX and hydrogels absorbed about 60 mg/g from the solution (data not shown). Loading efficiency is an important factor in drug delivery approaches. The materials with high loading efficiency reduce drug waste and make it possible to deliver a very high local dose by using only a small amount of polymer.
The responsiveness of SMA-modified hydrogels to the ionic strength and pH of the surrounding environment is useful for drug delivery applications. As is typical of polyelectrolyte hydrogels, SMA-modified hydrogels swell significantly in deionized water and dilute solutions, while behaving as a neutral polymer with limited swelling in high ionic strength or low pH solutions. Given these data, an ion-exchange mechanism is suggested for release of DOX from SMA-modified hydrogels in which the rate of release can be controlled by the ionic strength of the eluting solution. The main counter ion present in PBS and NaCl is Na+, which is exchanged with DOX bound to the charged hydrogels. The rate and extent of DOX release was shown to correlate well with the amount of SMA in the hydrogel formulation. With incorporation of SMA the rate of release was significantly reduced and only 20% of the loaded amount was released from HG-SMA300 after 15 days. However, most of the DOX absorbed by HG-0 was released in the first few days of study. Our results revealed that the burst release significantly decreased with incorporation of SMA in OPF hydrogels. There was almost no burst release with increasing concentrations of SMA to 30% in HG-SMA300. Comparison of the total DOX release at two different loading doses (50 and 200 μg/ml) suggested that loading dose did not have a significant effect on the release kinetics of DOX. The ionic strength of the salt solution had a significant effect on the extent and rate of DOX release from SMA-modified hydrogels. However, the effect of salt concentration was less significant at high concentrations of the salt (1M) due to a decrease in solubility of DOX in this solution. These data suggest that the release kinetics of DOX are not only influenced by the salt concentration but also by the solubility of the drug in the medium.
The antitumor efficacy of DOX released from the hydrogels was investigated by measuring MG63 osteosarcoma cell death over three days. It appears the process of DOX loading to the hydrogels had no effect on biological activity of the released DOX and it preserved its antitumor activity. In addition, the percentage of living cells in the presence of DOX released from HG-0 and HG-SMA50 was slightly less than other SMA-modified hydrogels. This could be due to the burst release from these hydrogels in the first days of treatment that reduced cell proliferation, leading to more cell death.
5. Conclusion
The present study demonstrates the incorporation of a negatively charged carboxylate salt monomer into the photocrosslinkable OPF hydrogel. The modified charged hydrogels possessed polyelectrolyte properties and were responsive to the ionic strength and pH of the surrounding environment. We also described the use of charged OPF hydrogels for DOX delivery. DOX can be efficiently loaded into the charged hydrogels with a loading efficiency of about 99% due to an electrostatic interaction between DOX and charged hydrogels. Uptake of DOX was a dynamic process that occurred within a few hours, depending upon the charge density of the hydrogel. We demonstrate that DOX was released from charged hydrogels in a controlled and sustained manner. The rate and extent of the release was controlled by the ionic strength of the solution and charge density of the hydrogels. Cell culture in the presence of charged hydrogels demonstrated good biocompatibility of charged hydrogels comparable to our negative control. Additionally, the process of DOX loading to the hydrogel had no effect on the biological activity of the released DOX and it preserved its antitumor effects.
Acknowledgments
The project described was supported by Grant Number R01 AR45871, and R01 EB003060 from NIH/NIAMs and Mayo foundation.
Footnotes
Conflicts of Interest
A non provisional patent has been filed for photocrosslinkable oligo(polyethylene glycol) fumarate used in this research, and this technology has been licensed to BonWrx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collier JH, Camp JP, Hudson TW, Schmidt CE. Synthesis and characterization of polypyrrole-hyaluronic acid composite biomaterials for tissue engineering applications. J Biomed Mater Res. 2000;50:574–584. doi: 10.1002/(sici)1097-4636(20000615)50:4<574::aid-jbm13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Kamada H, Tsutsumi Y, Yoshioka Y, Yamamoto Y, Kodaira H, Tsunoda S, et al. Design of a pH-sensitive polymeric carrier for drug release and its application in cancer therapy. Clin Cancer Res. 2004;10:2545–2550. doi: 10.1158/1078-0432.ccr-03-0544. [DOI] [PubMed] [Google Scholar]
- 3.Lai PS, Lou PJ, Peng CL, Pai CL, Yen WN, Huang MY, et al. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J Control Release. 2007;122:39–46. doi: 10.1016/j.jconrel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Hassan CM, Stewart JE, Peppas NA. Diffusional characteristics of freeze/thawed poly(vinyl alcohol) hydrogels: applications to protein controlled release from multilaminate devices. Eur J Pharm Biopharm. 2000;49:161–165. doi: 10.1016/s0939-6411(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 5.Minko T, Kopeckova P, Kopecek J. Comparison of the anticancer effect of free and HPMA copolymer-bound adriamycin in human ovarian carcinoma cells. Pharm Res. 1999;16:986–996. doi: 10.1023/a:1018959029186. [DOI] [PubMed] [Google Scholar]
- 6.Brannon-Peppas L, Peppas NA. Dynamic and equilibrium swelling behaviour of pH-sensitive hydrogels containing 2-hydroxyethyl methacrylate. Biomaterials. 1990;11:635–644. doi: 10.1016/0142-9612(90)90021-h. [DOI] [PubMed] [Google Scholar]
- 7.Chan AW, Neufeld RJ. Modeling the controllable pH-responsive swelling and pore size of networked alginate based biomaterials. Biomaterials. 2009;30:6119–6129. doi: 10.1016/j.biomaterials.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Chan AW, Whitney RA, Neufeld RJ. Semisynthesis of a controlled stimuli-responsive alginate hydrogel. Biomacromolecules. 2009;10:609–616. doi: 10.1021/bm801316z. [DOI] [PubMed] [Google Scholar]
- 9.Jiang B, Li B. Tunable drug loading and release from polypeptide multilayer nanofilms. Int J Nanomedicine. 2009;4:37–53. doi: 10.2147/ijn.s4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian F, Szymanski A, Gao J. Fabrication and characterization of controlled release poly(D,L-lactide-co-glycolide) millirods. J Biomed Mater Res. 2001;55:512–522. doi: 10.1002/1097-4636(20010615)55:4<512::aid-jbm1044>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 12.Qian F, Stowe N, Liu EH, Saidel GM, Gao J. Quantification of in vivo doxorubicin transport from PLGA millirods in thermoablated rat livers. J Control Release. 2003;91:157–166. doi: 10.1016/s0168-3659(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 13.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41:403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 14.Lowenthal RM. Alternative cancer treatments. Med J Aust. 1996;165:536–537. doi: 10.5694/j.1326-5377.1996.tb138635.x. [DOI] [PubMed] [Google Scholar]
- 15.Klein-Szanto AJ. Carcinogenic effects of chemotherapeutic compounds. Prog Clin Biol Res. 1992;374:167–174. [PubMed] [Google Scholar]
- 16.Lewis AL, Gonzalez MV, Leppard SW, Brown JE, Stratford PW, Phillips GJ, et al. Doxorubicin eluting beads - 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18:1691–1699. doi: 10.1007/s10856-007-3068-8. [DOI] [PubMed] [Google Scholar]
- 17.Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006;17:1335–1343. doi: 10.1097/01.RVI.0000228416.21560.7F. [DOI] [PubMed] [Google Scholar]
- 18.Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335–342. doi: 10.1097/01.RVI.0000195323.46152.B3. [DOI] [PubMed] [Google Scholar]
- 19.Jo S, Shin H, Mikos AG. Modification of oligo(poly(ethylene glycol) fumarate) macromer with a GRGD peptide for the preparation of functionalized polymer networks. Biomacromolecules. 2001;2:255–261. doi: 10.1021/bm000107e. [DOI] [PubMed] [Google Scholar]
- 20.Dadsetan M, Knight AM, Lu L, Windebank AJ, Yaszemski MJ. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials. 2009;30:3874–3881. doi: 10.1016/j.biomaterials.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dadsetan M, Szatkowski JP, Yaszemski MJ, Lu L. Characterization of photo-cross-linked oligo[poly(ethylene glycol) fumarate] hydrogels for cartilage tissue engineering. Biomacromolecules. 2007;8:1702–1709. doi: 10.1021/bm070052h. [DOI] [PubMed] [Google Scholar]


