Abstract
Chronic insulin resistance contributes to subclinical inflammation, thrombosis/impaired fibrinolysis, and dyslipidemia. The effect of dietary carbohydrate, specifically of glycemic index (GI) and glycemic load (GL), on established and emerging coronary heart disease (CHD) risk factors has not been elucidated fully. We conducted a randomized, cross-over feeding study of matched diets differing only in GI and GL in 24 overweight or obese but otherwise healthy men to investigate the effects on insulin sensitivity, inflammation, thrombosis/fibrinolysis, lipoproteins/lipids, and body composition. All meals for the high- and low-GI/GL diets were prepared in a metabolic kitchen. Each participant consumed both diets in random order for 4 weeks each, with a 4-week wash-out period in between. Each participant underwent a frequently-sampled intravenous glucose tolerance test for assessment of insulin sensitivity; blood sampling for the measurement of inflammatory markers, coagulation factors, and lipoproteins/lipids; and dual-energy x-ray absorptiometry for assessment of body composition at the beginning and end of each dietary period. There were no statistically significant differences in glucose metabolism factors, inflammatory markers, or coagulation factors following 4 weeks on the high- and low-GI/GL diets. The high-GI/GL diet resulted in a slightly greater reduction in fat mass and a slightly greater increase in lean mass compared to the low-GI/GL diet. The high-GI/GL diet resulted in significant, but unexpected, reductions in total and LDL cholesterol, while HDL cholesterol concentration was significantly reduced on the high-GI/GL diet compared to the low-GI/GL diet. Overall, high- and low-GI/GL diets of 4-weeks duration had no consistent effects on CHD risk factors in this group of overweight/obese men.
1. Introduction
Coronary heart disease (CHD) results in significant morbidity and mortality in the US [1]. Chronic insulin resistance likely plays an important role in the etiology of this disease by promoting subclinical inflammation and thrombosis/impaired fibrinolysis, in addition to its more established association with dyslipidemia [2-4]. Plasma mediators of chronic inflammation, such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), and other acute phase reactants such as fibrinogen and plasminogen activator inhibitor-1 (PAI-1), are some of the emerging risk factors and biomarkers being evaluated as early manifestations of insulin resistance [5].
Early intervention aimed at decreasing insulin resistance, including dietary intervention, may play a role in CHD risk reduction. Chronic excessive intake of carbohydrates may lead to chronic hyperinsulinemia, insulin resistance, and eventually to CHD [4]. However, carbohydrates elicit a wide spectrum of blood glucose and insulin responses, influenced by both the quality and quantity of the carbohydrate. Glycemic index (GI) is a ranking of foods based on their postprandial blood glucose responses and is a measure of carbohydrate quality [6]. Glycemic load (GL) is a measure that incorporates both the quantity and quality of dietary carbohydrates [7].
While observational studies have shown associations of GI and/or GL and markers of inflammation and thrombosis/impaired fibrinolysis [8-10], there is a paucity of randomized, controlled trials (RCTs) evaluating the effects of dietary interventions differing in GI and GL on comprehensive measures of insulin resistance, inflammation, and thrombosis/fibrinolysis. In addition, several RCTs that have been reported were conducted in participants with diabetes [11-13]. Relatively few studies have evaluated healthy participants, although existing data have confirmed the applicability of the concept of GI in healthy states for disease risk reduction [14]. Furthermore, previous studies may have been confounded by failure to match total energy intake and macronutrient content (most notably dietary fiber) between the low- and high-GI diets [13,15,16].
To address this, we conducted a randomized, cross-over, controlled feeding study of matched diets differing only in GI and GL in overweight or obese, but otherwise healthy, African-American and Caucasian men to investigate the effects of the diets on insulin sensitivity, inflammation, thrombosis/ fibrinolysis, lipoproteins/lipids, and body composition.
2. Materials and methods
2.1. Participants
Potential participants were recruited through flyers posted around the University of Alabama at Birmingham (UAB) campus, advertisements in the campus employee and student newspapers, and word of mouth. Inclusion criteria included men ages 20 to 50 years; body mass index (BMI) from 25 to 33 kg/m2; and ability to read and write English. Exclusion criteria included current chronic disease (CHD, diabetes, hypertension, kidney or liver disease, or uncontrolled thyroid disease); use of medications known to influence body composition or blood glucose, insulin, or lipid concentrations; use of anti-inflammatory medications; current smoking; greater than two hours of vigorous exercise per week; alcohol consumption greater than two drinks/day; illicit use of drugs; currently on a special diet; significant mental illness; or inability or unwillingness to submit to informed consent. Interested persons were screened for initial eligibility by telephone. Eligible persons attended a screening visit at the UAB General Clinical Research Center (GCRC) outpatient clinic following an overnight fast. At this visit, blood was drawn for screening laboratory tests (creatinine, albumin, total bilirubin, direct bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, thyroid stimulating hormone, glucose); height and weight were measured and BMI calculated; and questionnaires were completed (sociodemographic, medical history, medication use, and physical activity). Participants completed a 4-day food record during screening to determine general eating habits and assess compatibility with the study diets. Participants who were eligible following calculation of BMI, review of laboratory tests, and review of questionnaire responses were entered into the study. A total of 30 participants were enrolled in five separate waves. A total of 24 participants completed both dietary periods and all four overnight GCRC visits. The study was approved by the Institutional Review Board for Human Use at UAB, and all participants provided written informed consent.
2.2. Study design
In a cross-over design, participants consumed for 4 weeks each two study diets – a high-GI/GL diet and a low-GI/GL diet. The order of the diets was randomly determined. Participants were admitted to the GCRC for an overnight stay at the beginning and end of each dietary intervention period, for a total of four such visits. At each visit, participants were admitted during the evening, and height (first visit only), weight, and blood pressure were measured. Participants then were provided with dinner, followed by an overnight (12-h) fast. At approximately 0800 the following morning, participants underwent a frequently-sampled intravenous glucose tolerance test (FSIGT). Additional blood was drawn for the measurement of inflammatory markers, coagulation factors, and lipoproteins/lipids. Participants underwent dual-energy x-ray absorptiometry (DXA) following the FSIGT. Participants began their prescribed diet the same day and continued until the second overnight admission 4 weeks later, during which the same protocol was followed. There was a 4-week wash-out period between the two dietary intervention periods, during which participants were free to consume foods of their own choosing, without monitoring by study personnel. Following this period, participants had their third overnight admission and began the second 4-week dietary intervention period, culminating in the fourth overnight admission. During the second and fourth overnight admissions (the visits at the end of the four-week dietary intervention periods), participants received the dinner that corresponded to the diet to which they had been assigned during the preceding four weeks.
2.3. Study Diets
High- and low-GI/GL diets were designed to be isoenergetic within individual participants, with approximately equal macronutrient composition and dietary fiber content. Energy content of the diets was individualized to participants to ensure weight maintenance throughout the dietary intervention periods and was calculated using the Harris Benedict equation × 1.35 [17].The high-GI/GL diet was modeled after the Therapeutic Lifestyle Changes diet developed by the National Cholesterol Education Program [18]. The low-GI/GL diet was a modification of this diet, with the underlying principle being the replacement of high-GI carbohydrates with low-GI carbohydrate alternatives. GI and GL values were obtained from a published list of such values [19]. All dietary analyses, including determination of overall dietary GI and GL values, were conducted with Nutrition Data System for Research (NDSR) software [20].
All meals for the high- and low-GI/GL diets were prepared, stored, and dispensed in the Bionutrition Department of the GCRC. Meals for each diet were designed by GCRC research dietitians in collaboration with the principal investigator. Meals were prepared by research cooks in the GCRC metabolic kitchen under the direction of the Bionutrition Research Manager, a registered dietitian. Meals were prepared on a 4-day menu cycle with 12 meals. Four unique daily menus were designed for both the high- and low-GI/GL diets. The 4-day rotation ensured that participants did not consume the same dinner each Sunday, for example, which has promoted dietary adherence in other feeding studies conducted in the GCRC. Participants reported to the Bionutrition Department early in the morning three times a week to pick up meals. On Mondays, participants picked up meals for Monday and Tuesday; on Wednesdays, participants picked up meals for Wednesday and Thursday; and on Fridays, participants picked up meals for Friday, Saturday, and Sunday. Meals were packaged in two separate insulated containers. One container contained foods that were to remain frozen until use. The second container contained foods that were to be refrigerated until use, along with foods not requiring refrigeration.
Participants were provided with detailed instructions on the proper storage, selection (i.e., menus), and preparation of foods. Foods supplied required as little preparation prior to consumption as possible. In most cases, preparation entailed heating in a microwave oven. Participants were instructed to consume no foods or beverages outside of those provided by the study, with the exception of water, which was not limited on either diet. They also were instructed to consume all of the foods and beverages provided. In the event of a missing food item, the participant was instructed to substitute a food item from another day, and the missing item was replaced as quickly as possible. During each meal pick-up, participants were questioned about adherence to the study diets.
2.4. Anthropometry and body composition
Body height was measured, without footwear, to the nearest 0.1 cm with a calibrated, wall-mounted digital stadiometer (Heightronic model 235; Measurement Concepts, North Bend, WA). Body weight was measured, with participants wearing light clothing and no footwear, to the nearest 0.1 kg with a calibrated digital scale (Scale-Tronix model 6002; Carol Stream, IL). Besides being measured at the beginning and end of each dietary period, weight also was measured during participants' thrice weekly visits to the GCRC to pick up study meals. Any fluctuation of body weight of more than 2 kg from the previous weight was reported to study dietitians, who adjusted energy content of the participant's diet accordingly. Body composition was assessed at the beginning and end of each dietary period by DXA with a GE Lunar Prodigy bone densitometer (GE Healthcare, Waukesha, WI).
2.5. Frequently-sample intravenous glucose tolerance test
Whole-body insulin sensitivity was assessed on an in-patient basis in the GCRC after an overnight fast with an insulin-modified FSIGT. Prior to testing, flexible intravenous catheters were placed in the antecubital spaces of both arms. Three, 2.0-mL blood samples were taken over a 20-min period for determination of basal glucose and insulin concentrations (the average of the values was used for basal “fasting” concentrations). At time “0,” glucose (50% dextrose; 11.4 g/m2) was administered intravenously. Insulin (0.02 U/kg, Humulin, Eli Lilly and Co., Indianapolis, IN) was injected at 20 min post glucose injection. Blood samples (2.0 mL) were collected at the following times relative to glucose administration: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, and 180 min. Sera were stored at -85 °C until analyzed.
2.6. Laboratory analyses
Glucose and insulin analyses were performed in the Core Laboratory of the GCRC and the Clinical Nutrition Research Center (CNRC) at UAB. Serum glucose was measured with the glucose oxidase method using a SIRRUS analyzer, with an inter-assay coefficient of variation (CV) of 3%. Insulin was assayed with Linco Research, Inc. (St. Charles, MO) reagents, with an intra-assay CV of 4% and an inter-assay CV of 6%. Glucose and insulin values were entered into the minimal model of glucose dynamics (MINMOD computer program, Millenium version; © 2001, Richard N. Bergman, Los Angeles, CA) for determination of the insulin sensitivity index (Si), glucose effectiveness (Sg), and the acute insulin response to glucose (AIRg) [21-23]. AIRg was calculated as the incremental area under the curve for the first 10 min following glucose administration, as determined by the trapezoidal method. Intravenous glucose tolerance (Kg) was calculated as the inverse slope of time vs. the natural log of glucose concentration during minutes 8-19 following glucose administration. It was expressed in percent per minute (disappearance of glucose).
Inflammatory marker and coagulation factor assays were conducted at the Laboratory for Clinical Biochemistry Research within the College of Medicine at the University of Vermont. CRP was measured using the BNII nephelometer (Dade Behring, Inc., Deerfield, IL), utilizing a particle enhanced immunonepholometric assay. IL-6 was measured by ultra-sensitive ELISA (R&D Systems, Inc., Minneapolis, MN). TNF-α was measured by Luminex technology multiplex ELISA using the Human Serum Adipokine Panel B LINCOplex Kit (Linco Research, Inc.). Tumor necrosis factor- α receptor II (TNF-RII) was measured using an ultra-sensitive ELISA assay (R&D Systems, Inc.). Fibrinogen concentrations were quantified by the STAR automated coagulation analyzer (Diagnostica Stago, Inc., Parsippany, NJ) and the clotting method of Clauss [24]. PAI-1 assay was performed as a two-site ELISA. Inter-assay CVs for these assays ranged from 2% (CRP) to 15% (IL-6).
Lipoprotein/lipid analyses were performed in the Core Laboratory of the GCRC and CNRC at UAB. Serum total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured using a SIRRUS analyzer (Stanbio Laboratory, Boerne, TX). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation [25].
2.7. Statistical analyses
The primary purpose of the study was to assess the effect of high- and low-GI/GL diets on a variety of CHD risk factors, including measures of glucose metabolism, inflammatory markers, coagulation factors, lipids/lipoproteins, and body composition. This study was powered to detect a difference between the two diets with regard to change in serum glucose. Specifically, a two-sided t-test using 24 individuals in a cross-over design would achieve 80% power to detect a difference in the mean change in serum glucose between diets of 5.1 mg/dL, assuming a within root mean square error of 6.0 mg/dL and an alpha of 0.05.
Given the cross-over design of the study, analyses were conducted using the methods illustrated in Jones and Kenward [26]. Specifically, inferential tests were conducted for the effect of sequence (commonly referred to as cross-over effect), effect of the diets, and effect of time periods. The analysis strategy first was to examine for sequence effects. If this test was non-significant (P > .05), the test of the effect of diets was examined using information from both time periods. However when the test of sequence effect was statistically significant, this result was interpreted as indication of a carryover effect, whereby the effect of the first diet to which a participant was randomized had not completely “washed out” prior to commencing the second diet. When the test of sequence effect was statistically significant, the test of diet effect was conducted using only the data from the first time period. This was the case for HDL cholesterol because a statistical test indicated that, within the cross-over design, a significant carryover effect may have occurred. Therefore, for this specific variable, comparison of the two diets is based only upon data obtained in the first phase. The distributional assumption of normality was examined using histograms and normal probability plots and tested using Kolmogorov-Smirnov's test. In order to examine the robustness of our finding to the distributional assumption of normality, all statistical tests were recast as two-sample tests as illustrated by Hills and Armitage [27] and conducted using the non-parametric equivalent test (Wilcoxon Rank Sum) of the two-sample t-test. Because significance did not differ between the two approaches, only P values resulting from tests assuming normality are reported.
3. Results
3.1. Participants
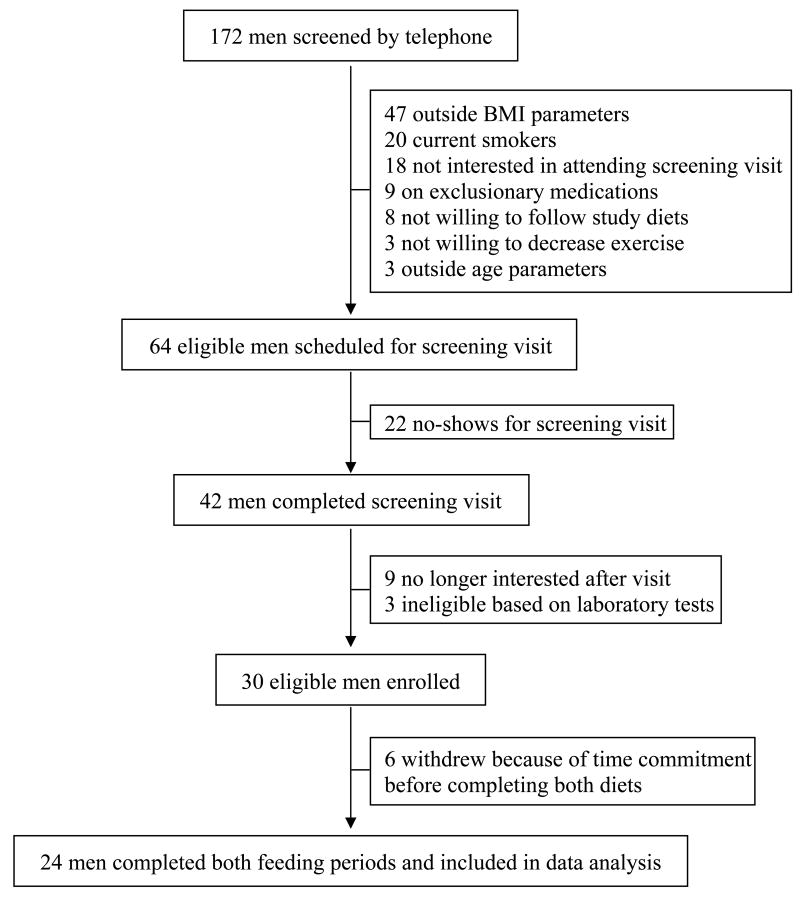
The study enrollment scheme is presented in Figure 1. A total of 172 men were screened by telephone for the study between September 2006 and April 2007. One hundred and eight men immediately were excluded because they did not meet eligibility requirements. The remaining 64 men were scheduled for a screening visit at the GCRC, 42 of whom completed this visit. Based on screening testes, 30 of these men were eligible and were entered into the study. Six men dropped out of the study before completing all four GCRC visits because of the time commitment required. Twenty four men completed both dietary intervention periods and all four overnight GCRC visits and were included in the data analysis. Participants included 14 Caucasian and 10 African-American men (Table 1). The mean age of participants was 34.5 ± 8.1 years, and mean BMI was 27.8 ±3.5 kg/m2, compared to a mean age of 25.0 ± 2.8 years (P = .0019 for difference) and mean BMI of 29.5 ± 4.3 kg/m2 (P = .2760) in the men who dropped out).
Fig. 1.
Study enrollment scheme
Table 1.
Characteristics of study participants at baseline
| Variable | All participants (n = 24) |
|---|---|
| Age (y) | 34.5 ±8.1 |
| Race | |
| Caucasian | 14 (58.3) |
| African American | 10 (41.7) |
| Weight (kg) | 90.5 ± 12.5 |
| BMI (kg/m2) | 27.8 ± 3.5 |
| Fat mass (%) | 26.9 ± 6.1 |
| Lean mass (%) | 69.2 ± 5.8 |
| Glucose (mg/dL) | 101.9 ± 8.6 |
| Insulin (mU/L) | 7.1 ± 2.7 |
| Si (× 10-4 × min-1 mU-1 × mL-1) | 4.0 ± 1.7 |
| Sg (min-1 × 10-2) | 1.8 ± 1.1 |
| AIRg (mU/L × min) | 736 ± 580 |
| Kg (%/min-1) | 1.7 ± 1.1 |
| CRP (mg/L) | 1.4 ± 1.5 |
| IL-6 (ng/L) | 2.8 ± 2.2 |
| TNF-α (ng/L) | 2.6 ± 1.8 |
| TNF-RII (mg/L) | 1.93 ± 0.42 |
| PAI-1 (μg/L) | 37.9 ± 39.6 |
| Fibrinogen (g/L) | 2.75 ± 0.53 |
| Total cholesterol (mg/dL) | 182.0 ± 41.9 |
| LDL cholesterol (mg/dL) | 117.8 ± 38.5 |
| HDL cholesterol (mg/dL) | 40.8 ± 8.7 |
| Triglycerides (mg/dL) | 125.8 ± 114.2 |
Data are shown as mean ± SD except for race, expressed as n (%).
3.2. Diets
Energy content of the low- and high-GI/GL diets was nearly identical and varied little over the 4-day menus for each diet (Table 2). Likewise, macronutrient composition of the diets was very similar. While there were no clinically meaningful differences between the two diets in total monounsaturated fatty acids or total trans-fatty acids, total saturated fatty acids were slightly lower (by approximately 3 g/d) and total polyunsaturated fatty acids were slightly higher (by approximately 5 g/d) in the high-GI/GL diet, although these differences did not reach statistical significance (data not shown). Total dietary fiber content was slightly higher (by approximately 2 g/d) in the low-GI/GL diet compared to the high-GI/GL diet, but this difference was not statistically significant. Mean GI in the low-GI/GL diet was 34.0% lower than that in the high-GI/GL diet (49.5 vs. 75.0, respectively; P = .0194) while mean GL in the low-GI/GL diet was 35.5% lower than that in the high-GI/GL diet (158.3 vs. 245.5, respectively; P = .0209). Participants were questioned by study dietitians about compliance with study diets during each visit to the GCRC to pick up their meals. Both diets were well tolerated by study participants. While two participants reported that the amount of food was more than they were accustomed to, and one said it was less than he was accustomed to, all participants reported upon questioning that they consumed all of their study foods and none reported that they consumed foods or beverages outside of the study diets.
Table 2.
Composition of the study diets
| Day | Energy (kcal) |
Fat (% of energy) |
Protein (% of energy) |
Carbohydrate (% of energy) |
Dietary fiber (g) |
GI | GL |
|---|---|---|---|---|---|---|---|
| Low-GI/GL load diet | |||||||
| 1,5 | 2483 | 29.6 | 18.2 | 54.8 | 19.2 | 50 | 161 |
| 2,6 | 2496 | 29.2 | 16.5 | 55.6 | 24.6 | 53 | 172 |
| 3,7 | 2480 | 28.6 | 18.4 | 54.7 | 27.6 | 45 | 141 |
| 4,8 | 2473 | 28.3 | 17.2 | 55.2 | 21.4 | 50 | 159 |
| Mean (SD) | 2483 ± 9.6 | 28.9 ± 0.6 | 17.6 ± 0.9 | 55.1 ± 0.4 | 23.2 ± 3.7 | 49.5 ± 3.3 | 158.3 ± 12.8 |
| High-GI/GL diet | |||||||
| 1,5 | 2529 | 30.1 | 16.5 | 54.8 | 16.1 | 76 | 250 |
| 2,6 | 2478 | 30.4 | 16.3 | 55.1 | 23.2 | 76 | 240 |
| 3,7 | 2482 | 27.0 | 16.5 | 58.4 | 26.2 | 69 | 233 |
| 4,8 | 2519 | 30.9 | 16.1 | 54.9 | 18.3 | 79 | 259 |
| Mean (SD) | 2502 ± 25.8 | 29.6 ± 1.8 | 16.4 ± 0.2 | 55.8 ± 1.7 | 21.0 ± 4.6 | 75.0 ± 4.2 | 245.5 ± 11.4 |
3.3. Anthropometry and body composition
As was the intent, body weight and BMI remained relatively stable on both diets over the dietary intervention periods, with no statistically significant differences in changes in weight or BMI between the groups (Table 3). Fat mass decreased slightly in both diet groups over the 4-week dietary periods. While the percentage reduction was significantly greater in the high-GI/GL group, the absolute difference between the groups was small (-1.1% vs. -0.5%, high-GI/GL and low-GI/GL diet, respectively).
Table 3.
Effects of the high- and low-GI/GL diets on body composition
| Variable | High-GI/GL diet | Low-GI/GL diet | P* | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | ||
| Weight (kg) | 88.3 ± 10.5 | 87.6 ± 10.9 | -0.7 ± 1.9 | 88.1 ± 11.1 | 87.1 ± 10.2 | -1.0 ± 1.6 | .7530 |
| BMI (kg/m2) | 27.5 ± 3.4 | 27.1 ±3.5 | -0.3 ± 0.6 | 27.4 ± 3.7 | 27.0 ± 3.3 | -0.4 ± 0.7 | .4777 |
| Fat mass (%) | 26.4 ± 6.3 | 25.3 ± 6.7 | -1.1 ± 1.3 | 26.0 ± 6.5 | 25.5 ± 6.6 | -0.5 ± 1.3 | .0244 |
| Lean mass (%) | 69.7 ± 6.1 | 70.8 ± 6.4 | 1.1 ± 1.3 | 70.1 ± 6.3 | 70.6 ± 6.4 | 0.5 ± 1.3 | .0175 |
Data are shown as mean ± SD.
P value for difference in change between high- and low-GI/GL diets.
3.4. Glucose metabolism factors
Glucose and insulin concentrations decreased slightly on both the high- and low-GI/GL diets (Table 4). While the reductions were slightly larger in magnitude on the low-GI/GL diet, the differences were not statistically significant. There were no significant differences in changes in Si, Sg, AIRg, or Kg between the two diets.
Table 4.
Effects of the high- and low-GI/GL diets on measures of glucose metabolism
| Variable | High-GI/GL diet | Low-GI/GL diet | P* | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | ||
| Glucose (mg/dL) | 101.9 ± 7.8 | 99.6 ± 6.3 | -2.2 ± 7.4 | 103.5 ± 8.0 | 98.4 ± 6.8 | -5.1 ± 6.96 | .1378 |
| Insulin (mU/L) | 7.2 ± 3.0 | 5.8 ± 2.9 | -1.4 ± 2.7 | 7.6 ± 4.8 | 5.7 ± 3.3 | -1.9 ± 4.5 | .4344 |
| Si (× 10-4 × min-1 mU-1 × mL-1) | 4.0 ± 1.7 | 3.7 ± 2.6 | -0.3 ± 2.1 | 5.0 ± 3.9 | 4.4 ± 2.4 | -0.6 ± 3.0 | .5548 |
| Sg (min-1 × 10-2) | 1.8 ± 0.9 | 1.6 ± 0.6 | -0.1 ± 0.9 | 1.9 ± 1.3 | 1.6 ± 1.0 | -0.3 ± 1.0 | .3640 |
| AIRg (mU/L × min) | 726 ± 475 | 737 ± 537 | 11 ± 450 | 742 ± 588 | 658 ± 570 | -85 ± 468 | .1881 |
| Kg (%/min-1) | 1.9 ± 1.0 | 1.6 ± 0.1 | -0.3 ± 1.1 | 2.0 ± 1.3 | 2.0 ± 1.3 | 0.0 ± 1.5 | .9876 |
Data are shown as mean ± SD.
P value for difference in change between high- and low-GI/GL diets.
3.5. Inflammatory markers and coagulation factors
There were no significant differences in changes observed in inflammatory markers (CRP, IL-6, TNF-α) or TNF-RII in response to the two dietary interventions (Table 5). Likewise, no significant differences in changes were noted between the diet groups for the coagulation factors (PAI-1, fibrinogen).
Table 5.
Effects of the high- and low-GI/GL diets on inflammatory markers and coagulation factors
| Variable | High-GI/GL diet | Low-GI/GL diet | P* | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | ||
| CRP (mg/L) | 2.1 ± 1.81 | 1.7 ± 1.9 | -0.4 ± 2.0 | 1.3 ± 1.2 | 1.3 ± 1.2 | 0.0 ± 0.7 | .7259 |
| IL-6 (ng/L) | 3.2 ± 2.8 | 3.1 ± 3.4 | -0.1 ± 3.5 | 2.5 ± 1.9 | 2.3 ± 1.7 | -0.2 ± 1.8 | .5762 |
| TNF-α (ng/L) | 2.7 ± 1.6 | 2.8 ± 1.7 | 0.1 ± 0.5 | 2.7 ± 1.7 | 2.6 ± 1.5 | -0.1 ± 0.7 | .5370 |
| TNF-RII (mg/L) | 1.89 ± 0.38 | 1.85 ± 0.38 | -0.04 ± 0.30 | 1.87 ± 0.41 | 1.80 ± 0.36 | -0.07 ± 0.21 | .7202 |
| PAI-1 (μg/L) | 28.0 ± 22.7 | 32.2 ± 29.2 | 4.2 ± 30.8 | 38.4 ± 38.7 | 38.8 ± 39.2 | 0.4 ± 52.8 | .9335 |
| Fibrinogen (g/L) | 2.88 ± 0.82 | 2.76 ± 0.83 | -0.12 ± 0.73 | 2.68 ± 0.56 | 2.64 ± 0.60 | -0.04 ± 0.49 | .9260 |
Data are shown as mean ± SD.
P value for difference in change between high- and low-GI/GL diets.
3.6. Lipoproteins/lipids
Concentrations of total, LDL, and HDL cholesterol decreased after four weeks on the high-GI/GL diet (Table 6). Total and LDL cholesterol concentrations increased on the low-GI/GL diet, while HDL cholesterol was virtually unchanged on the low-GI/GL diet. Overall, the changes in total, LDL, and HDL cholesterol were significantly different comparing the two diets, with the high-GI/GL diet resulting in improvements in total and LDL cholesterol, but worsening HDL cholesterol. There was no difference in the change in triglycerides between the two diets.
Table 6.
Effects of the high- and low-GI/GL diets on lipoproteins/lipids
| Variable | High-GI/GL diet | Low-GI/GL diet | P* | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | ||
| Total cholesterol (mg/dL) |
185.4 ± 39.9 | 171.4 ± 38.5 | -14.0 ± 25.1 | 170.3 ± 38.4 | 178.4 ± 30.8 | 8.1 ± 26.1 | .0013 |
| LDL cholesterol (mg/dL) |
120.8 ± 39.5 | 108.0 ± 34.9 | -12.8 ± 20.4 | 107.4 ± 34.4 | 113.4 ± 29.6 | 6.0 ± 21.37 | .0019 |
| HDL cholesterol (mg/dL) |
44.6 ± 7.5 | 40.2 ± 9.9 | -4.3 ± 4.6 | 38.5 ± 8.8 | 38.3 ± 11.1 | -0.1 ± 4.6 | .0447 |
| Triglycerides (mg/dL) |
111.5 ± 66.5 | 115.5 ± 79.2 | 4.0 ± 45.1 | 119.5 ± 112.9 | 134.4 ± 111.5 | 14.9 ± 52.3 | .3291 |
Data are shown as mean ± SD.
A statistical test indicated that within the cross-over design a significant carryover effect may have occurred for HDL cholesterol. This carryover effect implies that the outcomes observed in the second phase of the design could be dependent upon the first phase results. Therefore, for this specific variable, comparison of the two diets is based upon only data obtained in the first phase.
P value for difference in change between high- and low-GI/GL diets.
4. Discussion
In this randomized clinical trial, we observed no statistically significant differences in glucose metabolism factors, inflammatory markers, or coagulation factors following 4 weeks on high- or low-GI/GL diets. The high-GI/GL diet resulted in a slightly greater reduction in fat mass and a slightly greater increase in lean mass compared to the low-GI/GL diet. The high-GI/GL diet resulted in significant, but unexpected, reductions in total and LDL cholesterol, while HDL cholesterol concentration was significantly reduced on the high-GI/GL diet compared to the low-GI/GL diet. Overall, there were no consistent effects of high- and low-GI/GL diets on CHD risk factors in this group of overweight and obese men.
We observed no differences in glucose metabolism parameters between the high- and low-GI/GL diets. These results were in agreement with those from an RCT investigating the effects of 6-month high- and low-GL diets on glucose tolerance and inflammation in healthy but overweight participants, conducted by Pittas and colleagues [28]. However, the diets employed by Pittas and colleagues were energy-restricted, unlike the diets we employed. Several other RCTs also have shown no differences in changes in glucose, insulin, or HOMA-IR comparing high- and low-GI and/or GL diets [16,29-31].
Our CRP results were in general agreement with those from previous studies, including an RCT reported by McMillan-Price and colleagues, in which there were no differences in changes in CRP levels in overweight or obese participants assigned to one of four diets varying in GL for 12 weeks [30]. Likewise, there was no significant difference in change in CRP comparing low- and high-GI diets in the Canadian Trial of Carbohydrates in Diabetes [13].
Few previous intervention studies included IL-6 and TNF-α or its receptors. A clinical study showed that adipocyte TNF-α production was not influenced by dietary GI in an RCT of premenopausal women [32]. Similarly, in a short-term metabolic study in premenopausal, overweight women, the effects of high- and low-GL single meals on plasma levels of TNF-α and IL-6 did not differ [33]. In an observational study utilizing the Nurses' Health Study cohort, dietary GI, but not GL, was positively associated with TNF-RII levels [9]. We showed no significant difference in changes in PAI-1, a CHD risk factor (34), on the low- and high-GI/GL diets. This was in contrast to two previous RCTs which demonstrated beneficial effects of low-GI/GL diets on PAI-1 levels [35,36].
The cholesterol findings in this study were somewhat at odds with the results of previous low-GI/GL interventions, although previous results are somewhat mixed. There were no significant differences in changes in total, LDL, or HDL cholesterol after low-GI and/or GL diets in several RCTs conducted in obese or overweight men and women [15,16,36-38]. While there were no significant differences in the changes in total or HDL cholesterol in two RCTs of low-and high-GI diets in healthy overweight women, LDL cholesterol was significantly reduced in the low-GI compared to the high-GI diet [29,35]. There was a larger mean increase in HDL cholesterol in a reduced-GL diet relative to the control diet in overweight and obese adults [31]. It should be noted that it is possible that the slightly higher polyunsaturated fatty acid and slightly lower saturated fatty acid content of the high-GI/GL diet in the present study may have contributed to the improvements in total and LDL cholesterol on this diet.
Most previous studies have shown minimal effects of low-GI/GL interventions on measures of body composition [37,38]. Conversely, 12 weeks on a low-GL diet compared to a control diet lead to a greater reduction in fat mass (although the difference was small and not likely to be clinically meaningful), and there was a slightly greater reduction in fat-free mass in participants on the reduced-GL diet [31]. There was a significantly greater (albeit small) decrease in fat mass in a low-GI diet relative to a high-GI diet in overweight nondiabetic men [15]. Reduction in fat mass was significantly greater in the low-GI relative to the high-GL diet in a 10-week study of low- and high-GI diets in healthy overweight women [29].
This study had several strengths. In order to eliminate the effects of differences in other dietary components, the study diets were designed to be nearly equal in macronutrient content, especially in regard to dietary fiber. Many low-GI foods are high in soluble fiber (e.g., fruits and legumes), and soluble fiber potentially can affect many of the outcomes analyzed in this study (e.g., PAI-1 and blood lipids). Therefore, fiber potentially can confound any association between GI/GL and these outcomes, which statistical control may not eliminate [39]. Many previous studies either did not report fiber content of their study diets or made no attempt to equalize fiber intakes. Another strength of this study was that the low- and high-GI/GL diets were well characterized, with mean GI and GL reported for each day of each diet. In addition, study diets were provided to participants, offering some measure of control of dietary intake. Finally, body weight of participants was assessed during each visit to the GCRC to pick up meals. This allowed for any fluctuations in weight to be detected early and energy content of the diets to be adjusted to maintain body weight, preventing changes in body weight from potentially confounding results.
Limitations of the study include the relatively short dietary intervention period, the self-reporting of dietary compliance, and the small, all-male sample from which there was some attrition. It is possible that longer intervention periods may have allowed differences in the effects of the two diets on the various measures to become evident. Although strict compliance with the diets was universally reported by study participants, it is possible that some participants did not consume all of the study-provided foods and/or consumed non-study-provided foods but were hesitant to report this. This dietary noncompliance, especially if differential, could have biased the results. Finally, when considering the requirements of the study – the frequent visits to pick up study meals and the four required overnight GCRC visits – the attrition rate of 20% seems reasonable.
In summary, 4-week high- and low-GI/GL diets had little effect on glucose metabolism factors, inflammatory markers, or coagulation factors, and their effects on other risk factors were not consistent. However, future larger, longer-term studies of low-GI/GL diets in more diverse populations may be informative.
Acknowledgments
This study was supported by a grant from the General Mills Bell Institute of Health and Nutrition (Minneapolis, MN) and by Core facilities of the GCRC (M01-RR-00032), CNRC (P30-DK56336), and Diabetes Research and Training Center (P60-DK079626) at UAB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haasa N, et al. Heart disease and stroke statistics – 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Nesto R. C-reactive protein, its role in inflammation, Type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabetic Med. 2004;21:810–7. doi: 10.1111/j.1464-5491.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 3.Mavri A, Alessi MC, Juhan-Vague I. Hypofibrinolysis in the insulin resistance syndrome: implication in cardiovascular diseases. J Intern Med. 2004;255:448–56. doi: 10.1046/j.1365-2796.2003.01288.x. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–86. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109(25 Suppl 1):IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–7. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Manson J, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–8. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 9.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–11. doi: 10.2337/diacare.29.02.06.dc05-1903. [DOI] [PubMed] [Google Scholar]
- 10.Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, et al. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 2008;57:437–43. doi: 10.1016/j.metabol.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvi AE, Karlstrom BE, Granfeldt YE, Bjorck IE, Asp NL, Vessby BOH. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22:10–8. doi: 10.2337/diacare.22.1.10. [DOI] [PubMed] [Google Scholar]
- 12.Rizkalla SW, Taghrid L, Laromiguiere M, Huet D, Boillot J, Rigoir A, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men. Diabetes Care. 2004;27:1866–72. doi: 10.2337/diacare.27.8.1866. [DOI] [PubMed] [Google Scholar]
- 13.Wolever TMS, Gibbs AL, Mehling C, Chiasson JL, Connelly PW, Josse RG, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008;87:114–25. doi: 10.1093/ajcn/87.1.114. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins DJ, Wolever TM, Collier GR, Ocana A, Rao AV, Buckley G, et al. Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr. 1987;46:968–75. doi: 10.1093/ajcn/46.6.968. [DOI] [PubMed] [Google Scholar]
- 15.Bouche C, Rizkalla SW, Luo J, Vidal H, Veronese A, Pacher N, et al. Five-week, low-glycemic index diet decreases total fat mass and improves plasma lipid profile in moderately overweight nondiabetic men. Diabetes Care. 2002;25:822–8. doi: 10.2337/diacare.25.5.822. [DOI] [PubMed] [Google Scholar]
- 16.Frost GS, Brynes AE, Bovill-Taylor C, Dornhorst A. A prospective randomized trial to determine the efficacy of a low glycaemic index diet given in addition to healthy eating and weight loss advice in patients with coronary heart disease. Eur J Clin Nutr. 2004;58:121–7. doi: 10.1038/sj.ejcn.1601758. [DOI] [PubMed] [Google Scholar]
- 17.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institution; 1919. [Google Scholar]
- 18.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 19.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Schakel S, Sievert Y, Buzzard M. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–71. [PubMed] [Google Scholar]
- 21.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man. Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–67. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Computer Methods Programs Biomed. 1986;23:113–22. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 23.Yang YJ, Youn JH, Bergman RN. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol (Endocrinol Metab) 1987;253:E595–602. doi: 10.1152/ajpendo.1987.253.6.E595. [DOI] [PubMed] [Google Scholar]
- 24.Clauss A. A Gerrinnungs-physiologische schnell-methode zur bestimmung des fibrinogens. Acta Haematol. 1957;17:237. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Jones B, Kenward MG. Design and analysis of cross-over trials. 2nd. London: Chapman and Hall/CRC; 2003. [Google Scholar]
- 27.Hills M, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol. 1979;8:7–20. doi: 10.1111/j.1365-2125.1979.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittas AG, Roberts SB, Das SK, Gilhooly CH, Saltzman E, Golden J, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity. 2006;14:2200–9. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 29.Sloth B, Krog-Mikkelsen I, Flint A, Tetens I, Björk I, Vinoy S, et al. No difference in body weight decrease between a low-glycemic-index and a high-glycemic-index diet but reduced LDL cholesterol after 10-wk ad libitum intake of the low-glycemic-index diet. Am J Clin Nutr. 2004;80:337–47. doi: 10.1093/ajcn/80.2.337. [DOI] [PubMed] [Google Scholar]
- 30.McMillan-Price J, Petocz P, Atkinson F, O'Neill K, Samman S, Steinbeck K, et al. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166:1466–75. doi: 10.1001/archinte.166.14.1466. [DOI] [PubMed] [Google Scholar]
- 31.Maki KC, Rains TM, Kaden VN, Raneri KR, Davidson MH. Effects of a reduced-glycemic-load diet on body weight, body composition, and cardiovascular disease risk markers in overweight and obese adults. Am J Clin Nutr. 2007;85:724–34. doi: 10.1093/ajcn/85.3.724. [DOI] [PubMed] [Google Scholar]
- 32.Frost G, Leeds A, Trew G, Margara R, Dornhorst A. Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic diet. Metabolism. 1998;47:1245–51. doi: 10.1016/s0026-0495(98)90331-6. [DOI] [PubMed] [Google Scholar]
- 33.Motton DD, Keim NL, Tenorio FA, Horn WF, Rutledge JC. Postprandial monocyte activation in response to meals with high and low glycemic loads in overweight women. Am J Clin Nutr. 2007;85:60–5. doi: 10.1093/ajcn/85.1.60. [DOI] [PubMed] [Google Scholar]
- 34.Nordt TK, Peter K, Ruef J, Kubler W. Plasminogen activator inhibitor type-1 (PAI-1) and its role in cardiovascular disease. Thromb Haemost. 1999;82 1:14–8. [PubMed] [Google Scholar]
- 35.Jensen L, Slothe B, Krog-Mikkelsen I, Flint A, Raben A, Tholstrup T, et al. A low-glycemic-index diet reduces plasma plasminogen activator inhibitor-1 activity, but not tissue inhibitor of proteinases-1 or plasminogen activator inhibitor-1 protein, in overweight women. Am J Clin Nutr. 2008;87:97–105. doi: 10.1093/ajcn/87.1.97. [DOI] [PubMed] [Google Scholar]
- 36.Ebbeling CB, Leidig MM, Sinclair KB, Seger-Shippee LG, Feldman HA, Ludwig DS. Effects of an ad libitum low-glycemic load diet on cardiovascular disease risk factors in obese young adults. Am J Clin Nutr. 2005;81:976–82. doi: 10.1093/ajcn/81.5.976. [DOI] [PubMed] [Google Scholar]
- 37.De Rougemont A, Normand S, Nazare JA, Skilton MR, Sothier M, Vinoy S, et al. Beneficial effects of a 5-week low-glycaemic index regimen on weight control and cardiovascular risk factors in overweight non-diabetic subjects. Br J Nutr. 2007;98:1288–98. doi: 10.1017/S0007114507778674. [DOI] [PubMed] [Google Scholar]
- 38.Philippou E, McGowan BMC, Brynes AE, Dornhorst A, Leeds AR, Frost GS. The effect of a 12-week low glycaemic index diet on heart disease risk factors and 24 h glycaemic response in healthy middle-aged volunteers at risk of heart disease: a pilot study. Eur J Clin Nutr. 2008;62:145–9. doi: 10.1038/sj.ejcn.1602688. [DOI] [PubMed] [Google Scholar]
- 39.Pi-Sunyer FX. Glycemic index and disease. Am J Clin Nutr. 2002;76:290S–8S. doi: 10.1093/ajcn/76.1.264S. [DOI] [PubMed] [Google Scholar]