Abstract
The use of insulin pump therapy (continuous subcutaneous insulin infusion) has increased dramatically in youth with type 1 diabetes (T1D) in the past decade. In this review we provide background and practical clinical advice on insulin basal rates and bolus doses and on the advantages of pump therapy with exercise. Acute complications of T1D (hypoglycemia and diabetic ketoacidosis) in the context of pump therapy are reviewed. The advantages of pump therapy in the school setting and in hospitalized patients are discussed. Finally, diabetes management in the 21st century, in which pump therapy is combined with continuous glucose monitoring, and its potential for a closed-loop pancreas are presented.
Introduction
Insulin pump (continuous subcutaneous insulin infusion [CSII]) therapy became more widely accepted for youth with type 1 diabetes (T1D) in the mid-1990s after the availability of the rapid-acting insulin, insulin lispro. Previously, pediatric diabetologists were cautious about pump use in children, particularly as a result of the threefold increase in severe hypoglycemia reported among intensively treated patients in the Diabetes Control and Complications Trial.1 Of these, two-thirds used an insulin pump at some time, and all used regular insulin. With advances in insulin development and in pump features, however, the fear of severe hypoglycemia associated with intensive diabetes management has diminished.
It is impossible in this short article to describe all aspects of insulin pump care in youth (here defined as children 5–18 years of age) with diabetes. A more complete description, including our program for starting an insulin pump, is provided elsewhere.2 A consensus statement on insulin pump therapy in the pediatric age group may be helpful to healthcare providers.3 Table 1 lists some advantages and disadvantages of insulin pump use in children.
Table 1.
Advantages and Disadvantages of Insulin Pump Use in Children
| Advantages | Disadvantages |
|---|---|
| 1. Improved blood sugar control | 1. Remembering to give insulin boluses with food intake |
| 2. Insulin availability and convenience | 2. Ketonuria or ketoacidosis |
| 3. Use of multiple basal rates | 3. Psychological factors |
| 4. Use of temporary basal rates | 4. Expense |
| 5. Ease of administering multiple boluses | 5. Weight gain |
| 6. Reduction of hypoglycemia | 6. Skin infections |
| 7. Flexibility and freedom | 7. Insulin unavailability and instability |
| 8. Control of post-meal blood sugar/CGM values | 8. Infusion site locations and set changes |
| 9. Ease of adjusting insulin doses with exercise | 9. Physical/logistical considerations |
| 10. Ease of adjusting insulin doses with travel |
Adapted from Chase.2
Although insulin pump use is becoming more and more popular among families having a youth with T1D, it is not for everyone. In order for pump therapy to succeed, the youth as well as the parents must want the pump. Diabetes nurse-educators listed 12 other important factors to determine who should start pump therapy.4 They all agreed that doing adequate numbers of blood glucose (BG) tests per day was the most important criterion. Most pediatric centers require this for pump initiation, as this is not only a measure of compliance, but also a necessity for safety. Other criteria for initiation of insulin pump therapy in youth vary between centers. Rates of discontinuation of insulin pump therapy in youth, generally between 7% and 18% in the United States,5,6 may relate to the rigidity of criteria for initiating treatment.
Basal Insulin Rates
Basal insulin rates are important in controlling BG levels throughout the 24-h period. They are important in turning off glucose production by the liver and in preventing fat breakdown and ketogenesis. As only a rapid-acting insulin is used in the pump, a small dose is delivered approximately every 10 min, much as with human islet insulin production.7
Determining initial basal rates
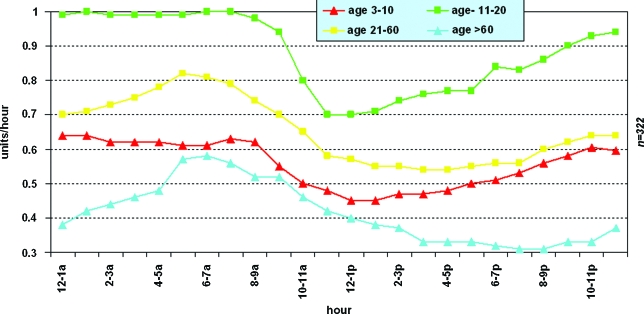
Hourly basal insulin rates vary for people of different ages, with the highest levels in adolescents8 (Fig. 1). This is presumably secondary to high levels of growth hormone and/or other counter-regulatory hormones. The total 24-h basal insulin dose is often determined using a slight reduction (10–20%) of the injected basal insulin (insulin glargine or insulin detemir). Other physicians calculate the average total daily dose of injectable insulin, reduce the total by 20–30%, and then give half of this dose as basal insulin.
FIG. 1.
Average hourly basal rate values by age group: age 11–20 years (dark squares), age 21–60 years (light squares), age 3–10 years (dark triangles), and age >60 years (light triangles). Reprinted with permission from Scheiner and Boyer.8 Color images available online at www.liebertonline.com/dia.
One of the advantages of insulin pump use in youth is the ability to administer multiple daily basal rates. In one study of youth using insulin pumps, use of more basal rates and younger age were the only two factors predictive of good glycemic control.9 Initially, slightly lower rates are often used during the night for safety. Generally, prepubertal children tend to have peak basal rates at 9–10 p.m., whereas pubertal patients have peak basal rates in the early morning hours.10 Following initiation of insulin pump therapy, we adjust basal rates on a daily basis as needed in the first week and then on a less frequent basis, focusing on identification of patterns. Basal rate changes will have their desired effect in 1–2 h, and therefore basal rate changes should be made with this understanding. In addition, higher or lower basal rates can be set on the alternate basal patterns and used on days previously shown to be related to longer periods of high or low BG levels (e.g., with exercise days, sick days, vacation days, menses, etc.).
Testing of basal rates
The first basal testing is usually done after hourly basal rates have stabilized. The method of doing this has been described previously.2 The nighttime/early-morning rates are evaluated first, as youth tend to vary their time of awakening. This is the time of greatest concern for parents and is the most important time to check. It is particularly important for college students who are often out of the home setting. It is helpful to have a parent or other person available to help test BG levels when breakfast is omitted and the person fasts (and often sleeps) until lunch. If BG levels are below or above range (usually 70–180 mg/dL), the condition is treated, the basal testing is discontinued, and the basal rates are adjusted accordingly. Further basal testing of this and other periods of the day can then be scheduled. Obviously, the use of continuous glucose monitoring (CGM) can be very helpful in facilitating testing of basal rates.
Temporary basal rates
Another advantage of using an insulin pump in youth is the ability to use temporary basal rates. We recently evaluated factors related to improvement in hemoglobin A1c (HbA1c) levels in youth using an insulin pump.11 The more frequent use of temporary basal rates was the number one factor relating to improvement in the HbA1c level. (Admittedly, this may just be a reflection of paying closer attention to the person's diabetes.) Short-term temporary basal rates for exercise and for hypoglycemia are very important and are discussed in the respective sections below. Similarly, the loss of basal rate insulin (e.g., dislodging/plugging of the infusion cannula) is discussed below under ketoacidosis.
Bolus Insulin Dosages
In addition to the use of basal insulin as discussed above, CSII therapy allows users to conveniently administer bolus insulin to control postprandial BG levels and to correct elevated BG levels. Danne et al.12 demonstrated that patients taking more of their daily insulin as boluses had significantly lower HbA1c levels than patients taking fewer boluses. Fortunately, most insulin pumps include a “smart pump” feature, which helps the user to calculate insulin bolus dosages based on a preset insulin-to-carbohydrate (I/C) ratio and a correction factor and calculates the insulin on-board from previous boluses. The user then enters the current BG value and the amount of carbohydrate to be consumed. He or she may then accept the recommended insulin dosage or alter it as desired. Special note should be made that different pumps use slightly different algorithms to calculate doses and that these differences can be a clinically significant issue.13
Bolus dosing for food
For patients already using injections of rapid-acting insulin before meals and carbohydrate counting, the pump bolus dosages for meals should be close to the previous insulin doses. The “Rule of 500” is useful for estimating the I/C ratio when previous values have not been determined. This involves determining the total insulin dose for the day and dividing this number into 500. Pump users can then fine-tune their I/C ratios for different times of the day to account for variations in insulin sensitivity. Some types of food (e.g., high fat, high carbohydrate) affect the postprandial glycemic profile differently and will not be well covered by a single dose of rapid-acting insulin. The insulin pump allows for special boluses (e.g., “extended combination bolus”) to accommodate these meals, with part of the insulin given as an immediate bolus and the other part over an extended time period.2,14
Individuals using CGM to monitor their glucose values may be able to look at trend graphs to evaluate bolus dosing. Others must evaluate the I/C ratio by testing the BG level 2 and 4 h after the meal. If the doses are set correctly, the postprandial glucose level should be within the American Diabetes Association recommended range of <180 mg/dL (<10 mmol/L).15
Timing of food boluses
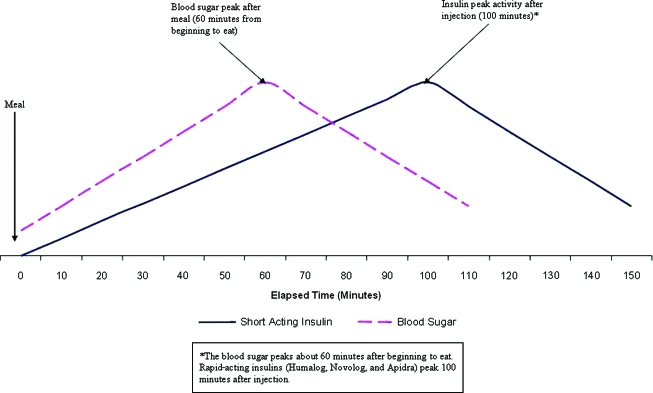
Although the availability of rapid-acting insulin analogs has contributed to improved control of postprandial glucose levels, these insulins peak approximately 90–100 min after administration (Fig. 2). Glucose levels, by contrast, peak approximately 60 min after food is consumed, although this will vary depending on macronutrient and fiber content. Cobry et al.16 have demonstrated that, because of this time discrepancy, pre-bolusing 20 min prior to a meal significantly reduces postprandial glucose excursions. It is advisable when the BG is >70 mg/dL for most patients with T1D to administer insulin boluses 20 min before eating. Insulin pump therapy facilitates the administration of multiple bolus insulin doses, which helps to make this goal more attainable. For young children, whose food intake may be unpredictable, a portion of the bolus can be given 20 min before eating to correct for any elevation in the BG level above target and to cover the carbohydrates that are sure to be consumed. Then, after the meal, a second bolus to cover any additional carbohydrates can easily be administered (without having to give another injection).
FIG. 2.
Blood sugar and insulin peaks. *The blood sugar peaks about 60 min after beginning to eat. Rapid-acting insulins peak 100 min after injection. Adapted from Chase.2 Color images available online at www.liebertonline.com/dia.
Missed boluses for food
Missing boluses for meals has been shown to contribute significantly to elevated HbA1c levels in children using CSII therapy.17 Cited as the most common reason for missing boluses, “forgetting” to bolus is a significant challenge for children and adolescents using insulin pumps. Even missing a bolus for afternoon snacks leads to higher and longer glycemic excursions.18 Pediatric care providers must emphasize the importance of bolusing for all carbohydrate intake in order to attain optimal glycemic control.
Bolus dosing for correction of hyperglycemia
For treatment of hyperglycemia, the individual's correction factor (sensitivity factor) must also be set to determine how much each unit of insulin will decrease the BG level. If unknown, this can be estimated by dividing the total daily insulin dose into 1,500 for insulin-resistant people or 1,700–2,000 for insulin-sensitive people.2 To evaluate the efficacy of the correction factor, the BG level should be tested 2–4 h after a correction dose is given. If the dosing is set correctly and other factors known to affect BG have been avoided, the value should have returned to the “target level” in this time. Moreover, we generally recommend patients begin by setting the duration of insulin action at 3 h with subsequent adjustments based on the desire to be more or less aggressive with correction doses.
Exercise and Insulin Pumps
Hypoglycemia incidence with exercise in T1D
Exercise is important for all youth, particularly in relation to body weight and cardiovascular health. One of the greatest advantages of insulin pump therapy in youth with T1D relates to the reduction of hypoglycemia during and after exercise.
The DirecNet study group used a standardized 60-min afternoon treadmill session, during which 11 of 50 youths had low BG values (<60 mg/dL).19 When these youths were followed during the night following the exercise day, 48% had low BG values. The same 50 children were followed after a different (randomized) non-exercise day, and only 28% experienced nocturnal hypoglycemia. Nocturnal hypoglycemia was more frequent during the night when the pre-bedtime BG was ≤130 mg/dL. Clearly, hypoglycemia during and after exercise continues to be a challenge in T1D, for which pump therapy provides therapeutic flexibility.
Physiology of exercise-induced hypoglycemia in youth with T1D
The physiologic mechanism of exercise-induced hypoglycemia in youth with T1D likely relates to three major factors:
Exercise enhances insulin sensitivity.
Insulin production diminishes to very low levels during strenuous exercise in people who do not have diabetes. In contrast, youth with T1D who have not reduced previously administered insulin (by shot or insulin pump) have relative hyperinsulinemia during exercise.
Youth with diabetes have diminished recognition of hypoglycemia and diminished production of counter-regulatory hormones.20,21
Prevention of hypoglycemia with exercise
Using a randomized crossover design, 49 children, 8–17 years old, using insulin pump therapy, used the same DirecNet structured exercise session referred to above on two different days.22 On a day in which the insulin was stopped during the exercise, 16% of the youths had BG values <70 mg/dL. On the other day, the insulin was continued, and 43% of subjects experienced hypoglycemia (P = 0.003). Clearly, the ability to diminish insulin levels during heavy afternoon exercise is easily possible with an insulin pump and provides a great advantage in reducing hypoglycemia during exercise. When the exercise is less strenuous, a lesser reduction (e.g., a 50% temporary basal rate) may be all that is needed. For some youth, the initial reduction in insulin may need to occur 15, 30, or 60 min prior to the exercise. This is because the current rapid-acting insulins peak approximately 95 min post-administration. In addition, if the exercise is 1–2 h after an insulin dose, a 15–30-g carbohydrate snack prior to the exercise may help to prevent hypoglycemia. Trial and error can be used to find the optimal management for a given individual and a given exercise, but requires additional BG monitoring.
Some youth experience delayed hypoglycemia during the night following days of heavy exercise. It has been shown that use of a 20% temporary basal rate reduction from 9 p.m. to 3 a.m. (in addition to discontinuing the insulin dose during the exercise) can be very helpful in preventing the nocturnal hypoglycemia.23
In summary, using the pump shutoff feature or temporary basal reduction can be very effective in decreasing hypoglycemia, both during exercise and when it occurs during the night following exercise. Similar modifications are more difficult with injection therapy. Additionally, as with making any changes in pump therapy, adjustments are often more successfully made based on repeat patterns rather than on single occurrences. A diligent person/family will find what works best for a given situation.
Acute Complications and Insulin Pump Use
Hypoglycemia
The fear of hypoglycemia is the number one fear among families having a child with T1D. It is often the limiting factor in the goal of attaining optimal glycemic control. Hypoglycemia prevention in youth is discussed in the above section on exercise and in the insulin pump and CGM section below. Almost every study, including one from our center,5 has reported fewer severe hypoglycemic events in the period of pump use compared to pre-pump therapy. This is likely in part related to elimination of insulins (e.g., NPH) that peak during the night when food is not being eaten and that vary in the timing of peak activity.
There is also the advantage of being able to “fine-tune” the basal insulin delivery to fit the individual child, with as many as 48 different basal rates possible in a day (although most youth use eight or less). Basal-rate checking was discussed earlier, and it is important to be certain rates are set correctly. Likewise, the use of temporary basal rates is important in “thinking ahead” to prevent hypoglycemia. Although exercise is the most obvious indication, reducing basal rates on a day with unexplained low glucose levels, less food intake, or sudden onset of an illness (e.g., vomiting, diarrhea) can be very beneficial. Obviously, with acute situations, it is not possible for youth using injection therapy to reduce the dose of long-acting insulin given earlier in the day or the previous night.
Diabetic ketoacidosis (DKA)
The incidence of DKA in youth using insulin pumps is lower than in non-pump users, although the characteristics of patients on each type of treatment may explain some of this difference. The incidence for all subjects in our clinic (injections or insulin pumps) was 8/100 patient-years.24 The incidence was significantly higher in subjects with underinsurance and associated problems. In contrast, the incidence of DKA among youths using an insulin pump at our clinic was 3.98/100 patient-years.5 Very few pump users have underinsurance (due to the high cost of insulin pump therapy). Thus, pump users are a select group, which may contribute to the decreased incidence of DKA in this population. The primary reason for episodes of DKA in youth using pumps relates to the loss of the rapid-acting insulin infusion due to the cannula dislodging or plugging. The use of a CGM can be helpful in providing an early warning of hyperglycemia and impending DKA.
Insulin Pump Use in the School Setting
Because youth may spend more of their awake time in a school setting than they do at home, safety while at school is very important and necessitates some level of education about insulin pumps (as well as about overall diabetes) for school personnel. This is especially true for younger children who may require supervision with pump data entry for carbohydrate intake or a BG level, as well as with insulin administration. In addition, someone in the school setting must have the ability to recognize and treat acute emergencies such as hypoglycemia and hyperglycemia with ketones. Table 2 outlines some of the issues that will need to be dealt with by the school.
Table 2.
Diabetes Care Topics to be Discussed in the School Setting
| Blood sugar testing—where and when to test |
| Record keeping |
| Management of hypoglycemia |
| • Glucagon injections |
| • Disconnecting from the pump |
| • Treatment of mild, moderate, and severe lows |
| Management of hyperglycemia |
| • Ketone testing |
| • Giving correction boluses using the pump |
| • Giving insulin injections |
| • Changing pump sites—where and when |
| Carbohydrate counting—who will decide insulin dosing for lunch and snacks |
| Exercise—when to adjust insulin and how to monitor |
| Safeguarding the pump when it is disconnected |
Adapted from Chase.2
Most states in the United States now require a school health plan for youth with T1D (see example in Chase2). No written plan can cover all possibilities, nor is a plan meant to override parental decisions or to place unwarranted burdens upon schools. A cooperative approach best serves the interests of the student and family, as well as of the school. Good communication and education are the keys to successful insulin pump use in the school setting. The American Diabetes Association has developed suggestions for care of children with diabetes in the school setting (www.diabetes.org), which may be helpful.
Insulin Pump Use in Hospitalized Patients
Several publications have shown the importance of aggressive diabetes management in patients hospitalized with serious medical conditions.25–28 Meticulous glycemic control in the hospital setting was initially reported to improve both morbidity and mortality in adults,24–28 although data for youth are limited.29 More recent studies30,31 have not shown a reduction in mortality as a result of intensive insulin therapy. Hypoglycemia is a concern and was clearly increased with intensive therapy,30,31 although the use of CGM (intravenous or subcutaneous) may help to circumvent this problem. Subcutaneous protocols have also been developed that may have value in specific circumstances.26,27
What then is the role of insulin pump use and subcutaneous insulin administration in youth with T1D who are hospitalized? We have previously advocated for people with T1D being allowed to continue to use their insulin pumps for elective and non-acute hospitalizations.32 If basal insulin rates are correctly set, fasting for elective surgical procedures is usually uneventful. However, some hospitals insist on pump removal, particularly for surgical procedures. The dose of injected insulin is then often calculated using outdated methods (e.g., sliding scales), resulting in variable glucose levels. Administration of intravenous insulin is frequently used in surgery and intensive care settings. Frequent BG monitoring (and/or use of CGM) then provides safe control of insulin delivery. Omission of insulin can result in hyperglycemia and is obviously dangerous. Unfortunately, research studies have not been done to compare outcomes using insulin pumps in non-acute hospitalizations.
Insulin Pump and CGM Use
At present, the combination of using an insulin pump together with CGM represents the optimal form of diabetes management, although challenges exist as with any new technology. Use of CGM, with warnings for high and low glucose levels, helps to maximize insulin pump benefits. A detailed description of how to use CGM data is provided elsewhere.2 As with pump initiation, it is crucial that the youth as well as the parents be committed to using CGM. If CGM is not consistently used at least 6 days a week, full benefit and HbA1c lowering are not likely to be achieved.33
There are advantages and disadvantages to implementing both devices in youth. The youth must wear two devices, which requires the use of two insertion sites. For children and adolescents, many of whom are sensitive about their diabetes, having multiple devices attached to the body can be a deterrent. Furthermore, some youth/families struggle with the vast quantities of information provided. Parents of youth must be careful not to allow the huge amounts of information from the CGM and insulin pump to negatively impact the child's normal life.
For youth who are unwilling to do frequent BG testing, CGM provides more complete information about the glucose patterns. However, youth are often disappointed when they learn that wearing a CGM device does not eliminate the need for fingerstick BG testing. The two types of data provided by CGM include real-time and retrospective data (Table 3). Both can be useful to youth/families with T1D. Using real-time CGM data, including trend arrows and alarms, the adjustment of bolus dosing and implementation of temporary basal rates may help to minimize glucose variability and to avoid hypoglycemic and hyperglycemic events. For youth who sleep through alarms during the night, many parents use a baby monitor placed near the CGM receiver to hear alarms themselves and to help the youth respond appropriately.
Table 3.
Real-Time and Retrospective CGM Data
| CGM data | Information gained |
|---|---|
| Real-Time | |
| 1. Real-time sensor glucose values | Real-time glucose values every 1–5 min |
| 2. Real-time trend graphs | Direction of change in glucose values |
| 3. Real-time trend arrows | Rate of change in glucose values |
| 4. Real-time alarms | (1) Threshold alarms indicate when a high or low glucose value has been reached |
| (2) Prediction alarms indicate that a high or low glucose value is likely to be reached | |
| (3) Rate of change alarms indicate when glucose levels are changing rapidly | |
| Retrospective | |
| 1. Trend graphs (Modal Day graphs) | Glucose values for previous hours or days |
| 2. Data table report | (1) Average glucose values |
| (2) SD values and highest and lowest glucose values | |
| (3) Percentage of glucose values above, below, and within range for different periods of the day | |
| 3. Glucose pie charts | Visual view of glucose values above, below, or within range for different periods of the day |
| 4. Events (if entered) | Data related to: |
| (1) Insulin | |
| (2) Meals | |
| (3) Exercise | |
| (4) Other |
Adapted from Chase.2
If the family will set a time once weekly to review the CGM data, events causing high glucose levels (e.g., missed insulin boluses for food) or low glucose levels can be evaluated, and a plan can be developed for future prevention. CGM may help the family to identify how different activities (sports, gym, sleepovers, etc.) impact glucose levels and to adjust accordingly. Education at a knowledgeable clinical center is a necessary component of CGM and insulin pump therapy, especially to enable users to make insulin adjustments in the home setting. Overall, the combination of an insulin pump and a CGM device provides many youths with T1D the best opportunity to achieve and/or maintain optimal glycemic control.
In conclusion, tremendous progress has been made in insulin pump therapy in recent decades. More extensive reviews of the clinical application of CSII are available.2 Insulin pump use has continued to increase in youth with T1D, and in the future, CSII combined with CGM holds promise for an artificial pancreas.
Acknowledgments
D.M.M. was supported in part by grant K23 DK075360 from the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Chase HP. Understanding Insulin Pumps and Continuous Glucose Monitors. 2nd. Denver: Children's Diabetes Foundation; 2010. [Feb;2010 ]. [Google Scholar]
- 3.Phillip M. Battelino T. Rodriguez H. Danne T. Kaufman F. European Society for Paediatric Endocrinology; Lawson Wilkins Pediatric Endocrine Society; International Society for Pediatric and Adolescent Diabetes; American Diabetes Association; European Association for the Study of Diabetes: Use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30:1653–1662. doi: 10.2337/dc07-9922. [DOI] [PubMed] [Google Scholar]
- 4.Lenhard MJ. Patient selection criteria for continuous subcutaneous insulin infusion (CSII) Infusystems USA. 2006;3(1):1–4. [Google Scholar]
- 5.Scrimgeour L. Cobry E. McFann K. Burdick P. Weimer C. Slover R. Chase HP. Improved glycemic control after long-term insulin pump use in pediatric patients with type 1 diabetes. Diabetes Technol Ther. 2007;9:421–428. doi: 10.1089/dia.2007.0214. [DOI] [PubMed] [Google Scholar]
- 6.Wood JR. Moreland EC. Volkening LK. Svoren BM. Butler DA. Laffel LM. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care. 2006;29:2355–2360. doi: 10.2337/dc06-1141. [DOI] [PubMed] [Google Scholar]
- 7.Porksen N. Hollingdal M. Juhl C. Butler P. Veldhuis JD. Schmitz O. Pulsatile insulin secretion: detection, regulation, role in diabetes. Diabetes. 2002 Feb;51(Suppl 1):S245–S254. doi: 10.2337/diabetes.51.2007.s245. [DOI] [PubMed] [Google Scholar]
- 8.Scheiner G. Boyer BA. Characteristics of basal insulin requirements by age and gender in Type-1 diabetes patients using insulin pump therapy. Diabetes Res Clin Pract. 2005;69:14–21. doi: 10.1016/j.diabres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Nabhan ZM. Rardin L. Meier J. Eugster EA. Dimeglio LA. Predictors of glycemic control on insulin pump therapy in children and adolescents with type I diabetes. Diabetes Res Clin Pract. 2006;74:217–221. doi: 10.1016/j.diabres.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Holterhus PM. Odendahl R. Oesingmann S. Lepler R. Wagner V. Hiort O. Holl R. Classification of distinct baseline insulin infusion patterns in children and adolescents with type 1 diabetes on continuous subcutaneous insulin infusion therapy. Diabetes Care. 2007;30:568–573. doi: 10.2337/dc06-2105. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson J. Chase HP. McFann K. Factors affecting improved glycemic control in youth using insulin pumps [abstract 1788-P] Diabetes. 2009;58(Suppl 1):A459. doi: 10.1111/j.1464-5491.2010.03068.x. [DOI] [PubMed] [Google Scholar]
- 12.Danne T. Battelino T. Jarosz-Chobot P. Kordonouri O. Pankowska E. Ludvigsson J. Schober E. Kaprio E. Saukkonen T. Nicolino M. Tubiana-Rufi N. Klinkert C. Haberland H. Vazeou A. Madacsy L. Zangen D. Cherubini V. Rabbone I. Toni S. de Beaufort C. Bakker-van Waarde. W van den. Berg N. Volkov I. Barrio R. Hanas R. Zumsteg U. Kuhlmann B. Aebi C. Schumacher U. Gschwend S. Hindmarsh P. Torres M. Shehadeh N. Phillip M. PedPump Study Group: Establishing glycaemic control with continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes: experience of the PedPump Study in 17 countries. Diabetologia. 2008;51:1594–1601. doi: 10.1007/s00125-008-1072-2. [DOI] [PubMed] [Google Scholar]
- 13.Zisser H. Robinson L. Bevier W. Dassau E. Ellingsen C. Doyle FJ. Jovanovic L. Bolus calculator: a review of four “smart” insulin pumps. Diabetes Technol Ther. 2008;10:441–444. doi: 10.1089/dia.2007.0284. [DOI] [PubMed] [Google Scholar]
- 14.Chase HP. Saib SZ. Mackenzie T. Hansen MM. Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19:317–321. doi: 10.1046/j.1464-5491.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 15.Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobry E. Messer L. VanderWel B. Chase HP. Timing of meal insulin boluses to achieve optimal postprandial glycemic control. Diabetes Technol Ther. 2010;12:173–177. doi: 10.1089/dia.2009.0112. [DOI] [PubMed] [Google Scholar]
- 17.Burdick J. Chase HP. Slover RH. Knievel K. Scrimgeour L. Maniatis AK. Klingensmith GJ. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113:e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 18.VanderWel B. Messer L. Horton L. McNair B. Cobry E. Chase HP. Missed insulin boluses for snacks in youth with type 1 diabetes. Diabetes Care. 2010;33:507–508. doi: 10.2337/dc09-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Diabetes Research in Children Network (DirecNet) Study Group: Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147:528–534. doi: 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Diabetes Research in Children (DirecNet) Study Group: Impaired overnight counterregulatory hormone responses to spontaneous hypoglycemia in children with type 1 diabetes. Pediatr Diabetes. 2007;8:199–205. doi: 10.1111/j.1399-5448.2007.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Diabetes Research in Children (DirecNet) Study Group: Blunted counter-regulatory hormone responses to hypoglycemia in young children and adolescents with well-controlled type 1 diabetes. Diabetes Care. 2009;32:1954–1959. doi: 10.2337/dc08-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Diabetes Research in Children Network (DirecNet) Study Group: Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29:2200–2204. doi: 10.2337/dc06-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taplin CE. Cobry E. Messer L. VanderWel B. McFann K. Chase HP. Fiallo-Scharer R. Prevention of post-exercise nocturnal hypoglycemia [abstract 2119-PO] Diabetes. 2009;58(Suppl 1):A546. [Google Scholar]
- 24.Rewers A. Chase HP. Mackenzie T. Walravens P. Roback M. Rewers M. Hamman RF. Klingensmith G. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287:2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- 25.Bode BW. Braithwaite SS. Steed RD. Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10(Suppl 2):71–80. doi: 10.4158/EP.10.S2.71. [DOI] [PubMed] [Google Scholar]
- 26.Moghissi ES. Hirsch IB. Hospital management of diabetes. Endocrinol Metab Clin North Am. 2005;34:99–116. doi: 10.1016/j.ecl.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Trence DL. Kelly JL. Hirsch IB. The rationale and management of hyperglycemia for in-patients with cardiovascular disease: time for change. J Clin Endocrinol Metab. 2003;88:2430–2437. doi: 10.1210/jc.2003-030347. [DOI] [PubMed] [Google Scholar]
- 28.van den Berghe G. Wouters P. Weekers F. Verwaest C. Bruyninckx F. Schetz M. Vlasselaers D. Ferdinande P. Lauwers P. Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 29.Wintergerst KA. Deiss D. Buckingham B. Cantwell M. Kache S. Agarwal S. Wilson DM. Steil G. Glucose control in pediatric intensive care unit patients using an insulin-glucose algorithm. Diabetes Technol Ther. 2007;9:211–222. doi: 10.1089/dia.2006.0031. [DOI] [PubMed] [Google Scholar]
- 30.Finfer S. Chittock DR. Su SY. Blair D. Foster D. Dhingra V. Bellomo R. Cook D. Dodek P. Henderson WR. Hebert PC. Heritier S. Heyland DK. McArthur C. McDonald E. Mitchell I. Myburgh JA. Norton R. Potter J. Robinson BG. Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 31.Griesdale DE. de Souza RJ. van Dam RM. Heyland DK. Cook DJ. Malhotra A. Dhaliwal R. Henderson WR. Chittock DR. Finfer S. Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chase HP. Don't take away my pump! Switching to injections may cause problems. Diabetes Forecast. 2005;58:55–56. [PubMed] [Google Scholar]
- 33.The Juvenile Diabetes Research Foundation (JDRF) Continuous Glucose Monitoring Study Group: Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]