Summation
The identification of tumor tissue biomarkers has led to the production, validation, and Food and Drug Administration-approval of a number of antibody-based targeted therapeutics in the past two decades. As a result of the significant role that these immunotherapeutics play in the management of cancer, and the potential utility of complementary imaging agents, immunoPET imaging has generated considerable interest. This update discusses the important factors to consider when designing a PET (positron emission tomography) imaging agent from the molecular target to the biological targeting molecule and radionuclide combination and also reviews recent preclinical and clinical findings in the immunoPET field. Although there are a variety of radionuclides that are currently utilized in PET studies, this update focuses on four of the positron emitters commonly used in labeling proteins: iodine-124, zirconium-89, copper-64, and fluorine-18. Notable advances in the preclinical setting include the continued development of immunoPET probes to predict the biodistribution of related radioimmunotherapeutics, the success of nontraditional radionuclide and antibody fragment combinations, the broader use of zirconium-89, and the recent emergence of 18F-labeled diabodies for same-day imaging. Antibody-based PET probes constitute a valuable class of molecular imaging agents, and the progress made preclinically should expedite the transition of these targeted diagnostics to clinical applications.
Key words: : antibody, cancer, molecular imaging, positron emission tomography (PET), radionuclide
Introduction
Antibodies that bind specifically to surface markers on tumor cells have formed the basis of an important class of molecularly targeted therapies. Antibody therapeutics, including unmodified, biologically active chimeric and/or human antibodies, as well as drug- and radionuclide-immunoconjugates, represent a multibillion-dollar market, with many additional candidates in development. Early on, the value of conjugation to “imaging” radionuclides for the detection and imaging of cancer and other diseases was also recognized, leading to approved antibody-based imaging agents. More recently, it has become clear that antibody-based imaging for determining the molecular “phenotype” of tumors and tissues in a noninvasive manner can play an important role in the development, evaluation, and implementation of molecularly targeted therapeutics. For example, imaging agents for noninvasive measuring of target levels in individuals have the potential to be helpful in selecting patients who are likely to respond to a given therapeutic, determining proper dosage, and assessing treatment response. In addition, such agents can also aid in initial tumor detection and provide information on the tissue of origin or biological potential of the lesion. Antibody specificity, coupled with the sensitivity and resolution offered by positron emission tomography (PET), has resulted in a growing immunoPET field. This update will discuss the factors that should be considered when designing an immunoPET agent, including the nature of the target to be imaged, the biological targeting molecule, and the positron-emitting radionuclide (Fig. 1) and also review novel probes that have been recently evaluated in the preclinical or clinical setting.
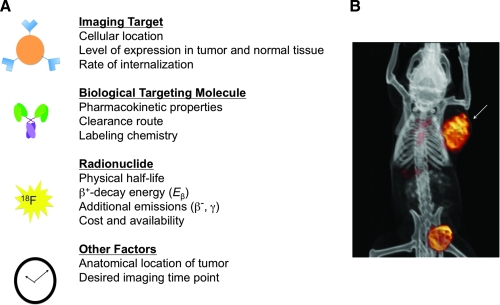
FIG. 1.
(A) Factors to consider when designing an immunoPET tracer. (B) Co-registered microPET and computed tomography images of a mouse bearing a subcutaneous PSCA+ LAPC-9 human prostate cancer xenograft (arrow) at 20 hours following intravenous injection of an 124I-labeled anti-PSCA minibody. Clearance of activity via the bladder is apparent. Image courtesy of Dr. Jeffrey V. Leyton. PET, positron emission tomography; PSCA, prostate stem cell antigen.
Imaging Target
Efforts continue to be directed toward genomic and proteomic analysis of normal and cancerous cells, resulting in an expanding list of biomarkers that are differentially expressed in the diseased state. Although these biomarkers could undoubtedly provide useful information in diagnostic and/or therapeutic applications, they must also satisfy additional criteria to be considered potential candidates for PET imaging. Ideally, the imaging target should be located on the cell surface so that it can be easily accessed by the PET probe. It should also be expressed at a high level on tumor tissue and not expressed at all or expressed at a low level on normal tissue. This will result in a signal intensity and target-to-background signal ratio that are both high enough to visualize the tumor on the image, while minimizing normal tissue exposure to the radionuclide. Likewise, cell surface proteins that undergo cleavage and are shed into the circulation do not make good molecular imaging targets. Noninternalizing targets are preferable, to avoid metabolism of the probe and possible loss of the radionuclide from the target cell upon ligand-induced receptor-mediated endocytosis, but more stable (residualizing) labeling methods are also available, which make imaging of internalizing targets possible as well. For example, conjugation of 64Cu via chelators such as 1,4,8,11-tetraazacyclotetradecane-N,N′,N′′,N′′′-tetraacetic acid and 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA),1 or 124I labeling using N-succinimidyl 4-iodobenzoate,2 radio-iodinated dilactitol-tyramine,3 or a radio-iodinated diethylenetriaminepentaacetic acid-appended peptide4 result in residualization of the radionuclide in the target cell.
Despite the growing list of tumor biomarkers that could serve as imaging targets, the majority of immunoPET agents in development are against well-established cell-surface molecules. Some of these molecules, such as human epidermal growth factor receptor-2 (HER2), epidermal growth factor receptor (EGFR), and CD20, are targets of Food and Drug Administration (FDA)-approved antibody-based therapeutics (trastuzumab, cetuximab, and rituximab, respectively).5 For other commonly targeted molecules, such as carcinoembryonic antigen (CEA) and prostate-specific membrane antigen (PSMA), FDA-approved imaging agents exist (Tc-99m arcitumomab [CEA-Scan] and In-111 capromab pendetide [ProstaScint], respectively),5 which could be improved upon; CEA-Scan is no longer commercially available,5 and single photon emission computed tomography (SPECT) imaging with the gamma ray-emitting ProstaScint does not produce the high-resolution images that PET is capable of generating. Further, ProstaScint is unable to image bone metastases.5
Biological Targeting Molecule
As previously mentioned, high-contrast images are produced as a result of high tracer uptake by the tumor, and lower tracer levels in normal organs and in the blood. When a PET tracer is injected intravenously, it travels through the circulatory system, coming into contact with various tissues. Assuming it is stable, the radioimmunoconjugate will bind specifically to cells that express the target of interest and will accumulate in the tumor and clear from the blood with kinetics that are characteristic of the biological targeting molecule. With a molecular weight of ∼150 kDa, intact antibodies will reside in the circulation for 1–3 weeks.6 Intact antibodies are well suited for targeted therapy because their long residence in the circulation allows for longer intervals between dosing. Not surprisingly, all commercially available, FDA-approved antibody-based therapeutics consist of intact antibodies.5 For immunoPET imaging, a targeting molecule with a long biological half-life usually requires scanning of a patient days after injection of the tracer. Disadvantages of this constraint include the need for an additional patient appointment, as well as the unnecessary exposure to radiation experienced by people in close proximity to the patient. Earlier scanning can be performed with smaller antibody fragments that retain the binding specificity of the parental antibody but are genetically engineered to have different pharmacokinetic properties, including tumor uptake and blood clearance.
The smallest fragment that retains the original binding site of the intact antibody is the single-chain variable fragment (scFv; 25–27 kDa), which consists of a variable heavy domain and a variable light domain joined by an amino acid linker. As a result of their monovalency and small size, scFvs clear quickly from the bloodstream (t1/2 β = 0.5–2 hours) and undergo low uptake by the tumor.6 Bivalent diabodies (noncovalent scFv dimers; 55 kDa) clear less rapidly than scFvs from the bloodstream (t1/2 β = 3–7 hours) and experience higher tumor uptake because of their increased avidity.6 Minibodies (noncovalent scFv-CH3 dimers; 80 kDa) clear less quickly (t1/2 β = 6–11 hours) because of their greater molecular weight and the presence of a constant domain, and accumulate at even higher levels in tumors.6
Recently, parallel efforts have been focused on developing nonimmunoglobulin protein scaffolds for a variety of applications, including imaging studies. These scaffolds are generally relatively small and highly stable.7 Of all the reported scaffolds, affibodies have shown the most promise as novel targeting molecules for immunoPET applications thus far. Affibody molecules are high-affinity, low-molecular-weight proteins that are derived from a domain of protein A.7 The affibody's small size results in rapid clearance from the blood, which, when combined with its high affinity, makes this protein well suited for same-day imaging.
There are several factors that must be taken into account when determining which of these biological targeting molecules is most appropriate for a particular immunoPET application. To begin with, the anatomical location of the tumor to be detected and observed needs to be considered. With the glomerular filtration threshold at ∼60–70 kDa, larger proteins (e.g., intact antibodies, minibodies) will clear through the liver, whereas smaller proteins (e.g., diabodies, scFvs, affibodies) will clear through the kidneys. The clearance route of the probe is of importance when attempting to image hepatic or renal carcinoma, or malignancies that are in the vicinity of these organs. The desirable amount of time between tracer injection and PET scan also affects selection of the format, as the residence time in the bloodstream of the probe dictates when high tumor-to-blood ratios can be attained and, therefore, high-contrast images can be produced. Quality images can typically be acquired no earlier than the day after tracer injection for minibody and intact antibody probes, whereas same-day imaging can be conducted with the smaller antibody fragments and affibodies. The radionuclide being conjugated as well as the nature of the conjugation (random vs. site-specific) also influences targeting molecule selection. Shorter-lived positron emitters are better paired with smaller molecules that have shorter biological half-lives, whereas longer-lived radioisotopes are better suited for coupling to larger molecules that have longer biological half-lives. These combinations, as well as alternative pairings, will be discussed in greater detail in the next section. Finally, random radiolabeling can often affect the immunoreactivity of the probe if the radionuclide (or chelator, in the case of indirect labeling with radiometals) is conjugated to a residue in the antigen-binding site of the molecule. Generally, smaller species have a greater chance of being adversely affected by random radiolabeling because a greater percentage of their amino acids are involved in target recognition. If a high immunoreactive fraction cannot be attained with a given protein, greater success may be achieved through reintroduction of constant domains. Alternatively, introduction of a cysteine residue in the smaller affibodies and diabodies (producing cysteine-modified diabodies) that is away from the antigen recognition site allows for site-specific labeling that should not interfere with the binding activity of the radioimmunoconjugate.
Radionuclide
To function as an immunoPET probe, the biological targeting molecule must be labeled with a positron (β+)-emitting radionuclide. The physical properties and availability of the radionuclide, biological half-life of the targeting species, type of molecular target (internalizing vs. noninternalizing), and location of the tumor all have some bearing on deciding which positron emitter is optimal. One radionuclide may have a well-matched physical half-life for the targeting molecule of interest, but its labeling may result in an immunoreactive fraction that is too small to be of use. Likewise, a radionuclide may be most appropriate for imaging a given internalizing target but not be useful for imaging a tumor in a particular anatomical location. It is therefore important to consider all of the above factors collectively when choosing a radionuclide for an imaging application.
The most common radionuclides currently utilized in preclinical and clinical PET studies (and those which will be the focus of this update), along with some of their physical properties, are listed in Table 1.2 As mentioned in the preceding section, the physical half-life (t1/2) of the radionuclide should be matched to the biological half-life of the protein to which it is being conjugated. For example, the short-lived (t1/2 = 110 minutes) 18F pairs well with affibodies and diabodies, whereas the longer-lived (t1/2 = 100.2 hours) 124I is typically more appropriate for intact antibodies. Success has been seen with unconventional pairings, however, resulting in many comparison studies involving different combinations of radionuclides and targeting molecules. Other physical properties that are significant are the energies associated with the β+-decay (Eβ) and gamma emissions (Eγ), if applicable. High β+-decay energy will result in poor-resolution images, whereas high Eγ requires patient isolation.5 Additional factors to consider when choosing a radionuclide include cost and accessibility. Because 18F is the most commonly used positron emitter in the clinical setting, it is often readily available. Because of its short half-life, however, 18F is not a feasible option if the cyclotron is not on the same site as or close to the radiolabeling and imaging facilities. Longer-lived radioisotopes that are produced commercially offer the advantage of transportation from remote locations, but unfortunately availability is sometimes limited.
Table 1.
Physical Properties of Positron-Emitting Radionuclides Commonly Used in Antibody Labeling
| Radionuclide | Half-life, t1/2 (hours) | β+max (MeV) (yields) | Eγ (MeV) (yields) |
|---|---|---|---|
| 124I | 100.2 | 2.14 (24%) | 0.60 (61%) |
| 89Zr | 78.4 | 0.90 (23%) | — |
| 64Cu | 12.7 | 0.66 (18%) | — |
| 18F | 1.83 | 0.63 (97%) | 0.14 (41%) |
As can be seen from Table 1, the most commonly used longer-lived PET imaging radionuclides fall into two broad classes: radiohalogens and radiometals. Radiohalogens are usually directly conjugated to the probe and can be removed from the targeting molecule upon receptor binding and internalization unless a more stable labeling method is used, as mentioned earlier. In addition, dehalogenases, such as deiodinases in the thyroid, result in tracer accumulation in nontarget tissue. In the case of 124I, this problem can be circumvented by using N-succinimidyl 4-iodobenzoate to increase the stability of the immunoconjugate.2 Radiometals, which are indirectly and typically more stably conjugated to the probe via a chelate, are usually chosen for applications involving internalizing targets.
The location of the tumor again becomes important when choosing the radionuclide portion of the PET probe because of the presence of proteins in various organs that will bind particular elements. Thyroid and stomach uptake of 124I prevents imaging of disease in these organs, and preclinical PET studies with this radionuclide require gastric gavaging of the animals with potassium perchlorate to block stomach uptake, and treatment of their drinking water to block thyroid uptake. In the liver, transchelation of radionuclides such as 64Cu to the metal-binding proteins superoxide dismutase and metallothionein precludes imaging of hepatic disease with radiometals.8
Preclinical Studies
Iodine-124
With its long half-life of 100.2 hours, 124I continues to be primarily employed in conjunction with minibodies and intact antibodies. A study by Bading et al.9 using an 124I-labeled anti-CEA intact antibody to serially image mice bearing LS-174T (human colon carcinoma; CEA+) tumors showed for the first time that, despite the high Eβ and true-coincidence gamma ray background, this radionuclide can be used for quantitative PET imaging. Blood curves derived from region of interest (ROI) analysis of PET images acquired at various time points agreed with tissue activity determined by blood sampling and counting, with differences in uptake less than 12% ID/g at each time point, and less than 6% ID/g from 24 hours onward. In a comparative study by Olafsen et al.,10 an anti-CD20 minibody labeled with 124I was shown to be a superior imaging agent to the same minibody conjugated to DOTA and subsequently labeled with 64Cu. High-contrast images of mice with both CD20-positive (B-cell lymphoma) and CD20-negative tumors were produced at 21 hours post-tracer injection as a result of a tumor-to-blood ratio of 4.8 ± 1.5 at this time point. A biodistribution study conducted after microPET scanning confirmed specific localization of the probe, with a tracer uptake value of 13% ± 3.4% ID/g for the positive tumor, compared with 1.9% ± 0.5% ID/g for the negative tumor. An antiprostate stem cell antigen (PSCA) minibody similarly labeled with 124I for imaging of androgen-dependent and androgen-independent prostate cancer in xenograft-bearing mice demonstrated visualization of the positive tumor as early as 4 hours postinjection, with high-contrast images produced at 21 hours.11 At this later time point, tracer uptake by the different PSCA-positive tumors ranged from 2.4% to 9.3% ID/g, and tumor-to-blood ratios varied from 0.7 to 2.8.
With the FDA approval of Y-90 ibritumomab tiuxetan (Zevalin) in 2002, and I-131 tositumomab (Bexxar) in 2003 for radioimmunotherapy of B-cell lymphomas, there has been an increasing interest in developing radioimmunotherapeutics as well as complementary imaging agents to predict the biodistribution of the therapeutics and determine appropriate dosing. Recently, a clinical trial was designed to evaluate the therapeutic 131I-L19-SIP, an scFv-CH4 dimer (∼80 kDa) targeting extra domain B of the tumor neovasculature marker fibronectin, labeled with a β-emitter.12 For the purposes of assessing the potential of this agent as a radioimmunotherapeutic, Tijink et al.13 have generated the related immunoPET imaging agent 124I-L19-SIP. In preclinical studies, the probe was able to specifically target and image the tumor neovasculature, with maximum tumor uptake of 11% ± 1.5% ID/g occurring at 6 hours. Despite this peak, better images were obtained at the later time points, as higher tumor-to-blood ratios were achieved (1.1 at 6 hours vs. 6.0 at 24 hours). Importantly, the biodistributions of 124I-L19-SIP and 131I-L19-SIP were similar, demonstrating the possible usefulness of 124I immunoPET probes for predicting tumor and normal organ uptake of 131I antibody-based therapeutics.
Although dogma would suggest that long-lived 124I would be best suited for labeling intact antibodies and large antibody fragments, work by Orlova et al.14 shows that this is not always the case. In their study, the anti-HER2 monoclonal antibody trastuzumab and the 7-kDa affibody molecule ZHER2:342 were labeled with 125I and 124I, respectively, using p-iodo-benzoate (PIB) as a linker. A solution of the two molecules was injected into three groups of mice bearing HER2-expressing NCI-N87 xenografts, and at various time points postinjection, groups of mice were sacrificed for an ex vivo biodistribution study. Unsurprisingly, tumor uptake of 125I-PIB-trastuzumab was consistently higher than that of 124I-PIB-ZHER2:342 (14% ± 7% IA/g vs. 4.7% ± 1.0% IA/g at 6 hours; 16% ± 3% IA/g vs. 1.4% ± 0.3% IA/g at 24 hours). Because of the faster clearance of the smaller affibody, however, higher tumor-to-nontumor ratios were achieved with 124I-PIB-ZHER2:342. Of particular importance, tumor-to-blood ratios were higher for 124I-PIB-ZHER2:342 than 125I-PIB-trastuzumab (8.0 vs. 0.78 at 6 hours; 16 vs. 1.2 at 24 hours). As a result, high-contrast images can be acquired with 124I-PIB-ZHER2:342. Further, both tumor-to-liver and tumor-to-lung ratios were higher for 124I-PIB-ZHER2:342 than 125I-PIB-trastuzumab. As the liver and lung are common sites of metastases in breast cancer patients, these results indicate that 124I-PIB-ZHER2:342 would not only be a better agent than 125I-PIB-trastuzumab for imaging primary cancer, but also could be used for visualizing metastatic cancer. It should be noted as well that high contrast is achieved as early as 6 hours postinjection of 124I-PIB-ZHER2:342, demonstrating that same-day imaging with this PET probe is possible.
Zirconium-89
With a slightly shorter half-life (78.4 hours) than 124I, 89Zr is another radionuclide that is typically selected for labeling large targeting molecules for preclinical PET studies. Labeling with radiometals such as 89Zr requires premodification of the antibody with a bifunctional chelating agent, and as this indirect labeling is frequently more stable than direct labeling with a halogen, radiometals are often more suitable for imaging of internalizing targets. With promising results in 2006 from the first clinical trial involving a 89Zr-labeled antibody (discussed in the next section), more attention has been focused on this positron emitter during the past couple of years. Previously, major drawbacks of 89Zr included its lack of availability and the complexity and time that premodification and labeling of antibodies entailed.2 Progress made in both of these areas, which will be discussed later in this section, has recently rendered 89Zr a more attractive radionuclide for PET studies.
In a comparative study by Perk et al.,15 anti-Met monoclonal antibody DN30 was labeled with either 89Zr or 131I. Doses consisting of approximately equal activities of the two probes (7.6 μCi 89Zr-DN30 and 10 μCi 131I-DN30) were then co-injected into mice bearing Met-positive tumors, and groups of animals were sacrificed for biodistribution studies at 1–5 days postinjection. Although the two tracer levels in the blood were comparable at each time point, tumor uptake of 89Zr-DN30 was increasingly greater than that of 131I-DN30 at each successive time point (12% ± 4.3% ID/g vs. 7.8% ± 3.1% ID/g at day 1; 20% ± 3.3% ID/g vs. 5.3% ± 1.0% ID/g at day 5), indicating that the probe is likely being internalized. Further, tumor uptake of 131I-DN30 was always significantly lower than blood activity, whereas tumor-to-blood ratios for 89Zr-DN30 consistently increased over time, from 0.71 at 24 hours to 2.2 at day 5. Higher tumor uptake values and tumor-to-blood ratios make 89Zr-DN30 the superior PET probe for imaging the internalizing Met receptor, and the authors suggest that 89Zr-DN30 immunoPET could be helpful in immunotherapy studies involving anti-Met therapeutic antibodies.
Preclinical evaluation of another 89Zr-labeled monoclonal antibody, 89Zr-trastuzumab, was carried out by Dijkers et al.16 In their study, equal amounts (100 μg) and activities (27 μCi) of 89Zr-trastuzumab and 111In-trastuzumab, a SPECT imaging agent that has been used to detect new tumors in breast cancer patients, were co-injected into mice bearing HER2-positive and HER2-negative xenografts. Biodistribution studies conducted at 1 and 6 days postinjection showed similar positive tumor uptake of 111In-trastuzumab (18% ± 1.9% ID/g at day 1; 39% ± 9.5% ID/g at day 6) and 89Zr-trastuzumab (19% ± 2.0% ID/g at day 1; 33% ± 7.6% ID/g at day 6). Blood levels of the two tracers at both time points were also comparable. Although these two probes exhibited similar targeting and biodistribution, greater spatial resolution and signal-to-noise ratio were achieved with 89Zr-trastuzumab, demonstrating its potential value as an imaging agent.
Previously, the lengthy and difficult procedure of separating 89Zr from its target material and the resulting variable radiochemical purity and low specific activity have made this radionuclide unappealing.17 Fortunately, the potential clinical utility of 89Zr has resulted in efforts to improve both production of clinical-grade 89Zr and 89Zr-labeling of monoclonal antibodies. On the production side, Holland et al.17 have established a standardized method for 89Zr isolation. In this study, both high radiochemical purity (>99%) and high specific activity (470–1195 Ci/mmol zirconium) were achieved. On the radiolabeling side, Perk et al.18 have accomplished easier and faster 89Zr labeling by using a novel chelate. In a previous study, Verel et al.19 modified desferrioxamine B (Df ) to produce tetrafluorophenol-N-succinyldesferal (TFP-N-sucDf ). TFP-N-sucDf was used to successfully couple 89Zr to the monoclonal antibody U36, but the multistep process required for production of TFP-N-sucDf precludes it from being a practical bifunctional chelate. In an effort to address this problem, Perk et al. evaluated the commercially available bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine (Df-Bz-NCS). In vivo immunoPET studies revealed that mAbs labeled with 89Zr using Df-Bz-NCS showed similar biodistribution and imaging capability as those labeled with 89Zr using TFP-N-sucDf. Collectively, these dramatic improvements in 89Zr production and labeling undoubtedly facilitate the use of 89Zr in the preclinical setting, making it a more practical and useful radionuclide for immunoPET studies.
Copper-64
Despite its relatively short half-life (12.7 hours), 64Cu is often chosen for labeling monoclonal antibodies that target internalizing cell surface molecules. Although this residualizing radionuclide cannot be used for immunoPET studies requiring long-term monitoring, it has been shown that high-contrast images using 64Cu-labeled intact antibodies can be obtained as early as 18 hours postinjection.20
Since the FDA approval of cetuximab (Erbitux) for the treatment of colorectal (2004) and head and neck (2006) cancers,5 EGFR has become a particularly interesting target for imaging purposes. Previous work by Perk et al.21 showed that 89Zr-cetuximab can accurately predict the biodistribution of the therapeutic radioimmunoconjugates 90Y- and 177Lu-labeled cetuximab as an immunoPET agent in mice. Recently, two different groups evaluated the potential of 64Cu-labeled cetuximab. In the first quantitative PET EGFR imaging study to date, 64Cu-DOTA-cetuximab was injected into seven xenograft models, and ROI analysis was carried out on PET images acquired at 48 hours postinjection to determine tumor tracer uptake.22 A positive linear correlation was found between tumor uptake values and EGFR expression levels determined by western blot. Further quantitative characterization of 64Cu-DOTA-cetuximab by Li et al.23 revealed a high binding affinity (KD = 0.28 M), and metabolite studies showed that the probe undergoes minimal metabolism in the blood (96% ± 2.9% authentic intact at 4 hours), but rapid metabolism in the liver (34% ± 13% at 4 hours) and tumor (16% ± 6.7% at 4 hours). Together, these studies indicate that 64Cu-DOTA-cetuximab could be useful as a PET probe in immunotherapy studies. Applications could include selecting patients for cetuximab-based therapies, estimating effective doses, and monitoring treatment response.
A 64Cu-labeled anti-CEA minibody24 and cysteine-modified diabody25 have been evaluated preclinically, and the list has now been extended to include a 64Cu-labeled intact antibody. In a study by Li et al.,26 a humanized anti-CEA intact antibody, hT84.66-M5A (M5A), was labeled with 64Cu using the chelator 1,4,7-tris(carboxymethyl)-10-(vinylsulfone)-1,4,7,10-tetraazacyclododecane (DO3A-VS). The 64Cu-labeled conjugate (NH-DO3A-VS-M5A or SH-DO3A-VS-M5A) was then injected into mice bearing CEA-expressing LS-174T tumors. Serial imaging was performed from 1 to 48 hours postinjection, and groups of mice were sacrificed at different time points for a biodistribution study. High-contrast images were acquired as early as 22 hours, and the biodistribution data confirmed specific and comparable targeting of the two probes, with tumor uptake around 40% ID/g and a tumor-to-blood ratio of ∼2.5 at 24 hours. To compare DO3A-VS with the conventional chelate DOTA, Li et al. also evaluated the biodistribution of DOTA-M5A. The authors concluded that the tumor uptake of DOTA-M5A was similar to the uptake values of the DO3A-VS-M5A conjugates, but liver uptake of DOTA-M5A was significantly higher, suggesting that DO3A-VS could offer an advantage over DOTA.
In the first to-date PSMA PET imaging study, Elsässer-Beile et al.27 labeled a DOTA-conjugated intact anti-PSMA antibody (3/A12) with 64Cu for evaluation in mice bearing PSMA-positive prostate carcinoma xenografts. Images were acquired at 3, 24, and 48 hours postinjection, and mice were sacrificed after the final scan for ex vivo γ-counting of organs. A high tumor-to-background ratio was achieved as early as 3 hours (3.3 ± 1.3), and high-quality images were obtained by 24 hours. The biodistribution study confirmed specific targeting of the probe, with 35% ± 8.0% ID/g in the PSMA-positive tumor, 13% ± 2.7% ID/g in the PSMA-negative tumor, and 16% ± 10% ID/g in the blood. These findings show promise for 64Cu-DOTA-3/A12 as a PET probe for imaging primary prostate cancer. It is also important to note that 3/A12 binds an extracellular domain of PSMA, a characteristic that may allow this probe to detect bone metastases as well. ProstaScint, the FDA-approved anti-PSMA SPECT imaging agent, is unable to localize and image these lesions, most likely because of its recognition of an intracellular epitope of PSMA.
Fluorine-18
The extremely short half-life (110 minutes) of 18F precludes its use for labeling of large targeting molecules, but makes the radionuclide an ideal match for smaller diabodies and affibodies. The fact that 18F is often more readily available than other positron emitters because of its routine use in the clinical setting, and the ability to perform same-day PET imaging with 18F-labeled probes make diabodies and affibodies labeled with 18F two new exciting classes of imaging agents.
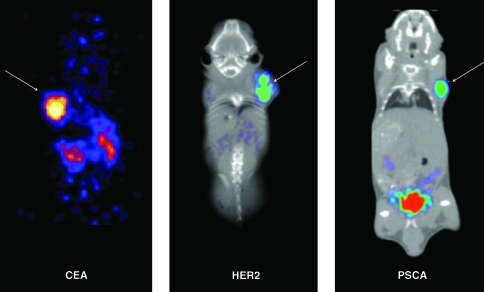
In a study by Cai et al.,28 an anti-CEA diabody (T84.66 diabody) was labeled with N-succinimidyl-4-[18F]-fluorobenzoate ([18F]SFB) for imaging of LS-174T xenografts in mice (Fig. 2). At various time points postinjection, mice were imaged and biodistribution studies were conducted. Images at 30 minutes showed high tumor uptake, and high-contrast images were observed as early as 1 hour. Tumor uptake was 2.7% ± 1.1% ID/g at 1 hour and declined over time, but rapid blood clearance of the probe resulted in increasing tumor-to-blood ratios from 1 hour (1.3 ± 0.59) to 6 hours (6.8 ± 2.3). Liver and spleen uptake (6.3% ± 1.8% and 8.2% ± 2.5% ID/g, respectively, at 1 hour) was also seen, as was high uptake by the kidneys (17% ± 1.2% ID/g at 1 hour), indicative of expected renal clearance for the 55-kDa 18F-FB-T84.66. In two similar studies, Sirk et al.29 and Lepin et al.30 labeled an anti-HER2 diabody and an anti-PSCA diabody, respectively, with [18F]SFB synthesized by a one-pot, remote-controlled method. Evaluation of 18F-FB-anti-HER2 diabody in mice bearing MCF7-HER2 transfected xenografts and 18F-FB-anti-PSCA diabody in mice bearing LAPC-9 xenografts revealed tumor visualization at 4 hours postinjection (Fig. 2). These studies show promise for 18F-labeled diabodies as immunoPET imaging agents, with the latter two demonstrating success of a rapid and simplified radiolabeling method that should increase the appeal of 18F for labeling biologicals.
FIG. 2.
18F-FB diabody microPET (and co-registered computed tomography images in the middle and left panels) of mice bearing subcutaneous xenografts (arrows) expressing different oncogenic biomarkers. Left panel: 18F-FB-T84.66 diabody targeting a CEA-positive LS-174T tumor.28 Middle panel: 18F-FB-anti-HER2 diabody targeting a HER2-positive MCF7-HER2 tumor. Image courtesy of Dr. Shannon J. Sirk. Right panel: 18F-FB-anti-PSCA diabody targeting a PSCA-positive LAPC-9 tumor. Image courtesy of Dr. Eric J. Lepin. PET, positron emission tomography; CEA, carcinoembryonic antigen; HER2, human epidermal growth factor receptor-2; PSCA, prostate stem cell antigen.
Two 18F-labeled affibody molecules have also been recently validated in preclinical studies. Namavari et al.31 aminooxy-functionalized a 14-kDa anti-HER2 dimeric affibody and site-specifically labeled the molecule with 4-[18F]fluorobenzaldehyde via oxime chemistry for imaging of mice bearing HER2-positive SKOV3 xenografts. Despite modest tumor uptake (1.8% ± 0.40% ID/g), high-contrast images were produced at 2 hours because of the very rapid clearance of the small probe. Tracer uptake by the tumor peaked at 1 hour (2.2% ± 0.51% ID/g), but the blood clearance resulted in the maximum tumor-to-blood ratio (3.0 ± 0.48) occurring at 3 hours. High tracer uptake was also seen in the liver, kidneys, and intestines. In a study by Kiesewetter et al.,32 a cysteine-modified 7-kDa anti-HER2 affibody was labeled with N-[2-(4-[18F]fluorobenzamido)ethyl]maleimide vial maleimide chemistry for imaging of mice bearing HER2-positive BT474 tumors. Similar to the findings by Namavari et al., this study showed that tumors could be distinguished on images acquired at 2 hours postinjection. A biodistribution study at the same time point showed extremely high tracer uptake by the tumor (23% ID/g), with the kidney being the only other organ with significant uptake (10% ID/g). The results from these two affibody studies indicate that high-affinity, rapidly clearing 18F-labeled affibodies could be a platform for same-day imaging of tumors.
Clinical Studies
Promising immunoPET agents that have been validated in preclinical studies continue to advance slowly to the clinical setting. As mentioned in the preceding section, the first clinical study involving zirconium-89 was conducted in 2006. In this study, Börjesson et al.19 evaluated the safety and imaging capability of 89Zr-labeled chimeric U36 (cmAb U36), an anti-CD44v6 intact antibody that had previously been shown to detect small squamous cell carcinoma of the head and neck (HNSCC) in mice.33 89Zr-cmAb U36 immunoPET (up to 6 days postinjection) as well as computed tomography (CT) and/or magnetic resonance imaging (MRI) scans were acquired for the 20 enrolled HNSCC patients prior to surgery. In addition, 6 patients were also examined by 18F-fluoro-2-deoxy-d-glucose (18F-FDG) PET. 89Zr-U36 immunoPET sensitivities (73% for sides of the neck and 72% for lymph node levels) and accuracies (93% for sides and 76% for levels) were comparable to those of CT/MRI (sensitivity of 73% for sides and 60% for levels; accuracy of 90% for sides and 80% for levels) and 18F-FDG PET (sensitivity of 62% for sides and accuracy of 88% for levels). Not surprisingly, superior immunoPET images were obtained at the later time points (72 or 144 hours vs. 1 or 24 hours), and the authors suggest that better immunoPET image analysis might be made possible by fusing immunoPET images with anatomical CT or MRI images. A dosimetry study with the same group of patients showed that a 10 mg dose of cmAb U36 labeled with 89Zr (74.9 ± 0.6 MBq) was well tolerated, with no patients exhibiting adverse effects and only 2 patients displaying a human anti-chimeric antibody response.34 Importantly, there was good agreement between tracer uptake values determined from ROI analysis of immunoPET scans and obtained from blood sampling (difference of 0.2% ± 16.9% ID/g for patients <100 kg) and tumor biopsy (difference of −8.4% ± 34.5% ID/g), demonstrating the potential of quantitative 89Zr-cmAb U36 immunoPET. In another 89Zr pilot clinical imaging study, Perk et al.35 labeled the anti-CD20 murine monoclonal antibody ibritumomab tiuxetan (Zevalin) for evaluation in a patient with B-cell non-Hodgkin's lymphoma. At 2 hours postinjection of 74 MBq 89Zr-Zevalin (1.8 mg Zevalin in total), an immunoPET image revealed mostly blood pool activity, whereas a second image acquired at day 3 showed all tumors that had previously been detected with 18F-FDG PET. With these clinical findings and the aforementioned progress in 89Zr production and antibody labeling, it is likely that more 89Zr immunoPET agents will enter the clinical setting in the near future.
In the first clinical PET study involving patients with renal tumors, Divgi et al.36 radiolabeled chimeric G250 (cG250) with 124I for presurgical PET imaging of 26 individuals with renal masses. An antibody against carbonic anhydrase IX, a target overexpressed in clear-cell renal carcinoma (CCRC), cG250 has been previously evaluated in advanced renal cell carcinoma patients as a stand-alone therapeutic,37,38 a radioimmunotherapeutic,39 and as part of a combination therapy regimen.40 In this phase I clinical study, patients were given an intravenous infusion of 185 MBq (10 mg) 124I-cG250 and imaged at 7 days postinjection, just prior to surgery and subsequent histopathology. 124I-cG250 immunoPET had a sensitivity of 94% (15/16 patients with CCRC had positive PET scans) and a specificity of 100% (9/9 patients who did not have CCRC had negative PET scans). As a result of these findings, the authors conclude that 124I-cG250 immunoPET could be useful in noninvasively classifying tumors and deciding the best treatment option for patients.
Conclusions
The emergence of FDA-approved targeted therapies for cancer has resulted in recent renewed interest in molecular imaging, especially immunoPET. The high sensitivity and resolution of PET makes this imaging modality particularly attractive, and the specificity of antibodies and their derivatives makes this class of biological molecules well suited for targeting a variety of tumor markers. As discussed in this update, there are many factors that need to be considered when designing a preclinical immunoPET study. The properties of the targeting molecule and radionuclide, the type of target, and the desirable imaging time point are all important pieces of the imaging puzzle. To achieve high tumor uptake and tumor-to-blood ratios, which will result in high-contrast images, these factors must be considered collectively. Although conventional targeting protein and radionuclide combinations (e.g., intact antibodies and 124I, diabodies and 18F) continue to be evaluated, alternative pairings (e.g., intact antibodies and 64Cu, affibodies and 124I) have demonstrated unanticipated success preclinically and represent exceptions to immunoPET dogma. Review of the literature clearly shows that progress is being made in the preclinical setting, but that translation to the clinic continues to be slow. With recent developments in radionuclide production and availability, as well as improved radiolabeling methods, movement of immunoPET imaging agents into the clinical setting should be accelerated.
Acknowledgments
The authors thank Drs. Jeffrey V. Leyton, Shannon J. Sirk, and Eric. J. Lepin for providing images. Funding support was provided by the UCLA SPORE in Prostate Cancer (NIH CA 092131), the UCLA Center for In Vivo Imaging in Cancer Biology (NIH CA 086306), the Stanford Center for Nanotechnology Excellence (NIH CA 119367), and a Dr. Ursula Mandel Scholarship. Anna M. Wu is a member of the UCLA Jonsson Comprehensive Cancer Center (NIH CA 016042).
Disclosure Statement
Anna Wu consults for ImaginAb, Inc.
About the Authors

Katelyn E. McCabe received her B.A. from Amherst College in 2003 and is currently a Ph.D. candidate under the guidance of Anna M. Wu in the Department of Molecular and Medical Pharmacology at the University of California, Los Angeles (UCLA) David Geffen School of Medicine. Her research includes the engineering, modification, and evaluation of antibodies for diagnostic and therapeutic applications.

Anna M. Wu is a professor in the Departments of Molecular and Medical Pharmacology, and Pathology and Laboratory Medicine, at the UCLA David Geffen School of Medicine. She is a member of the Crump Institute for Molecular Imaging and the California NanoSystems Institute and also serves as the Director of the Cancer Molecular Imaging Program in the Jonsson Comprehensive Cancer Center at UCLA. She received her A.B. degree in Biochemical Sciences from Harvard University and a Ph.D. in Molecular Biophysics and Biochemistry from Yale University. She carried out her postdoctoral studies at Yale University and at the University of California, San Francisco. Her research focuses on engineered proteins (including antibodies) for targeting and imaging applications in cancer, including the use of single photon emission computed tomography, positron emission tomography, optical, and multimodality approaches.
References
- 1.Shokeen M. Anderson CJ. Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET) Acc Chem Res. 2009;42:832. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayak TK. Brechbiel MW. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug Chem. 2009;20:825. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein R. Goldenberg DM. Thorpe SR, et al. Effects of radiolabeling monoclonal antibodies with a residualizing iodine radiolabel on the accretion of radioisotope in tumors. Cancer Res. 1995;55:3132. [PubMed] [Google Scholar]
- 4.Stein R. Govindan SV. Mattes MJ, et al. Improved iodine radiolabels for monoclonal antibody therapy. Cancer Res. 2003;63:111. [PubMed] [Google Scholar]
- 5.Boswell CA. Brechbiel MW. Development of radioimmunotherapeutic and diagnostic antibodies: An inside-out view. Nucl Med Biol. 2007;34:757. doi: 10.1016/j.nucmedbio.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu AM. Olafsen T. Antibodies for molecular imaging of cancer. Cancer J. 2008;14:191. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 7.Gronwall C. Stahl S. Engineered affinity proteins—Generation and applications. J Biotechnol. 2009;140:254. doi: 10.1016/j.jbiotec.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CJ. Ferdani R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biother Radiopharm. 2009;24:379. doi: 10.1089/cbr.2009.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bading JR. Hörling M. Williams LE, et al. Quantitative serial imaging of an 124I anti-CEA monoclonal antibody in tumor-bearing mice. Cancer Biother Radiopharm. 2008;23:399. doi: 10.1089/cbr.2007.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olafsen T. Betting D. Kenanova VE, et al. Recombinant anti-CD20 antibody fragments for small-animal PET imaging of B-cell lymphomas. J Nucl Med. 2009;50:1500. doi: 10.2967/jnumed.108.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leyton JV. Olafsen T. Lepin EJ, et al. Humanized radioiodinated minibody for imaging of prostate stem cell antigen-expressing tumors. Clin Cancer Res. 2008;14:7488. doi: 10.1158/1078-0432.CCR-07-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schliemann C. Neri D. Antibody-based targeting of the tumor vasculature. Biochim Biophys Acta. 2007;1776:175. doi: 10.1016/j.bbcan.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Tijink BM. Perk LR. Budde M, et al. 124I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of 131I-L19-SIP radioimmunotherapy. Eur J Nucl Med Mol Imaging. 2009;36:1235. doi: 10.1007/s00259-009-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orlova A. Wållberg H. Stone-Elander S. Tolmachev V. On the selection of a tracer for PET imaging of HER2-expressing tumors: Direct comparison of a 124I-labeled Affibody molecule and trastuzumab in a murine xenografts model. J Nucl Med. 2009;50:417. doi: 10.2967/jnumed.108.057919. [DOI] [PubMed] [Google Scholar]
- 15.Perk LR. Stigter-van Walsum M. Visser GWM, et al. Quantitative PET imaging of Met-expressing human cancer xenografts with 89Zr-labelled monoclonal antibody DN30. Eur J Nucl Med Mol Imaging. 2008;35:1857. doi: 10.1007/s00259-008-0774-5. [DOI] [PubMed] [Google Scholar]
- 16.Dijkers ECF. Kosterink JGW. Rademaker AP, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009;50:974. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- 17.Holland JP. Sheh Y. Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36:729. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perk LR. Vosjan MJWD. Visser GWM, et al. p-Isothiocyanatobenzyl-desferrioxamine: A new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur J Nucl Med Mol Imaging. 2009;37:250. doi: 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verel I. Visser GWM. Boellaard R, et al. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med. 2003;44:1271. [PubMed] [Google Scholar]
- 20.Philpott GW. Schwarz SW. Anderson CJ, et al. RadioimmunoPET: Detection of colorectal carcinoma with positron-emitting copper-64-labeled monoclonal antibody. J Nucl Med. 1995;36:1818. [PubMed] [Google Scholar]
- 21.Perk LR. Visser GWM. Vosjan MJWD, et al. 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med. 2005;46:1898. [PubMed] [Google Scholar]
- 22.Cai W. Chen K. He L, et al. Quantitative PET of EGFR expression in xenografts-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging. 2007;34:850. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- 23.Li WP. Meyer LA. Capretto DA, et al. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biother Radiopharm. 2008;23:158. doi: 10.1089/cbr.2007.0444. [DOI] [PubMed] [Google Scholar]
- 24.Wu AM. Yazaki PJ. Tsai S-W, et al. High-resolution microPET imaging of carcinoembryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. Proc Natl Acad Sci USA. 2000;97:8495. doi: 10.1073/pnas.150228297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olafsen T. Cheung C-W. Yazaki PJ, et al. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng Des Sel. 2004;17:21. doi: 10.1093/protein/gzh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L. Bading J. Yazaki PJ, et al. A versatile bifunctional chelate for radiolabeling humanized anti-CEA antibody with In-111 and Cu-64 at either thiol or amino groups: PET imaging of CEA-positive tumors with whole antibodies. Bioconjug Chem. 2008;19:89. doi: 10.1021/bc700161p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsässer-Beile U. Reischl G. Wiehr S, et al. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J Nucl Med. 2009;50:606. doi: 10.2967/jnumed.108.058487. [DOI] [PubMed] [Google Scholar]
- 28.Cai W. Olafsen T. Zhang X, et al. PET imaging of colorectal cancer in xenografts-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J Nucl Med. 2007;48:304. [PubMed] [Google Scholar]
- 29.Sirk SJ. Olma S. Shen CK, et al. MicroPET imaging of HER2+ tumors using F-18-labeled anti-HER2 diabody. J Nucl Med. 2009;50(suppl2):128. (abstract). [Google Scholar]
- 30.Lepin EJ. Olma S. Wang M, et al. 18F-Labeled diabodies for microPET imaging of prostate stem cell antigen (PSCA)-expressing xenografts [abstr]. Proceedings of the 2009 World Molecular Imaging Congress, Montreal, Canada, September 23–26. Mol Imaging Biol. 2009;12(suppl1):J682. [Google Scholar]
- 31.Namavari M. De Jesus OP. Cheng Z, et al. Direct site-specific radiolabeling of an Affibody protein with 4-[18F]fluorobenzaldehyde via oxime chemistry. Mol Imaging Biol. 2008;10:177. doi: 10.1007/s11307-008-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiesewetter DO. Krämer-Marek G. Ma Y, et al. Radiolabeling of HER2 specific Affibody® molecule with F-18. J Fluorine Chem. 2008;129:799. doi: 10.1016/j.jfluchem.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Börjesson PKE. Jauw YWS. Boellaard R, et al. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin Cancer Res. 2006;12:2133. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- 34.Börjesson PKE. Jauw YWS. de Bree R, et al. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J Nucl Med. 2009;50:1828. doi: 10.2967/jnumed.109.065862. [DOI] [PubMed] [Google Scholar]
- 35.Perk LR. Visser OJ. Stigter-van Walsum M, et al. Preparation and evaluation of 89Zr-Zevalin for monitoring of 90Y-Zevalin biodistribution with positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:1337. doi: 10.1007/s00259-006-0160-0. [DOI] [PubMed] [Google Scholar]
- 36.Divgi CR. Pandit-Taskar N. Jungbluth AA, et al. Preoperative characterization of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: A phase I trial. Lancet Oncol. 2007;8:304. doi: 10.1016/S1470-2045(07)70044-X. [DOI] [PubMed] [Google Scholar]
- 37.Bleumer I. Knuth A. Oosterwijk E, et al. A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Brit J Cancer. 2004;90:985. doi: 10.1038/sj.bjc.6601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis ID. Wiseman GA. Lee F-T, et al. A phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinoma. Cancer Immun. 2007;7:13. [PMC free article] [PubMed] [Google Scholar]
- 39.Divgi CR. O'Donoghue JA. Welt S, et al. Phase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancer. J Nucl Med. 2004;45:1412. [PubMed] [Google Scholar]
- 40.Bleumer I. Oosterwijk E. Oosterwijk-Wakka JC, et al. A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol. 2006;175:57. doi: 10.1016/S0022-5347(05)00040-6. [DOI] [PubMed] [Google Scholar]