Summation
Radioimmunotherapy (RIT) has emerged as one of the most promising treatment options, particularly for hematologic malignancies. However, this approach has generally been limited by a suboptimal therapeutic index (target-to-nontarget ratio) and an inability to deliver sufficient radiation doses to tumors selectively. Pretargeted RIT (PRIT) circumvents these limitations by separating the targeting vehicle from the subsequently administered therapeutic radioisotope, which binds to the tumor-localized antibody or is quickly excreted if unbound. A growing number of preclinical proof-of-principle studies demonstrate that PRIT is feasible and safe and provides improved directed radionuclide delivery to malignant cells compared with conventional RIT while sparing normal cells from nonspecific radiotoxicity. Early phase clinical studies corroborate these preclinical findings and suggest better efficacy and lesser toxicities in patients with hematologic and other malignancies. With continued research, PRIT-based treatment strategies promise to become cornerstones to improved outcomes for cancer patients despite their complexities.
Key words: antibody, cancer, immunotherapy, leukemia, lymphoma, pretargeting, radioimmunotherapy
Introduction
Monoclonal antibodies have revolutionized oncology as vehicles to deliver radionuclides to tumor cells for diagnostic or therapeutic purposes. Undoubtedly, radioimmunotherapy (RIT) has emerged as one of the most exciting treatment modalities, particularly for hematologic malignancies.1,2 For example, anti-CD20 RIT, either alone or combined with chemotherapy and/or hematopoietic cell transplantation (HCT), has dramatically improved the outcome of patients with indolent B-cell non-Hodgkin lymphomas, and yields promise for aggressive lymphomas.1–3 Similarly, encouraging results have been obtained with RIT targeting CD33, CD45, or CD66 together with high-dose chemotherapy and HCT for patients with acute leukemias.1 Conceptually, RIT may be advantageous over external-beam radiation methods, at least partly because DNA damage accumulates through prolonged, low dose-rate radiation exposure resulting from extended tumor residence of the targeting vehicle. Unfortunately, current conventional RIT regimens are often limited by heterogeneous distributions of antibodies in targeted tumors and suboptimal therapeutic indices (target-to-nontarget ratios).4,5 And although lymphomas and leukemias are exquisitely radiosensitive, many patients still relapse after RIT-based therapy. Several strategies have been explored to overcome these hurdles and allow higher tumor radiation doses. Some approaches aimed to improve the effectiveness of the administered radiation dose. This was accomplished through radiosensitization of malignant cells, increased radiation dose rates, augmentation of tumor antigen expression, or enhancement of tumor localization via compartmental administration, increase of vascular permeability, or biological response modifiers.6,7 Alternatively, an attempt was made to improve the therapeutic index by optimizing radionuclide delivery to tumors while limiting normal organ radiation. Thereby, higher doses of absorbed radiation can be achieved in the tumor before dose-limiting toxicities occur. Strategies to accomplish this include dose fractionation, removal of circulating radioactivity that has not localized to the tumor site, and pretargeted RIT (PRIT).7 Among these approaches, PRIT may be the most advanced and promising at the current time. Initially used to enhance imaging capabilities,8 its potential for therapeutic applications was revealed when Axworthy et al. demonstrated that PRIT yielded radiation localized to a tumor, which rivaled that of a directly radiolabeled antibody.9 An increasing number of preclinical and clinical studies have subsequently shown that PRIT indeed improves the ratio of tumor-to-nontumor radiation and provides encouraging results in the treatment of hematologic and other malignancies.
The Concept of Pretargeting
Radiolabeled antibodies or other large molecules circulate in the blood for prolonged periods of time. They expose normal organs, especially the radiosensitive bone marrow, to radiation and limit the radiation dose that can be safely administered. At the same time, impeded extravasation may cause slow tumor accretion.10 In contrast, antibody fragments clear the plasma faster, localize more rapidly to tumors, and may penetrate tumors better, although substantial uptake in normal tissue remains problematic.11–14 With PRIT, administration of the prolonged-circulating antibody construct (“targeting vehicle”) is separated from the therapeutic radioisotope (“effector”). Conceptually, this sequential administration allows for maximal antibody targeting to take place prior to delivery of the therapeutic radionuclide while maintaining targeting specificity. If the effector has higher penetration, clearance, and diffusion rates than the targeting antibody, more rapid tumor radionuclide localization and higher tumor selectivity can be achieved.5 PRIT is attractive because whole-body radiation is substantially decreased because of radionuclide delivery via small molecules that yield rapid tumor uptake and fast renal excretion of nontumor bound radioactivity. To avoid binding of a sizable portion of the effector to the antibody in the blood, synthetic chasers (“clearing agents”) have been introduced as an additional refinement to PRIT. Administered at peak tumor uptake, they remove unbound targeting vehicle lingering in the bloodstream before application of the radioactive moiety.15–19 The potential advantages and disadvantages of PRIT systems are summarized in Table 1.
Table 1.
Potential Advantages and Disadvantages of Pretargeted Radioimmunotherapy Systems
| Advantages |
| • Favorable biodistribution and blood clearance |
| • High therapeutic ratio (high target to-nontarget organ ratio) |
| • Flexibility with respect to isotope and antigen target |
| • Multiple effector dosing possible |
| • Sequential amplification possible |
| Disadvantages |
| • Ab constructs may be difficult to manufacture |
| • Monovalent (to tumor antigen) Ab fragment constructs may decrease tumor residence time |
| • Tetravalent constructs (e.g., streptavidin) may crosslink antibody–antigens and internalize |
| • Immunogenicity (e.g., streptavidin) |
| • Hapten release from processed Ab may poison tumor-associated conjugate |
| • Perceived as complex with respect to dosing and timing |
| • Rapidly internalized antigens are nontargetable |
Ab, antibody.
Pretargeting Approaches
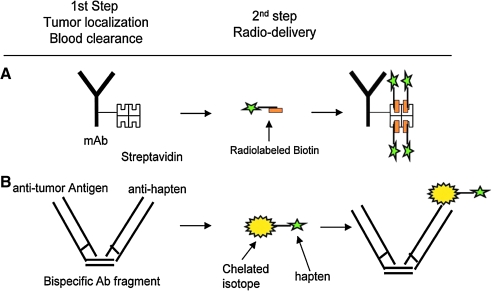
Since initial conception, several PRIT approaches have been described.8,19–22 All are based on a modified unlabeled monoclonal antibody or antibody fragment that recognizes a tumor antigen and a rapidly diffusing small radiolabeled molecule (effector), which is quickly excreted if left unbound. Currently, the most popular PRIT techniques use either the hapten/antibody (bifunctional antibody) or the biotin/(strept)avidin system (see examples in Fig. 1), but DNA/DNA systems have been used as well.19,20 Other systems, such as the recombinant human enzyme dihydrofolate reductase combined with its high-affinity inhibitor methotrexate, have been proposed but have not undergone extensive testing.23
FIG. 1.
Examples of pretargeted radioimmunotherapy approaches. (A) Divalent antibody–streptavidin conjugate followed by delivery of radiolabeled biotin; (B) bispecific monovalent antibody–antihapten conjugate followed by injection of a chelated isotope bound to a small, low-molecular-weight hapten. Reproduced with permission from Pagel.101
Hapten/antibody systems
Antihapten monoclonal antibodies recognizing metal ions via bifunctional chelating agents were first raised by Reardan et al.24 These initial studies on the benzyl ethylenediaminetetraacetic acid (EDTA) chelate of indium provided the basis for the development of metal-specific monoclonal antibodies against cobalt and gallium chelates.20,25,26 However, many metal chelates distribute rapidly in the extracellular fluid and have prompt renal clearance and are thus suitable candidates for use in PRIT systems.20 Interestingly, Reardan et al. envisioned antibodies with dual antigen specificity, which simultaneously bind a metal chelate and a physiological antigen,24 thereby foreseeing the hapten/antibody system. Indeed, only 1 year later, Goodwin et al. used hapten antibodies for radionuclide delivery.27,28 Initially, the antibody and radionuclide were administered concomitantly but, in later studies, in sequential fashion, establishing the concept of PRIT.27,28
The initial bispecific monoclonal antibodies were chemically produced and typically based on Fab′ or F(ab′)2-equivalent molecules. These constructs required several days to clear from the blood before the radiolabeled effector could be administered in a two-step procedure. However, even trace amounts of circulating nonbound antibody can bind effector molecules immediately. Thus, some investigators have used a three-step approach with a “chase” to remove circulating antibody.20,28 This was accomplished without significant reduction of target-bound antibody with a multivalent antigen bound covalently to a slowly diffusible serum protein. The chase is then rapidly removed by the hepatic reticuloendothelial system after crosslinking and formation of aggregates in the plasma.20,27,28 An alternative approach to clear circulating nonbound bispecific antibody entails antibody biotinylation and injection of avidin before hapten administration; this strategy can significantly improve tumor-to-blood ratios without limiting tumor uptake.29
The basic hapten/antibody PRIT system has been refined and methodologically improved over the years, most notably with respect to antibody size and valency of tumor and hapten binding. Molecular engineering has enabled the preparation of antibody fragments with smaller weights than F(ab')2 fragments, such as single-chain variable fragments (scFv) or diabodies; it has also allowed the generation of constructs with higher valencies, such as triabodies and tetrabodies, which showed higher tumor retention than monovalent constructs.30–32 Smaller constructs may offer pharmacological advantages as they penetrate tumors more effectively than larger chemically conjugated bispecific antibodies. This leads to more uniform distribution of radioactivity within the tumor, an important factor for the efficacy of targeted radionuclides.30–32
A critical parameter of the hapten/antibody system is the affinity toward the hapten.33 Without a chase, the effector may be trapped by excess circulating unbound antibody if the affinity is too strong, thereby reducing tumor localization and clearance.27 However, suboptimal tumor localization would be obtained if the affinity is too low. Le Doussal et al. also demonstrated that hapten valency is a major determinant for tumor uptake and effector retention.33 Specifically, bivalent hapten effectors are markedly superior to those with a single hapten as they have a higher affinity to cell-bound than unbound bispecific antibody.33–35 This affinity enhancement system (AES) is believed to be due to the ability of the bivalent hapten to crosslink pretargeted macromolecules at the tumor site; as a more stable complex is formed, the tumor residence time is prolonged and the tumor-to-nontumor ratio is improved.33–38 Limited data also suggest cooperative binding at target surfaces if two distinct bispecific antibodies are used, each recognizing a different target antigen and hapten.10
Considerable flexibility of the hapten/antibody system was gained by the incorporation of antibodies that recognize a “universal” molecule such as histamine succinyl glycine (HSG).39 As binding occurs to the universal hapten rather than the effector moiety (e.g., diethylenetriaminepentaacetic acid [DTPA]), various radionuclides can be incorporated into a basic core peptide.22,40–42 A number of different constructs based on this universal hapten binding system have been developed and expressed in bacterial, yeast, or mammalian systems.30,31,43 Finally, significant progress has been achieved in the engineering of recombinant, humanized bispecific antibodies. Most significantly, a recent system uses bispecific trimeric Fab′-based recombinant constructs made by a modular procedure of antibody and protein engineering called the “dock and lock” (DNL) method.10,43–45 DNL takes advantage of the naturally occurring interaction between the dimerization and docking domain (DDD) of the regulatory subunit of human cAMP-dependent protein kinase A and the anchoring domains (ADs) of A-kinase anchoring proteins.10,43 One Fab′ fragment is generated to contain the DDD, whereas the other is fused to the AD. When combined, they form stably tethered, noncovalent complexes with well-defined orientation and fully retained functions of the individual components. These complexes are subsequently covalently linked via disulfide bonds by incorporation of cysteine residues into the natural DDD and AD sequences.10,43,45
Biotin/(strept)avidin system
The second popular PRIT approach takes advantage of the ultrahigh affinity (strept)avidin–biotin system, a system widely used in scientific applications.5,20,46,47 Both the glycosylated 66-kDa protein, avidin, and the nonglycosylated 53-kDa bacterial protein from Streptomyces avidinii, steptavidin, have been used for PRIT. Despite its immunogenicity, streptavidin is preferred for the antibody conjugate because of its slower blood clearance.48 Avidins are proteins that bind up to four biotin moieties with high affinity and specificity: The dissociation constant of this complex is in the order of 10−15 M,49 that is, about 1 million-fold stronger than most antibody/antigen interactions. However, the binding sites for biotin occur in pairs fairly close to each other, causing steric hindrance to the binding of more than two biotinylated molecules.47 Avidins are thus expected to bind less than four biotinylated molecules. An insufficient distance between an avidin and the surface of the biotinylated molecule might also result in unsatisfactory binding. For adequate binding capability to avidin, the conjugate's biotin and chelating sites must be separated by a spacer, because steric hindrance occurs to a varying degree between the sites.5,50
Biotin is present naturally in serum and tissues. Experimental mice thus require a biotin-deficient diet before biotin/(strept)avidin-based PRIT studies can be conducted.51 However, humans have lower blood biotin concentrations, and biotin-based systems can be used without prior reduction of endogenous biotin levels.32 New biotin derivatives with preserved (strept)avidin-binding properties were developed to protect against biotinidases.32,52–55
Theoretically, the biotin moiety could be conjugated to the antibody or carry the radionuclides, and both approaches have been evaluated for PRIT.32 Theoretical modeling suggests that the combination of (strept)avidin-conjugated targeting vehicles and biotin-labeled effectors offers pharmacokinetic advantages over biotin-conjugated targeting vehicles with (strept)avidin-labeled effectors in the absence of antigen internalization.56 If (strept)avidin is conjugated to the antibody, a clearing agent may be necessary to remove the circulating (strept)avidin conjugate before administration of radiolabeled biotin because of their large size.32 Initially proposed clearing agents were based on polyvalent macromolecules that remained largely confined to the serum compartment and were removed by the liver via the reticuloendothelial system.15,57 Improved biotin-containing agents were subsequently synthesized. These newer agents remove residual circulating (strept)avidin conjugates through the hepatic asialoglycoprotein receptors (Ashwell receptors). This receptor recognizes ligands with terminal clustered galactosyl or N-galactosyl residues and facilitates their rapid endocytosis into liver cells and clearance from the bloodstream.16,57,58 The first-generation clearing agents utilizing the Ashwell receptor were based on human serum albumin as a scaffold to derivatize a small number of biotin molecules with a high load of galactose residues.57 However, albumin-based clearing agents reduced the biotin-binding capacity of tumors, most likely as a result of their catabolic degradation with release of free biotin or low–molecular-weight biotin adducts that then bound (strept)avidin-labeled antibody.57 Second-generation clearing agents consisted of a defined dendrimeric synthetic molecule with 16 modified N-acetyl galactosamine residues and biotin to bind circulating (strept)avidin-containing proteins.57 Advantages of these agents include reproducible in vivo behavior, lack of biotin release following liver uptake, and lack of competition for tumor targeting by radiolabeled 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic (DOTA)–biotin. Radiolabeling of biotin or (strept)avidin is done either directly to the protein or, indirectly, by covalently linking to a chelating agent that then binds a suitable radionuclide.5 The most commonly used chelators include polyaminocarboxylic acids (e.g., EDTA and DTPA) and their chemically modified derivatives such as DOTA.5 The latter forms stable complexes with yttrium-90 (90Y)59 and is widely used as an effector in PRIT. However, alternative β- or α-emitting radionuclides can be used, as DOTA forms highly stable complexes with other isotopes such as 64Cu, 67Ga, 149Pm, 166Ho, 177Lu, 212Pb, 212Bi, and 213Bi.60,61 Alternative carriers for biotin and radionuclides have also been developed, for example, modified polylysine for improved pharmacokinetics with intraperitoneal administration of radionuclides such as 211At.62,63
In the second approach, biotin-conjugated antibody is used to target the tumor. Antibody biotinylation is easily accomplished; importantly, addition of a few molecules of biotin (molecular weight: 244 Da) does not alter immunoreactivity, plasma kinetics, or rates of antibody permeation and diffusion and only minimally increases the molecular weight of the targeting vehicle.5 Theoretically, radiolabeled (strept)avidin could be used as an effector, in which case an avidin chase increases tumor localization.64 However, as radiolabeled (strept)avidin has an unfavorable biodistribution, a strategy was devised that includes a clearing and bridging step and allows the use of radiolabeled biotin. Herein, avidin is used as a clearing agent to remove circulating biotin-labeled antibody. Afterward, streptavidin is administered to bind to biotin conjugate attached to the tumor; through multiple binding sites, this agent generates a bridge between the antibody and subsequently administered radiolabeled biotin.32
A recent improvement in PRIT technology has been the enhanced uniformity of the streptavidin-antibody targeting molecule such as a protein between an engineered tetravalent scFv and streptavidin monomers (scFv[4]SA).57,65–67 These fusion proteins have been validated for various antibodies and can be expressed as soluble tetramers in the periplasmic space of Escherichia coli. They retain full antigen and biotin-binding capabilities of their parent molecules and have tumor targeting ability comparable to whole IgG antibodies. However, they provide an economic advantage for protein production and are scaleable to large quantities. In fact, the encouraging preclinical results have prompted cGMP production of an anti-CD45 fusion protein for PRIT trials to be conducted in patients with advanced myeloid leukemias.
DNA/DNA systems
This system is the most recent and has received the least attention thus far but is anticipated to have low immunogenicity.32 It explores complementary hybridization (Watson–Crick pairing) of DNA and other oligomers, particularly phosphorodiamidate morpholino oligomers (MORFs), as a recognition system in tumor pretargeting.68–75
Advantages and disadvantages
The advantages and disadvantages of individual PRIT strategies have been discussed in recent reviews.10,19,76,77 The biotin/(strept)avidin system has several advantages, including the availability of multiple biotin-binding sites on (strept)-avidin, the high-affinity binding between (strept)avidin and biotin, its flexibility of use, and the commercial availability of reagents and suitable linkers. One downside of the biotin/streptavidin system may be the necessity of a clearing step and the immunogenicity of streptavidin; this results in the development of anti-streptavidin antibody responses after initial exposure in a majority of patients, even in heavily pretreated patients with hematologic malignancies.78,79 However, human anti-mouse antibody (HAMA) responses have been observed in patients treated with murine bispecific antibodies80; the incidence of anti-antibody responses declines with increasing degrees of humanization of these murine antibodies.81 A clearing step may not be required if bispecific antibodies are used, but natural clearance of these constructs may require several days before the radionuclide can be administered.
Preclinical PRIT Studies
Both hapten/antibody-based and biotin/(strept)avidin-based PRIT systems have been studied preclinically for hematologic malignancies and solid tumors; these studies are summarized below. By comparison, the experience with DNA/DNA-based systems is sparse, with only limited data documenting successful tumor targeting so far.82
Animal models of hematologic malignancies
Lymphoma
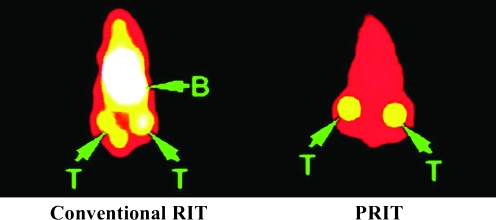
Several antigens have proven useful for conventional RIT in clinical lymphoma trials, most notably CD20, CD22, and HLA-DR (Fig. 2).32,83 Building on these observations, the biodistribution of radiation delivered to tumor and normal organs in murine xenograft models using streptavidin/biotin-based PRIT targeting these antigens was investigated. For all three antigens, the pretargeting approach resulted in biodistributions that were far superior to those obtained with conventional RIT17,84–87; examples of conventional RIT and PRIT studies targeting CD20 are shown in Figure 3. The streptavidin–antibody conjugate achieving the best tumor uptake and tumor-to-normal organ ratios varied depending on the target antigen expression of the cell line used, with anti-CD20 (1F5) and anti-HLA-DR (Lym-1), yielding the most promising biodistributions. The best tumor-to-normal organ ratios of absorbed radioactivity using the PRIT approach were observed when individual streptavidin–antibody conjugates rather than combinations with all three conjugates were used.
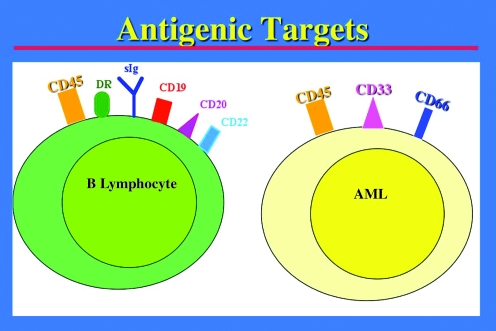
FIG. 2.
Target antigens for radioimmunotherapy and pretargeted radioimmunotherapy on B-cell lymphocytes and acute myeloid leukemia cells.
FIG. 3.
Comparison of CD20-targeting conventional RIT and PRIT in human lymphoma xenografts. Gamma camera images of Ramos xenograft-bearing athymic mice injected with either directly 111In-labeled anti-CD20 antibody (1F5; at left) or pretargeted 1F5–streptavidin conjugate followed 24 hours later by a clearing agent and then by 111In-1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic-biotin (at right). Arrows indicate radioactivity in tumors (T) and blood pool (B) at 24 hours after injection of 111In-reagents. Bladder activity is also seen in the conventional RIT mouse. Reproduced with permission from Subbiah et al.84 RIT, radioimmunotherapy; PRIT, pretargeted RIT.
Although the aforementioned studies demonstrated that PRIT afforded superior biodistributions compared with conventional RIT, it was important to document that improved targeting also translated into enhanced efficacy and diminished toxicity. Therefore, the utility of streptavidin–antibody conjugates targeting CD20, CD22, and/or HLA-DR with an N-acetyl galactosamine dendrimeric clearing agent and 90Y-DOTA–biotin for treatment of human lymphoma xenografts in athymic mice was investigated.17,84,88 Comparative studies demonstrated marked superiority of PRIT for each of the antigenic targets with more complete tumor regressions and longer mouse survival relative to conventional one-step RIT.17,84,88 In line with the biodistribution studies, the streptavidin–antibody conjugate yielding the best tumor regression and progression-free survival after PRIT varied depending on the antigen expression levels of the lymphoma cell line. This observation emphasizes the importance of antigen density for selection of the PRIT reagent with maximal therapeutic benefit. Of note, CD20 and HLA-DR proved superior to CD22 (HD39) in the eradication of all three lymphoma xenografts.88 It has been speculated that the suboptimal outcomes with anti-CD22 PRIT is related to the rapid internalization of the CD22 antigen after streptavidin–antibody conjugate binding.89 The best survival of mice was obtained with single streptavidin–antibody conjugate rather than combinations of conjugates. This was explained by enhanced toxicity and impaired efficacy of the clearing agent, which was overwhelmed by the amount of circulating streptavidin–antibody conjugates. Although CD45 is mainly focused as a target for leukemia, this antigen may also be useful for PRIT of lymphoma as suggested by studies with a tetravalent anti-CD45 scFvSA fusion protein in nude mice bearing human lymphoma xenografts.67
Another target that has been exploited for PRIT of lymphomas and leukemias using the biotin/(strept)avidin system is CD25 (interleukin-2 receptor α chain).90 Initial studies with a chemically linked humanized anti-Tac antibody–streptavidin conjugate and 213Bi-DOTA–biotin found significant growth inhibition of adult T-cell leukemia cells xenografted in mice and prolongation of survival of these animals compared with conventional RIT.91 Subsequent studies explored genetically engineered tetrameric scFvs of anti-Tac antibody fused to streptavidin.90 This construct was used in mouse models of anaplastic large cell lymphoma and adult T-cell leukemia with 90Y-DOTA–biotin or 213Bi-DOTA–biotin, respectively; in both models, CD25-targeting PRIT effectively reduced tumors and prolonged survival of experimental animals.90
In parallel to these biotin/(strept)avidin systems, investigators have explored PRIT for lymphoma using bispecific antibodies. Sharkey et al. initially used a bispecific antibody consisting of an anti-CD20 Fab′ fragment and an antihapten Fab′ fragment and found improved efficacy in nude mice bearing human lymphoma cell line tumors.92 These investigators have subsequently developed a refined PRIT approach with a humanized construct, TF4, consisting of two anti-CD20 Fab′s and one anti-HSG Fab′ that was developed by the DNL method.93 Compared with a bivalent construct or directly radiolabeled antibody, this trivalent bispecific antibody combined with a 90Y-DOTA peptide yielded vastly improved tumor uptake and tumor-to-blood ratios and reduced hematopoietic toxicity in a murine xenograft model.93 Together with improved cure rates of animals bearing xenograft tumors, these data suggested an improved therapeutic index of PRIT with the trivalent construct relative to the bivalent construct or conventional RIT.
Unlike the experience with the biotin/(strept)avidin system in lymphoma models, the simultaneous use of two different bispecific antibodies, recognizing two different antigens and two different haptens (CD10/HSG and CD20/DTPA-In), enhanced targeting specificity in a mouse model of human Burkitt lymphoma, possibly because of binding cooperativity.94 An interesting question for future studies is whether PRIT can be improved by dual therapy with unlabeled antibodies. This possibility was suggested by recent experiments with Burkitt lymphoma-bearing nude mice, which showed that unlabeled antibodies against CD20 (veltuzumab) but not against CD22 (epratuzumab) or CD74 (milatuzumab) improved the therapeutic response achieved with 90Y-hapten-peptide PRIT.95 Benefits of such an approach may largely depend on exact experimental conditions, as indicated by the observation that tumor uptake was significantly reduced when unlabeled veltuzumab was administered in advance of the radiolabeled veltuzumab or bispecific antibody injection. Likewise, the net effect on PRIT efficacy, that is, improvement or worsening, depended on the amount of predosed unlabeled veltuzumab.95
Leukemia
Early experience of HCT in humans demonstrated the effectiveness of total body irradiation (TBI) as an antileukemic agent when used in the setting of HCT.96 Subsequently, several studies documented the importance of the TBI dose for therapeutic response. For example, two separate randomized trials, one in acute and one in chronic myeloid leukemia, showed that the relapse rate could be decreased by increasing the TBI dose from 12 to 15.75 Gy, indicating a step dose–response of leukemia cells to radiation.97–99 However, this benefit was offset by increased severe or fatal toxicities that principally involved lung, liver, and mucous membranes and lead to increased early transplant-related mortality.97–99 Nevertheless, these studies suggested the possibility of improved outcomes if additional radiation therapy could be directed to hematopoietic and lymphoid tissue while sparing normal organs; this notion formed the rational basis for RIT in leukemias. The most commonly targeted antigens on leukemias are CD33, CD45, and CD66 (Fig. 2).100,101 CD45 (leukocyte common antigen) is absent on nonhematopoietic cells but expressed on almost all hematopoietic cell lineages except mature thrombocytes, erythrocytes, and some of their progenitors.102,103 Most hematologic malignancies, including 85%–90% of acute lymphoid and myeloid leukemias, express CD45.103–106 This antigen is expressed in a relatively high copy number (200,000 binding sites per cell) and is not appreciably internalized or shed after ligand binding.107,108 Clinical studies with conventional anti-CD45 RIT have yielded encouraging results; nevertheless, many patients relapse despite myeloablative doses of radionuclides,109–111 suggesting that this approach might be more successful if the therapeutic index could be improved.
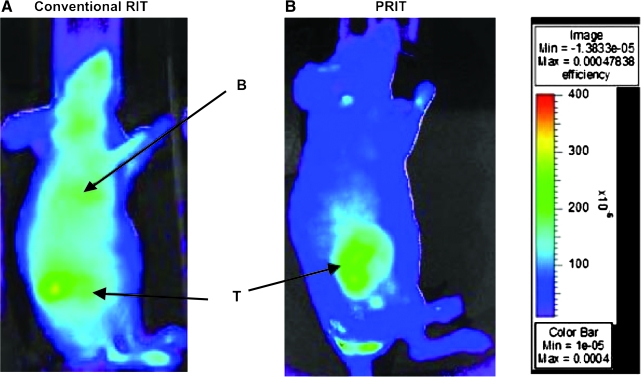
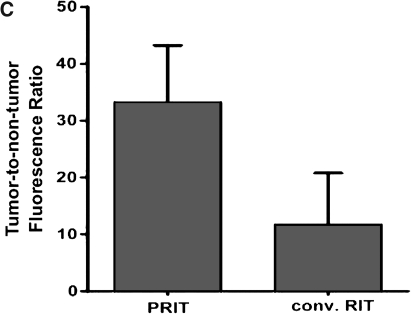
The value of PRIT targeting CD45 for leukemia has been recently assessed by directly comparing conventional anti-CD45 RIT with an anti-CD45–streptavidin conjugate in combination with a dendrimeric synthetic biotinylated clearing agent and radiolabeled biotin.112 Fluorescent imaging studies demonstrated significantly superior localization to xenografted human leukemia cell nodules with PRIT (Fig. 4). In parallel, PRIT improved tumor-to-blood and tumor-to-normal organ radioactivity concentration ratios by as much as 15-fold and resulted in markedly enhanced therapeutic efficacy.112 This was achieved despite the fact that the concentration of activity in leukemia xenografts decreased with the PRIT strategy by 43% at 24 hours compared with conventional RIT, suggesting that the clearing agent can remove significant amounts of tumor-localized streptavidin–antibody conjugate.112 To counterbalance the limitations of xenograft models, two separate syngeneic murine systems were also studied, and markedly improved hematopoietic organ-to-nonhematopoietic organ radioactivity concentrations and absorbed dose ratios were found using PRIT.112,113 This effect was primarily the result of elimination of the initial nonspecific radioactivity from circulating blood associated with conventional RIT. Radiation dose calculations suggested that PRIT could deliver twice as much radiation absorbed dose to the marrow and five times more to the spleen relative to conventional RIT.112
FIG. 4.
Fluorescent images of HEL xenograft-bearing athymic mice treated with either conventional RIT or PRIT. Mice were injected with either (A) 1.4 nmol anti-human CD45 antibody directly labeled with fluorophore or (B) pretargeted anti-human CD45 antibody–streptavidin conjugate (1.4 nmol) followed 22 hours later by a clearing agent and then by 100 mg R-phycoerythrin-biotin. Images are shown at same camera intensity settings. Images of mice are shown at 12 hours after injection of the fluorophore. Arrows indicate fluorophore in tumor (T) and blood pool (B). (C) Tumor–to–whole-body fluorescence ratios at 8 hours after injection in conventional RIT and PRIT mice. Reproduced with permission from Pagel et al.112 PRIT, pretargeted radioimmunotherapy.
Together, these murine studies demonstrated the superiority of PRIT over conventional RIT and indicated that anti-CD45 PRIT may allow intensification of targeted radiotherapy to sites of leukemic involvement with diminished toxicity. These findings were corroborated by studies in nonhuman primates (Macaca fascicularis), showing that administration of an anti-CD45 streptavidin fusion protein (BC8 scFv[4]SA) with radio-DOTA–biotin significantly improved the target-to-normal organ ratio of absorbed radiation compared with directly radiolabeled bivalent CD45 antibody.114 PRIT generated superior target-to-normal organ ratios in the blood, lung, and liver compared with conventional RIT controls. The fusion protein demonstrated superior retention in target tissues relative to comparable directly radiolabeled bivalent anti-CD45 RIT. The timepoint of administration of the second-step radiolabeled ligand significantly affected the biodistribution of radioactivity in target tissues. Rapid α-phase blood clearance of the fusion protein from the circulation obviated the need for a synthetic clearing agent in this model. The kidney was the only nontarget organ that showed higher levels of measured radiation in the pretargeted animal, suggesting that nonspecific radiation exposure to the kidney may define the dose-limiting toxicity with this two-step PRIT approach. Overall, the results from these experimental animal studies support translation of the anti-CD45 PRIT strategy to clinical trials for patients with both leukemia and lymphoma.114
Animal models of solid tumors
PRIT targeting carcioembryonic antigen
PRIT strategies were extensively evaluated in animal models of carcioembryonic antigen (CEA)–expressing tumors, particularly with bispecific antibodies. Using a bispecific antibody recognizing CEA and DTPA-In, initial studies demonstrated the superiority of PRIT over conventional RIT with respect to biodistribution and tumor growth inhibition115; a high-affinity bivalent hapten (AES reagent) provided high efficiency and selectivity.37 Although absolute tumor uptake was lower, such approaches resulted in much higher tumor-to-blood ratios compared with directly labeled anti-CEA antibodies because of rapid background clearance.116 In experimental mice bearing LS174T colon carcinomas, a bispecific antibody recognizing CEA and DTPA-In cured one third of treated animals and suppressed tumor growth for up to 8 months.117 In comparison, an 131I-labeled anti-CEA F(ab′)2 construct at similar radioactivity resulted in severe toxicity and death or recurrent disease in surviving animals after only a short period of time.117
A major focus of preclinical and clinical studies using CEA-directed PRIT has been medullary thyroid cancer.118 For example, significant antitumor efficacy but limited toxicity was observed with a bispecific anti-CEA × anti-DTPA-In antibody and an 131I-labeled bivalent hapten in experimental mice bearing medullary thyroid cancers, even in repeated treatments.119 Later, combination studies in athymic mice suggested the potential value of paclitaxel as a radiosensitizer with additive or synergistic activity when combined with this bispecific antibody.118 Immunohistological studies performed on xenografted colon or medullary thyroid carcinomas at their minimal relative post-treatment volume showed persistence of antigen expression in most cases. This suggested that repeated treatment early after the tumor reached its minimal relative volume could improve the efficacy of this approach.120 Although many studies of CEA-expressing tumors used 131I, other radionuclides were also suitable in this system.35,121
CEA targeting with a trivalent bispecific antibody binding the HSG hapten was explored for both imaging as well as therapeutic purposes.31,122 Sharkey et al. demonstrated improved tumor-to-blood ratios (by up to 40-fold or more) in mice bearing human colon cancer xenografts while increasing tumor uptake 10-fold with a trivalent PRIT construct compared with directly labeled anti-CEA Fab′ fragments.123 Subsequent therapeutic studies were conducted with a 90Y-labeled di-HSG–DOTA peptide.124 Compared with control animals treated with 90Y-labeled anti-CEA IgG, mice bearing human colon-cancer xenografts received more radioactivity to tumors and had significantly longer times to tumor progression when treated with PRIT. These comparative studies also highlighted the differences in expected dose-limiting toxicities between the two approaches, namely, hematologic toxicity in the conventional RIT approach and renal toxicity in the PRIT approach.124
CEA was also explored as a therapeutic target in preclinical studies using the biotin/(strept)avidin system and 153Sm.125 Herein, the therapeutic efficacy of PRIT and RIT appeared similar in comparative studies, but target-to-nontarget ratios were increased with PRIT while toxicity was reduced.125
PRIT targeting the epithelial cell adhesion molecule
An important construct in the development of PRIT strategies used the NR-LU-10 antibody. NR-LU-10 targets epithelial cell-adhesion molecule (EPCAM), which is expressed in various epithelial tumors, including colon, ovary, lung, and breast carcinomas.126,127 Initial preclinical experiments used a murine monoclonal antibody fused to streptavidin.16 In these landmark studies, Axworthy et al. reported 80%–100% cures with no recurrence for over 1 year in subcutaneous small-cell, breast, and colon carcinoma xenografts by using 90Y-DOTA–biotin at doses of up to 800 μCi/mouse, with negligible hematologic toxicity. In contrast, 200 μCi was dose limiting because of marrow toxicity with conventionally labeled antibodies.16 Promising tumor-to-blood ratios resulting from extremely rapid tumor uptake and blood clearance have also been obtained with this streptavidin–antibody construct when combined with 64Cu-DOTA–biotin.128 Subsequently, a modified targeting vehicle consisting of a tetrameric scFv fusion protein between humanized NR-LU-10 and streptavidin was developed.66 Similar to the initial vehicle, this construct resulted in cures in most mice with established subcutaneous human small cell lung or colon cancer xenografts when combined with a single administration of 90Y-DOTA–biotin at a dose of 800 μCi/mouse.
PRIT targeting mucin 1 glycoprotein
The anti-mucin 1 glycoprotein (MUC1) monoclonal antibody, PAM4, recognizes an epitope that is accessible in the majority of invasive pancreatic adenocarcinomas, including early stage disease, but not in normal pancreas or pancreatitis, suggesting its potential value as a tumor-targeting antibody.129,130 However, although PAM4 has been successfully used to image pancreatic cancers, and preclinical models of RIT using PAM4 appeared promising, early clinical results from a phase 1 trial with conventional 90Y-labeled RIT were relatively modest.131,132 PRIT approaches with PAM4, using a bivalent and, subsequently, a trivalent bispecific antibody that binds the HSG hapten (TF10), have recently demonstrated improved imaging abilities relative to conventional RIT.133,134 The latter showed an approximately 200-fold improved tumor-to-blood ratio when compared with directly labeled PAM4 IgG antibody.134 Given the flexibility of the HSG hapten, this system could be modified for therapeutic purposes, and radiation dose estimates suggested that PRIT with 90Y-labeled TF10 would provide a greater antitumor effect than 90Y-labeled PAM4.134 Another multivalent bispecific antibody targeting MUC1, which is composed of single chain antibody fragments covalently attached to a multifunctional polyethylene glycol scaffold, has recently been developed.135 Such approaches may be particularly interesting in combination with gemcitabine, a first-line drug of choice for pancreatic cancer that can significantly radiosensitize tumor cells.132
PRIT targeting tumor-associated glycoprotein 72 and mesothelin
A tetramer composed of four scFvs fused to a streptavidin molecule has proven to be quite effective in animal models of human colon cancer xenograft-bearing mice using an anti–tumor-associated glycoprotein 72 (TAG-72) antibody (CC49 fusion protein).136 These studies also demonstrated that gemcitabine could be combined with PRIT (400 μCi of 90Y-DOTA–biotin) as a radiosensitizer without increased hematologic toxicity.136 The same CC49 fusion construct has subsequently been explored for intraperitoneal PRIT.137 After intraperitoneal administration of the CC49 fusion protein and intravenous (i.v.) administration of a synthetic clearing agent, DOTA–biotin complexed with 111In-, 90Y-, or 177Lu was injected either i.v. or intraperitoneally. Compared with the i.v. route, intraperitoneal administration of radiolabeled DOTA–biotin resulted in superior radiolocalization. Moreover, therapeutic studies demonstrated prolonged survival of tumor-bearing mice treated with either 90Y-DOTA–biotin at doses of 400–600 μCi or 177Lu-DOTA–biotin at doses of 600–800 μCi compared with control animals, suggesting value of this approach for further clinical development.137 As a potential limitation of CC49-based therapies, renal radiotoxicity has been noted by several investigators.138 However, chemical modification of the streptavidin antibody construct, more specifically succinylation, may reduce kidney uptake of radiolabeled biotin, presumably by inhibiting reuptake of the fusion protein in the proximal renal tubules, and may improve the renal tolerability of CC49.138
A similar tetramer was developed to target mesothelin-overexpressing cancers.139 This anti-mesothelin tetravalent scFv-streptavidin fusion protein (SS1scFvSA) was tested in mice bearing subcutaneous tumors of the human epidermoid cancer cell line A431 stably transfected with mesothelin. These studies demonstrated tumor localization of SS1scFvSA and significant effects on tumors, with tumor-free survival of 86% of treated mice for 110 days. In contrast, all untreated mice died with a median survival of only 16 days.139
PRIT targeting extracellular matrix proteins
Several approaches have targeted extracellular matrix proteins in an attempt to target solid tumors. Such targets include tenascin C140 and splice variant fibronectin. For example, Moosmayer et al.141 developed a bispecific antibody (AP39xm679) consisting of a scFv (AP39) recognizing extra domain B (ED-B) fibronectin coupled to a HSG hapten-binding Fab′ fragment. Comparative studies with a directly radioiodinated divalent anti-ED-B antibody fragment showed that AP39xm679 followed by 111In-HSG–DOTA yielded superior tumor uptake in mice bearing human glioblastoma xenografts. Extrapolations suggested that PRIT could improve the therapeutic efficacy over threefold relative to conventional RIT.141
PRIT targeting carbonic anhydrase isoform IX
PRIT has yielded excellent results with a bispecific antibody (G250xDTIn-1) in several mouse models of renal cell carcinoma.142,143 In this construct, the hapten-binding arm is derived from a monoclonal antibody, DTIn-1, which reacts specifically with DTPA loaded with indium. The hybridoma cell fusion partner produces the antirenal cell carcinoma antibody, G250, which targets anticarbonic anhydrase isoform IX.144,145 Studies with this construct have explored various radionuclides and found beneficial properties with 188Re. This radionuclide yielded significantly higher tumor uptake than 131I, although tumor uptake and retention of 131I could be improved with a peptidase-resistant bivalent peptide consisting of four d-amino acids rather than the previously used radiolabeled l-amino acid peptide.146,147 Interestingly, these preclinical studies found no correlation between target antigen expression and tumor uptake of the radionuclide, pointing to the importance of other tumor characteristics, such as vascular permeability, for PRIT efficacy.142
PRIT targeting the Lewis antigen
Another set of studies have explored PRIT strategies using a monoclonal antibody (B3) directed at Lewisy and polyfucosylated-Lewisx, which are found on many mucinous carcinomas of the colon, stomach, and ovary but only on a limited number of normal tissues.148 A B3/streptavidin conjugate in combination with a synthetic clearing agent and 90Y-DOTA–biotin was tested in mice bearing A431 tumor xenografts, a human epidermoid carcinoma cell line expressing the antigen recognized by B3.149 Compared with various controls, B3-streptavidin-based PRIT resulted in cure of a significant proportion of mice and dramatic prolongation of survival times.149
Clinical PRIT Studies
As summarized above, the accumulated wealth of preclinical studies indicates compelling advantages of PRIT over RIT to target radiation to malignant cells and supports the clinical development of this approach to induce tumor responses, diminish relapse rates, and improve survival. In fact, a small number of studies exploring PRIT in the clinic have already been conducted (Table 2).
Table 2.
Clinical Studies Using Pretargeted Radioimmunotherapy
| Study | N | Disease | Targeting vehicle | Effector | Other therapy | Results |
|---|---|---|---|---|---|---|
| Weiden et al.150 | 10 | Recurrent/relapsed B-cell non-Hodgkin's lymphoma | Anti-CD20 Ab-SA | 90Y-DOTA–biotin | None | 6 objective tumor responses in 7 patients treated with 90Y-DOTA–biotin: 3 CR and 1 PR |
| Forero et al.151 | 15 | Recurrent/relapsed B-cell non-Hodgkin's lymphoma | Anti-CD20 (scFv[4])SA | 90Y-DOTA–biotin | None | 14 evaluable patients: 2 CR (lasting 91 and 325 days), 1 PR (lasting 297 days), and 3 SD. No significant hematologic toxicity related to PRIT; substantial HAMA in 3 patients |
| Kraeber-Bodéré et al.153 | 26 | Recurrent medullary thyroid cancer | Anti-CEA × anti-DTPA-In (bsAb) | 131I-labeled bivalent hapten | None | 17 evaluable patients: 4 with pain reliefs, 5 with minor tumor responses, and 4 with biological responses (decrease of thyrocalcitonin). HAMA in 9 patients; bone marrow suppression as the dose-limiting toxicity |
| Vuillez et al.154 | 14 | Relapsed small cell lung carcinoma | Anti-CEA × anti-DTPA-In (bsAb) | 131I-labeled bivalent hapten | Autologous stem cell support (if >150 mCi) | 12 evaluable patients: 2 partial responses (1 almost complete for 3 months) and 1 SD (>24 months); bone marrow suppression as the dose-limiting toxicity |
| Kraeber-Bodéré et al.81,155 | 22 | CEA-producing tumors: colon/rectum/lung/pleura (13), medullary thyroid cancer (9) | Anti-CEA × anti-DTPA-In (chimeric bsAb) | 131I-labeled bivalent hapten | None | 20 evaluable patients: 9 with SD (3–12+ months); toxicity and efficacy higher in 75 mg/m2 than 40 mg/m2 bsAb dose. HAMA in 4 patients |
| Paganelli et al.79 | 48 | Residual or recurrent grade III and grade IV gliomas | Anti-tenascin moAb-biotin | 90Y-DOTA–biotin | None | 12 objective responses after 2 months of therapy, including 3 CR; 52% with SD |
| Grana et al.162 | 37 | High-grade glioma: grade III (17), grade IV (20). Use in adjuvant setting after surgery/radiotherapy | Anti-tenascin moAb-biotin | 90Y-DOTA–biotin | None | 19 patients received PRIT and 18 nontreated controls; improved outcome in both glioma III and glioma IV subgroups |
| Paganelli et al.163 | 24 | Recurrent glioma. Local PRIT administration catheter placed in tumor cavity | Anti-tenascin moAb-biotin | 90Y-DOTA–biotin | None | 6 objective responses (2 PR and 4 minor responses) and 12 SD |
| Knox et al.160 | 25 | Metastatic colon cancer | Anti-EPCAM moAb-SA | 90Y-DOTA–biotin | None | 2 PR and 4 SD |
Ab-SA, antibody streptavidin; 90Y, yttrium-90; DOTA, 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic; CR, complete remission; PR, partial remission; scFv, single-chain variable fragment; SD, stable disease; PRIT, pretargeted radioimmunotherapy; HAMA, human anti-mouse antibody; CEA, carcioembryonic antigen; DTPA, diethylenetriaminepentaacetic acid; bsAb, bispecific antibody; moAb, monoclonal antibody; EPCAM, epithelial cell adhesion molecule.
Clinical studies of hematologic malignancies
Lymphoma
The feasibility of anti-CD20 PRIT has been established in two early phase clinical trials for patients with advanced B-cell non-Hodgkin's lymphoma.150,151 The first study used the chimeric anti-CD20 antibody C2B8 (rituximab) that was chemically conjugated to streptavidin followed by a synthetic clearing agent and DOTA–biotin labeled with either 111In for imaging or 90Y (30 or 50 mCi/m2) for therapy.150 All 10 enrolled patients received prior therapy including high-dose chemotherapy and HCT, 131I-tositumomab, or prior rituximab. In 3 patients, the C2B8/streptavidin conjugate was directly radiolabeled with trace amounts of 186Re to assess the pharmacokinetics and biodistribution of the targeting vehicle. These imaging studies confirmed that the conjugate localized to known tumor sites and that the clearing agent removed >95% of the conjugate from the circulation. DOTA–biotin resulted in tumor targeting within 10 minutes after the injection and identified previously unknown disease sites in several patients. The estimated tumor–to–whole-body dose ratio of 38:1 achieved with PRIT was higher than previously reported with conventional RIT.150 Six (6) of 7 patients who received 90Y-DOTA–biotin achieved objective tumor regression, including three complete remissions (CRs) and one partial remission (PR). The regimen was safe and well tolerated; only grade 1/2 nonhematologic toxicity was observed, with the most prevalent toxicity being fatigue. Five (5) of the 7 patients experienced transient grade 3 hematologic toxicity. Delayed and transient humoral responses were recorded in 6 of the 10 patients.150 A subsequent phase 1 clinical study studied a tetrameric single-chain anti-CD20-streptavidin fusion protein (B9E9FP)65 at two different doses (160 and 320 mg/m2) in 15 patients with relapsed or refractory B-cell non-Hodgkin lymphoma; 14 of these 15 patients not only received 111In-DOTA–biotin but also 90Y-DOTA–biotin (15 mCi/m2).151 The synthetic clearing agent was highly efficacious in reducing plasma levels of circulating B9E9FP within 6 hours. The ratio of average tumor–to–whole-body radiation dose was 49:1. No significant drug-associated hematologic toxicities were noted, and nonhematologic toxicities were mild and transient. The study also provided some evidence of clinical efficacy. Specifically, among 14 evaluable patients, 2 patients achieved CRs of 91 and 325 days' duration, respectively, and 1 patient had a PR that lasted 297 days, for an overall response rate of 21%. Three (3) additional patients had stable disease (SD).151 Three (3) patients had substantial immune responses directed to both streptavidin and the V-region of B9E9FP, and 5 patients had transient low-titer antibody responses; these responses appeared more frequent in patients receiving a higher dose of B9E9FP.151
In contrast to the more limited success of tenascin-targeting PRIT in glioma patients with active disease, Palumbo et al. recently reported the successful treatment of a patient with anaplastic large cell lymphoma with this strategy.152 This 6-year-old heavily pretreated girl relapsed 57 days after cord blood HCT with an endobronchial tumor that was CD20 negative but highly expressed tenascin. The patient initially received therapy with cyclosphosphamide and vinblastine but had endobronchial and pulmonary tumor relapses. Subsequently, the patient received biotinylated ST2146 (tentumomob) followed by 90Y-DOTA–biotin as a compassionate therapy and experienced a CR that was ongoing at 10 months at the time of reporting.152
Clinical studies of solid tumors
PRIT targeting CEA
The first clinical experience using a bispecific antibody-AES-based approach was reported on 26 patients with recurrent medullary thyroid cancer.153 This phase 1/2 trial used escalating doses of anti-CEA × anti-DTPA-In, followed 4 days later by a 131I-labeled bivalent hapten. The maximum tolerated activity was 48 mCi/m2 (1.3 GBq/m2), with bone-marrow suppression being the dose-limiting toxicity. Grade 3 or 4 hematologic toxicities occurred in 7 patients, most of them with bone metastases.153 Tumor responses were relatively modest: Among 17 evaluable patients, five minor tumor responses and four biological responses with decreases of thyrocalcitonin were observed. However, 9 patients developed HAMA responses.153 The same bispecific antibody system and schedule have been used in a similar phase 1/2 trial that studied 14 patients with relapsed small-cell lung carcinoma.154 These patients received 1.48–6.66 GBq (40–180 mCi) of 131I; autologous HCT was used if ≥150 mCi was administered. Bone-marrow suppression was again noted as the main toxicity, with grade 2 leukopenia in 2 patients and grade 3 or 4 thrombocytopenia in 3 patients. The estimated tumor dose was 2.6–32.2 cGy/mCi (0.7–8.7 Gy/BGq). Among 12 evaluable patients, 2 PR and 1 SD lasting for more than 24 months were observed. Efficiency and toxicity were dose related and the maximal tolerable dose without hematologic rescue was 150 mCi (5.6 GBq).154
Later studies used a chimeric bispecific antibody to decrease the immunogenicity and HAMA responses. Thirty-five (35) patients with CEA-producing tumors were treated with hMN-14 × m734, followed by 131I-hapten.81 This study established a dose of the bispecific antibody of 40 mg/m2 with administration of the hapten after 5 days as optimal for rapid hapten clearance and reduced hematologic toxicity without compromising tumor uptake.81 Clinical results were subsequently reported for 22 patients with cancer of the colon, rectum, lung, or pleura (13 patients) or medullary thyroid cancer (9 patients) receiving either 75 mg/m2 (11 patients) or 40 mg/m2 (11 patients) of the targeting vehicle and 1.9–5.5 GBq of 131I-di-DTPA. Among 20 evaluable patients, SD for 3–12 + months was noted in 9 patients, 6 of whom had medullary thyroid cancer.155 Both toxicity and efficacy were dose-dependent, with significantly higher rates of disease stabilization (64% vs. 22%), bone-marrow suppression, and hepatotoxicity with the higher dose.155 As predicted, the likelihood of HAMA development appeared reduced relative to previous constructs, but human anti-human responses were observed in 4 patients.155
A follow-up study subsequently compared the outcome of 29 evaluable patients with recurrent medullary thyroid carcinoma with that of 39 contemporaneous untreated patients.156 In another nonrandomized and retrospective study, control patients were selected based on comparable serum calcitonin doubling time, a prognostic indicator of survival.157 This study indicated that anti-CEA PRIT induced long-term disease stabilization in a significant number of patients, including 47% of high-risk patients. Furthermore, although there was no survival advantage in the entire study cohort, median survival was significantly longer in PRIT patients than control patients in the high-risk subgroup (110 versus 61 months; p < 0.03),156 providing first evidence for the therapeutic efficacy of PRIT in metastatic, progressive solid tumors.
PRIT targeting TAG-72
Another PRIT approach for the treatment of colon cancer employed the CC49 fusion protein, the streptavidin-containing tetramer targeting TAG-72. Forero-Torres et al studied 9 patients with metastatic colorectal cancer who were given 160 mg/m2 of CC49 fusion protein, a synthetic clearing agent, and 5 mCi of 111In-DOTA–biotin for imaging and dosimetry; 5 patients also received 10 mCi/m2 of 90Y-DOTA–biotin. The study confirmed the preclinical findings of rapid tumor localization of DOTA–biotin and rapid biphasic plasma clearance; high tumor-to-marrow and tumor–to–whole-body radiation doses were achieved, with reduced radiation to normal organs from circulating radionuclide and absence of infusion-related renal, hepatic, or hematologic toxicities.158,159 Based on previous data obtained with 90Y-labeled CC49, it was estimated that PRIT resulted in about 8–11-fold higher tumor-to-normal organ dose ratios.159
PRIT targeting EPCAM
The biotin/(strept)avidin system was explored in early studies targeting the EPCAM antigen. An initial phase 1 study enrolled 43 patients with EPCAM-positive epithelial malignancies including colorectal, lung, pancreas, gastroesophageal, bladder, breast, and ovarian cancers.78 Using a streptavidin conjugate of the murine NR-LU-10 antibody, Breitz et al. found a tumor-to-marrow dose ratio of 63:1, which was favorably comparable to the 6:1 ratio reported previously for conventional RIT.78 This PRIT approach proved feasible and safe, with no significant adverse events observed after initial administration of any of the components. In a subsequent phase 2 trial, 25 patients with metastatic colon carcinoma received the same targeting vehicle, a clearing agent, and 90Y-DOTA–biotin (110 mCi/m2).160 Although this study confirmed the validity of the PRIT concept, the clinical results were modest with 2 CR and 4 SD.160 111In-based images identified the intestinal tract and the kidneys as potential organs at risk of clinically significant radiation toxicity161; these findings correlated well with observed toxicities (16% grade 4 diarrhea, 8% delayed renal toxicity).160,161
PRIT targeting tenascin
Tenascin was explored as a target in PRIT studies of glioma patients. A phase 1/2 study used a biotinylated antitenascin monoclonal antibody in combination with avidin and streptavidin as clearing agent and 90Y-labeled biotin (2.22–2.96 GBq/m2) in 48 patients with residual or recurrent grade III or IV glioma.79 Twelve (12) patients showed an objective reduction in tumor mass at 2 months after treatment, including 3 patients with CR; 8 of these 12 patients had responses that lasted for more than 12 months. An additional 52% of patients had SD. A subsequent open, controlled, nonrandomized study enrolled 17 patients with grade III and 20 patients with grade IV glioma in the adjuvant setting after receiving surgery and radiotherapy (all patients) and platinum-based chemotherapy (9 patients). At the time of the study, patients were tumor-free, as confirmed by neuroradiological examination. Nineteen (19) patients received PRIT with 90Y-DOTA–biotin, whereas 18 patients received no further treatment and served as controls. The outcome was better for patients receiving PRIT in thegrade III and grade IV glioma subgroups. Specifically, among the grade IV patients, 5 of 8 treated and 12 of 12 untreated patients died, yielding an estimated median overall survival of 33.5 versus 8 months in treated and untreated patients, respectively. Among the grade III glioma patients, 2 of 11 treated and 4 of 6 untreated patients died, yielding a significantly different estimated median overall survival (not reached versus 33 months).162 As an alternative approach to systemic PRIT, Paganelli et al. studied 24 patients with recurrent glioma who underwent a second debulking surgery. The biotinylated antitenascin monoclonal antibody, the clearing agent, and the 90Y-DOTA–biotin were administered through a catheter implanted into the surgical resection cavity.163 Two radionuclide doses from 0.555 to 1.110 GBq were given to each patient at 8–10 weeks apart; further dose escalation was limited by neurological toxicity. A total of 6 patients showed an objective response, including 2 PR and 4 minor responses, and 12 patients showed SD.163
As of December 28, 2009, at least three studies investigating PRIT-based approaches were registered at ClinicalTrials.Gov. The first study (NCT00860860) investigates the toxicity, safety, and pharmacokinetics of an anti-CEA × antihapten bispecific antibody (TF2) with the 177Lu-labeled di-HSG–DOTA peptide IMP-288 in patients with colorectal cancer. The second study (NCT00467506) assesses the efficacy and toxicity of an anti-CEA × anti-DTPA bispecific antibody with 131I-labeled di-DTPA peptide in patients with recurrent medullary thyroid carcinoma. Finally, the third study (NCT00988715) investigates an anti-CD45 streptavidin fusion protein and 90Y-DOTA–biotin in combination with TBI and peripheral blood HCT for patients with high-risk acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndromes.
Conclusions and Perspective
Research over the last two decades has established the feasibility and safety of PRIT and demonstrated its ability to improve directed delivery of radiation to malignant cells in preclinical as well as early phase clinical studies. Despite these encouraging results, PRIT faces challenges before its widespread clinical use. Importantly, PRIT remains a complex, multidisciplinary effort; the requirement for injections of multiple reagents at specified time intervals may undermine its general acceptance. Controlled clinical trials will define preferred PRIT strategies and, ultimately, determine whether the addition of PRIT indeed improves patient outcomes. Future studies will explore alternative antigenic targets and radionuclides, for example, α-emitters with short half-lives, to improve therapeutic results. Additional improvements may also come from the use of other novel strategies, such as the integration of unlabeled antibodies into PRIT-based therapies.95 Undoubtedly, PRIT will remain an exciting avenue because of constant methodological refinements. With continued research, it holds the potential to establish itself as an important treatment modality for hematologic and other malignancies for small-volume disease, as radiation boost in combination with other treatments such as stem-cell rescue, or as adjuvant therapy.
Acknowledgments
This work was supported by the NCI/NIH grants (PO1-CA44991, R01-CA76287, R01-CA109663, R01-CA136639, K08-CA95448, and K23-CA137161), the Leukemia and Lymphoma Society (SCOR grant no. 7040-08), and the Frederick Kullman and Penny E. Petersen Memorial Funds. O.W.P. was supported by an endowed chair from James and Shirley Raisbeck. J.M.P. was supported by career development awards from the Lymphoma Research Foundation and the Damon Runyon Cancer Foundation.
Disclosure Statement
No competing financial interests exist.
About the Authors

Roland B. Walter received his medical degree in 1997 from the University of Zürich in Switzerland and completed his internal medicine residency in Switzerland. In 2002, he joined the Fred Hutchinson Cancer Research Center, in Seattle, WA, to be trained as a physician/scientist in hematology/oncology. He earned a doctorate degree in experimental pathology from the University of Washington in Seattle in 2006 and finished a fellowship in hematology at the same institution in 2007.
Dr. Walter's primary research interest is CD33-targeted immunotherapy in AML; he is also the principal investigator in several clinical trials on AML. To support his translational AML studies, he has received several career development awards and research grants. Dr. Walter is currently an assistant member in the Clinical Research Division at the Fred Hutchinson Cancer Research Center and an assistant professor of medicine in the Division of Hematology at the University of Washington.

Oliver W. Press is a member of the Fred Hutchinson Cancer Research Center and a professor of medicine at the University of Washington in both in Seattle. After earning a doctoral degree in biological structure from the University of Washington, Dr. Press received his medical degree from the same institution. He completed an internship and residency training at the Massachusetts General Hospital and a fellowship at the Harvard Medical School in Boston before returning to Seattle to continue his career.
Dr. Press's research is centered on the investigation of novel treatments for hematologic malignancies, particularly the use of radiolabeled monoclonal antibodies. He is co-chair of the National Cancer Institute's Lymphoma Steering Committee, a member of the Scientific Advisory Board and the Executive Committee of the Lymphoma Research Foundation, as well as chairman of the Follicular Lymphoma Consortium. He has served as a principal investigator for many clinical trials and NIH grants and has written more than 250 original peer-reviewed articles, review papers, editorials, and other publications on the treatment of hematologic malignancies.
John M. Pagel is an assistant professor in the Division of Oncology at the University of Washington and an assistant member at the Fred Hutchinson Cancer Research Center in Seattle, WA. In addition, Dr. Pagel is a member of several professional societies, including the American Association of Cancer Research, the American Society of Hematology, and the American Association of Clinical Oncology. Dr. Pagel has published many research articles in a variety of publications, including Blood, Journal of Clinical Oncology, Bone Marrow Transplantation, and the Proceedings of the National Academy of Sciences. He has received numerous awards, including the ASCO Young Investigator Award, Lymphoma Research Foundation Career Development Award, and a Damon Runyon Clinical Investigator Award. Dr. Pagel earned his medical degree from the Boston University School of Medicine in Boston, MA, and a doctorate of philosophy in microbiology and molecular genetics from the University of California, Irvine.
References
- 1.Park SI. Press OW. Radioimmunotherapy for treatment of B-cell lymphomas and other hematologic malignancies. Curr Opin Hematol. 2007;14:632. doi: 10.1097/MOH.0b013e3282efb17c. [DOI] [PubMed] [Google Scholar]
- 2.DeNardo GL. DeNardo SJ. Balhorn R. Systemic radiotherapy can cure lymphoma: A paradigm for other malignancies? Cancer Biother Radiopharm. 2008;23:383. doi: 10.1089/cbr.2007.0523-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green DJ. Pagel JM. Pantelias A, et al. Pretargeted radioimmunotherapy for B-cell lymphomas. Clin Cancer Res. 2007;13:5598s. doi: 10.1158/1078-0432.CCR-07-1223. [DOI] [PubMed] [Google Scholar]
- 4.Strand SE. Zanzonico P. Johnson TK. Pharmacokinetic modeling. Med Phys. 1993;20:515. doi: 10.1118/1.597047. [DOI] [PubMed] [Google Scholar]
- 5.Chinol M. Grana C. Gennari R, et al. Pretargeted radio of cancer. In: Abrams PG, editor; Fritzberg AR, editor. Radioimmunotherapy of Cancer. New York: Marcel Dekker; 2000. p. 169. [Google Scholar]
- 6.Slavin-Chiorini DC. Horan Hand P. Greiner JW. Antibodies and novel constructs for tumor targeting. In: Abrams PG, editor; Fritzberg AR, editor. Radioimmunotherapy of Cancer. New York: Marcel Dekker; 2000. p. 137. [Google Scholar]
- 7.Pagel JM. Boerman OC. Breitz HB, et al. Targeted radionuclide therapy of cancer. In: Oldham RK, editor; Dillman RO, editor. Principles of Cancer Biotherapy. 5th. New York: Springer; 2009. p. 463. [Google Scholar]
- 8.Chang CH. Sharkey RM. Rossi EA, et al. Molecular advances in pretargeting radioimmunotherapy with bispecific antibodies. Mol Cancer Ther. 2002;1:553. [PubMed] [Google Scholar]
- 9.Axworthy DB. Fritzberg AR. Hylarides MD, et al. Preclinical evaluation of an anti-tumor monoclonal antibody/streptavidin conjugate for pretargeted 90Y radioimmunotherapy in a mouse xenograft model [abstr] J Immunother. 1994;16:158. [Google Scholar]
- 10.Goldenberg DM. Chatal JF. Barbet J, et al. Cancer imaging and therapy with bispecific antibody pretargeting. Update Cancer Ther. 2007;2:19. doi: 10.1016/j.uct.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milenic DE. Yokota T. Filpula DR, et al. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991;51:6363. [PubMed] [Google Scholar]
- 12.Yokota T. Milenic DE. Whitlow M, et al. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402. [PubMed] [Google Scholar]
- 13.Schott ME. Milenic DE. Yokota T, et al. Differential metabolic patterns of iodinated versus radiometal chelated anticarcinoma single-chain Fv molecules. Cancer Res. 1992;52:6413. [PubMed] [Google Scholar]
- 14.Buist MR. Kenemans P. den Hollander W, et al. Kinetics and tissue distribution of the radiolabeled chimeric monoclonal antibody MOv18 IgG and F(ab′)2 fragments in ovarian carcinoma patients. Cancer Res. 1993;53:5413. [PubMed] [Google Scholar]
- 15.Goodwin DA. Meares CF. Pretargeting: General principles; October 10–12, 1996. Cancer. 1997;80(suppl):2675. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2675::aid-cncr45>3.3.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Axworthy DB. Reno JM. Hylarides MD, et al. Cure of human carcinoma xenografts by a single dose of pretargeted yttrium-90 with negligible toxicity. Proc Natl Acad Sci U S A. 2000;97:1802. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Press OW. Corcoran M. Subbiah K, et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood. 2001;98:2535. doi: 10.1182/blood.v98.8.2535. [DOI] [PubMed] [Google Scholar]
- 18.Pagel JM. Hedin N. Subbiah K, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg DM. Sharkey RM. Paganelli G, et al. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin DA. Meares CF. Advances in pretargeting biotechnology. Biotechnol Adv. 2001;19:435. doi: 10.1016/s0734-9750(01)00065-9. [DOI] [PubMed] [Google Scholar]
- 21.Boerman OC. van Schaijk FG. Oyen WJ, et al. Pretargeted radioimmunotherapy of cancer: Progress step by step. J Nucl Med. 2003;44:400. [PubMed] [Google Scholar]
- 22.Sharkey RM. Goldenberg DM. Novel radioimmunopharmaceuticals for cancer imaging and therapy. Curr Opin Investig Drugs. 2008;9:1302. [PubMed] [Google Scholar]
- 23.Hawkins GA. McCabe RP. Kim CH, et al. Delivery of radionuclides to pretargeted monoclonal antibodies using dihydrofolate reductase and methotrexate in an affinity system. Cancer Res. 1993;53(suppl):2368. [PubMed] [Google Scholar]
- 24.Reardan DT. Meares CF. Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316:265. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 25.Schuhmacher J. Klivenyi G. Matys R, et al. Multistep tumor targeting in nude mice using bispecific antibodies and a gallium chelate suitable for immunoscintigraphy with positron emission tomography. Cancer Res. 1995;55:115. [PubMed] [Google Scholar]
- 26.Klivenyi G. Schuhmacher J. Patzelt E, et al. Gallium-68 chelate imaging of human colon carcinoma xenografts pretargeted with bispecific anti-CD44V6/anti-gallium chelate antibodies. J Nucl Med. 1998;39:1769. [PubMed] [Google Scholar]
- 27.Goodwin DA. Meares CF. McTigue M, et al. Monoclonal antibody hapten radiopharmaceutical delivery. Nucl Med Commun. 1986;7:569. doi: 10.1097/00006231-198608000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin DA. Meares CF. McCall MJ, et al. Pre-targeted immunoscintigraphy of murine tumors with indium-111-labeled bifunctional haptens. J Nucl Med. 1988;29:226. [PubMed] [Google Scholar]
- 29.Mirallié E. Saï-Maurel C. Faivre-Chauvet A, et al. Improved pretargeted delivery of radiolabelled hapten to human tumour xenograft in mice by avidin chase of circulating bispecific antibody. Eur J Nucl Med Mol Imaging. 2005;32:901. doi: 10.1007/s00259-005-1811-2. [DOI] [PubMed] [Google Scholar]
- 30.Rossi EA. Sharkey RM. McBride W, et al. Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin Cancer Res. 2003;9:3886S. [PubMed] [Google Scholar]
- 31.Rossi EA. Chang CH. Losman MJ, et al. Pretargeting of carcinoembryonic antigen-expressing cancers with a trivalent bispecific fusion protein produced in myeloma cells. Clin Cancer Res. 2005;11(suppl):7122s. doi: 10.1158/1078-0432.CCR-1004-0020. [DOI] [PubMed] [Google Scholar]
- 32.Sharkey RM. Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(suppl):115S. [PubMed] [Google Scholar]
- 33.Le Doussal JM. Martin M. Gautherot E, et al. In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: Enhanced divalent hapten affinity for cell-bound antibody conjugate. J Nucl Med. 1989;30:1358. [PubMed] [Google Scholar]
- 34.Barbet J. Kraeber-Bodere F. Vuillez JP, et al. Pretargeting with the affinity enhancement system for radioimmunotherapy. Cancer Biother Radiopharm. 1999;14:153. doi: 10.1089/cbr.1999.14.153. [DOI] [PubMed] [Google Scholar]
- 35.Karacay H. McBride WJ. Griffiths GL, et al. Experimental pretargeting studies of cancer with a humanized anti-CEA x murine anti-[In-DTPA] bispecific antibody construct and a (99m)Tc-/(188)Re-labeled peptide. Bioconjug Chem. 2000;11:842. doi: 10.1021/bc0000379. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin DA. Meares CF. McTigue M, et al. Pretargeted immunoscintigraphy: Effect of hapten valency on murine tumor uptake. J Nucl Med. 1992;33:2006. [PubMed] [Google Scholar]
- 37.Gautherot E. Le Doussal JM. Bouhou J, et al. Delivery of therapeutic doses of radioiodine using bispecific antibody-targeted bivalent haptens. J Nucl Med. 1998;39:1937. [PubMed] [Google Scholar]
- 38.Boerman OC. Kranenborg MH. Oosterwijk E, et al. Pretargeting of renal cell carcinoma: Improved tumor targeting with a bivalent chelate. Cancer Res. 1999;59:4400. [PubMed] [Google Scholar]
- 39.Morel A. Darmon M. Delaage M. Recognition of imidazole and histamine derivatives by monoclonal antibodies. Mol Immunol. 1990;27:995. doi: 10.1016/0161-5890(90)90122-g. [DOI] [PubMed] [Google Scholar]
- 40.Janevik-Ivanovska E. Gautherot E. Hillairet de Boisferon M, et al. Bivalent hapten-bearing peptides designed for iodine-131 pretargeted radioimmunotherapy. Bioconjug Chem. 1997;8:526. doi: 10.1021/bc970083h. [DOI] [PubMed] [Google Scholar]
- 41.Sharkey RM. McBride WJ. Karacay H, et al. A universal pretargeting system for cancer detection and therapy using bispecific antibody. Cancer Res. 2003;63:354. [PubMed] [Google Scholar]
- 42.Morandeau L. Benoist E. Loussouarn A, et al. Synthesis of new bivalent peptides for applications in the Affinity Enhancement System. Bioconjug Chem. 2005;16:184. doi: 10.1021/bc0497721. [DOI] [PubMed] [Google Scholar]
- 43.Rossi EA. Goldenberg DM. Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CH. Rossi EA. Goldenberg DM. The dock and lock method: A novel platform technology for building multivalent, multifunctional structures of defined composition with retained bioactivity. Clin Cancer Res. 2007;13(suppl):5586s. doi: 10.1158/1078-0432.CCR-07-1217. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg DM. Rossi EA. Sharkey RM, et al. Multifunctional antibodies by the dock-and-lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med. 2008;49:158. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 46.Hnatowich DJ. Virzi F. Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294. [PubMed] [Google Scholar]
- 47.Wilchek M. Bayer EA. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988;171:1. doi: 10.1016/0003-2697(88)90120-0. [DOI] [PubMed] [Google Scholar]
- 48.Sung C. van Osdol WW. Saga T, et al. Streptavidin distribution in metastatic tumors pretargeted with a biotinylated monoclonal antibody: Theoretical and experimental pharmacokinetics. Cancer Res. 1994;54:2166. [PubMed] [Google Scholar]
- 49.Green NM. Avidin. 1. The use of (14-C)biotin for kinetic studies and for assay. Biochem J. 1963;89:585. doi: 10.1042/bj0890585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodwin DA. Meares CF. Osen M. Biological properties of biotin-chelate conjugates for pretargeted diagnosis and therapy with the avidin/biotin system. J Nucl Med. 1998;39:1813. [PubMed] [Google Scholar]
- 51.Sharkey RM. Karacay H. Griffiths GL, et al. Development of a streptavidin–anti-carcinoembryonic antigen antibody, radiolabeled biotin pretargeting method for radioimmunotherapy of colorectal cancer: Studies in a human colon cancer xenograft model. Bioconjug Chem. 1997;8:595. doi: 10.1021/bc970101v. [DOI] [PubMed] [Google Scholar]
- 52.Chauhan J. Dakshinamurti K. Purification and characterization of human serum biotinidase. J Biol Chem. 1986;261:4268. [PubMed] [Google Scholar]
- 53.Foulon CF. Alston KL. Zalutsky MR. Astatine-211-labeled biotin conjugates resistant to biotinidase for use in pretargeted radioimmunotherapy. Nucl Med Biol. 1998;25:81. doi: 10.1016/s0969-8051(97)00166-2. [DOI] [PubMed] [Google Scholar]
- 54.Sabatino G. Chinol M. Paganelli G, et al. A new biotin derivative-DOTA conjugate as a candidate for pretargeted diagnosis and therapy of tumors. J Med Chem. 2003;46:3170. doi: 10.1021/jm030789z. [DOI] [PubMed] [Google Scholar]
- 55.Urbano N. Papi S. Ginanneschi M, et al. Evaluation of a new biotin-DOTA conjugate for pretargeted antibody-guided radioimmunotherapy (PAGRIT) Eur J Nucl Med Mol Imaging. 2007;34:68. doi: 10.1007/s00259-006-0124-4. [DOI] [PubMed] [Google Scholar]
- 56.Sung C. van Osdol WW. Pharmacokinetic comparison of direct antibody targeting with pretargeting protocols based on streptavidin–biotin binding. J Nucl Med. 1995;36:867. [PubMed] [Google Scholar]
- 57.Theodore LJ. Fritzberg AR. Schultz JE, et al. Evolution of a pretarget radioimmunotherapeutic regimen. In: Abrams PG, editor; Fritzberg AR, editor. Radioimmunotherapy of Cancer. New York: Marcel Dekker; 2000. p. 195. [Google Scholar]
- 58.Ashwell G. Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 59.Moi MK. Meares CF. DeNardo SJ. The peptide way to macrocyclic bifunctional chelating agents: Synthesis of 2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecane N,N′,N′′,N′′′-tetraacetic acid and the study of its yttrium (III) complex. J Am Chem Soc. 1988;10:6266. doi: 10.1021/ja00226a063. [DOI] [PubMed] [Google Scholar]
- 60.Su FM. Beaumier P. Axworthy D, et al. Pretargeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl Med Biol. 2005;32:741. doi: 10.1016/j.nucmedbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Yao Z. Zhang M. Garmestani K, et al. Pretargeted alpha emitting radioimmunotherapy using (213)Bi 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid-biotin. Clin Cancer Res. 2004;10:3137. doi: 10.1158/1078-0432.ccr-03-0171. [DOI] [PubMed] [Google Scholar]
- 62.Lindegren S. Andersson H. Jacobsson L, et al. Synthesis and biodistribution of 211At-labeled, biotinylated, and charge-modified poly-l-lysine: Evaluation for use as an effector molecule in pretargeted intraperitoneal tumor therapy. Bioconjug Chem. 2002;13:502. doi: 10.1021/bc010054d. [DOI] [PubMed] [Google Scholar]
- 63.Lindegren S. Karlsson B. Jacobsson L, et al. (211)At-labeled and biotinylated effector molecules for pretargeted radioimmunotherapy using poly-l- and poly-d-Lysine as multicarriers. Clin Cancer Res. 2003;9:3873S. [PubMed] [Google Scholar]
- 64.Yao Z. Zhang M. Kobayashi H, et al. Improved targeting of radiolabeled streptavidin in tumors pretargeted with biotinylated monoclonal antibodies through an avidin chase. J Nucl Med. 1995;36:837. [PubMed] [Google Scholar]
- 65.Schultz J. Lin Y. Sanderson J, et al. A tetravalent single-chain antibody–streptavidin fusion protein for pretargeted lymphoma therapy. Cancer Res. 2000;60:6663. [PubMed] [Google Scholar]
- 66.Goshorn S. Sanderson J. Axworthy D, et al. Preclinical evaluation of a humanized NR-LU-10 antibody-streptavidin fusion protein for pretargeted cancer therapy. Cancer Biother Radiopharm. 2001;16:109. doi: 10.1089/108497801300189209. [DOI] [PubMed] [Google Scholar]
- 67.Lin Y. Pagel JM. Axworthy D, et al. A genetically engineered anti-CD45 single-chain antibody–streptavidin fusion protein for pretargeted radioimmunotherapy of hematologic malignancies. Cancer Res. 2006;66:3884. doi: 10.1158/0008-5472.CAN-05-3443. [DOI] [PubMed] [Google Scholar]
- 68.Bos ES. Kuijpers WH. Meesters-Winters M, et al. In vitro evaluation of DNA-DNA hybridization as a two-step approach in radioimmunotherapy of cancer. Cancer Res. 1994;54:3479. [PubMed] [Google Scholar]
- 69.Kuijpers WH. Bos ES. Kaspersen FM, et al. Specific recognition of antibody-oligonucleotide conjugates by radiolabeled antisense nucleotides: A novel approach for two-step radioimmunotherapy of cancer. Bioconjug Chem. 1993;4:94. doi: 10.1021/bc00019a013. [DOI] [PubMed] [Google Scholar]
- 70.Liu CB. Liu GZ. Liu N, et al. Radiolabeling morpholinos with 90Y, 111In, 188Re and 99mTc. Nucl Med Biol. 2003;30:207. doi: 10.1016/s0969-8051(02)00389-x. [DOI] [PubMed] [Google Scholar]
- 71.Liu G. Liu C. Zhang S, et al. Investigations of 99mTc morpholino pretargeting in mice. Nucl Med Commun. 2003;24:697. doi: 10.1097/00006231-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 72.Liu G. Dou S. Mardirossian G, et al. Successful radiotherapy of tumor in pretargeted mice by 188Re-radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analogue. Clin Cancer Res. 2006;12:4958. doi: 10.1158/1078-0432.CCR-06-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He J. Rusckowski M. Wang Y, et al. Optical pretargeting of tumor with fluorescent MORF oligomers. Mol Imaging Biol. 2007;9:17. doi: 10.1007/s11307-006-0071-2. [DOI] [PubMed] [Google Scholar]
- 74.He J. Liu G. Dou S, et al. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug Chem. 2007;18:983. doi: 10.1021/bc060208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu G. Dou S. He J, et al. Predicting the biodistribution of radiolabeled cMORF effector in MORF-pretargeted mice. Eur J Nucl Med Mol Imaging. 2007;34:237. doi: 10.1007/s00259-006-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldenberg DM. Chang CH. Sharkey RM, et al. Radioimmunotherapy: Is avidin–biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30:777. doi: 10.1007/s00259-002-1089-6. [DOI] [PubMed] [Google Scholar]
- 77.Paganelli G. Chinol M. Radioimmunotherapy: Is avidin–biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30:773. doi: 10.1007/s00259-002-1090-0. [DOI] [PubMed] [Google Scholar]
- 78.Breitz HB. Weiden PL. Beaumier PL, et al. Clinical optimization of pretargeted radioimmunotherapy with antibody–streptavidin conjugate and 90Y-DOTA-biotin. J Nucl Med. 2000;41:131. [PubMed] [Google Scholar]
- 79.Paganelli G. Grana C. Chinol M, et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur J Nucl Med. 1999;26:348. doi: 10.1007/s002590050397. [DOI] [PubMed] [Google Scholar]
- 80.Le Doussal JM. Chetanneau A. Gruaz-Guyon A, et al. Bispecific monoclonal antibody-mediated targeting of an indium-111-labeled DTPA dimer to primary colorectal tumors: Pharmacokinetics, biodistribution, scintigraphy and immune response. J Nucl Med. 1993;34:1662. [PubMed] [Google Scholar]
- 81.Kraeber-Bodéré F. Faivre-Chauvet A. Ferrer L, et al. Pharmacokinetics and dosimetry studies for optimization of anti-carcinoembryonic antigen Xanti-hapten bispecific antibody-mediated pretargeting of Iodine-131-labeled hapten in a phase I radioimmunotherapy trial. Clin Cancer Res. 2003;9(suppl):3973S. [PubMed] [Google Scholar]
- 82.Liu G. Dou S. Pretorius PH, et al. Pretargeting CWR22 prostate tumor in mice with MORF-B72.3 antibody and radiolabeled cMORF. Eur J Nucl Med Mol Imaging. 2008;35:272. doi: 10.1007/s00259-007-0606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goldenberg DM. Targeted therapy of cancer with radiolabeled antibodies. J Nucl Med. 2002;43:693. [PubMed] [Google Scholar]
- 84.Subbiah K. Hamlin DK. Pagel JM, et al. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med. 2003;44:437. [PubMed] [Google Scholar]
- 85.Pagel JM. Lin Y. Hedin N, et al. Comparison of a tetravalent single-chain antibody–streptavidin fusion protein and an antibody-streptavidin chemical conjugate for pretargeted anti-CD20 radioimmunotherapy of B-cell lymphomas. Blood. 2006;108:328. doi: 10.1182/blood-2005-11-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pagel JM. Pantelias A. Hedin N, et al. Evaluation of CD20, CD22, and HLA-DR targeting for radioimmunotherapy of B-cell lymphomas. Cancer Res. 2007;67:5921. doi: 10.1158/0008-5472.CAN-07-0080. [DOI] [PubMed] [Google Scholar]
- 87.Pantelias A. Pagel JM. Hedin N, et al. Comparative biodistributions of pretargeted radioimmunoconjugates targeting CD20, CD22, and DR molecules on human B-cell lymphomas. Blood. 2007;109:4980. doi: 10.1182/blood-2006-11-056895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pagel JM. Orgun N. Hamlin DK, et al. A comparative analysis of conventional and pretargeted radioimmunotherapy of B-cell lymphomas by targeting CD20, CD22, and HLA-DR singly and in combinations. Blood. 2009;113:4903. doi: 10.1182/blood-2008-11-187401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Press OW. Farr AG. Borroz KI, et al. Endocytosis and degradation of monoclonal antibodies targeting human B-cell malignancies. Cancer Res. 1989;49:4906. [PubMed] [Google Scholar]
- 90.Zhang M. Zhang Z. Garmestani K, et al. Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc Natl Acad Sci U S A. 2003;100:1891. doi: 10.1073/pnas.0437788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang M. Yao Z. Garmestani K, et al. Pretargeting radioimmunotherapy of a murine model of adult T-cell leukemia with the alpha-emitting radionuclide, bismuth 213. Blood. 2002;100:208. doi: 10.1182/blood-2002-01-0107. [DOI] [PubMed] [Google Scholar]
- 92.Sharkey RM. Karacay H. Chang CH, et al. Improved therapy of non-Hodgkin's lymphoma xenografts using radionuclides pretargeted with a new anti-CD20 bispecific antibody. Leukemia. 2005;19:1064. doi: 10.1038/sj.leu.2403751. [DOI] [PubMed] [Google Scholar]
- 93.Sharkey RM. Karacay H. Litwin S, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin's lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res. 2008;68:5282. doi: 10.1158/0008-5472.CAN-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hillairet de Boisferon M. Raguin O. Dussaillant M, et al. Enhanced targeting specificity to tumor cells by simultaneous recognition of two antigens. Bioconjug Chem. 2000;11:452. doi: 10.1021/bc9901090. [DOI] [PubMed] [Google Scholar]
- 95.Sharkey RM. Karacay H. Johnson CR, et al. Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma. J Nucl Med. 2009;50:444. doi: 10.2967/jnumed.108.058602. [DOI] [PubMed] [Google Scholar]
- 96.Thomas ED. Storb R. Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:832. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 97.Clift RA. Buckner CD. Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: A randomized trial of two irradiation regimens. Blood. 1990;76:1867. [PubMed] [Google Scholar]
- 98.Clift RA. Buckner CD. Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: A randomized trial of two irradiation regimens. Blood. 1991;77:1660. [PubMed] [Google Scholar]
- 99.Clift RA. Buckner CD. Appelbaum FR, et al. Long-term follow-up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92:1455. [PubMed] [Google Scholar]
- 100.Kotzerke J. Bunjes D. Scheinberg DA. Radioimmunoconjugates in acute leukemia treatment: The future is radiant. Bone Marrow Transplant. 2005;36:1021. doi: 10.1038/sj.bmt.1705182. [DOI] [PubMed] [Google Scholar]
- 101.Pagel JM. Radioimmunotherapeutic approaches for leukemia: The past, present and future. Cytotherapy. 2008;10:13. doi: 10.1080/14653240701679881. [DOI] [PubMed] [Google Scholar]
- 102.Omary MB. Trowbridge IS. Battifora HA. Human homologue of murine T200 glycoprotein. J Exp Med. 1980;152:842. doi: 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dahlke MH. Larsen SR. Rasko JE, et al. The biology of CD45 and its use as a therapeutic target. Leuk Lymphoma. 2004;45:229. doi: 10.1080/1042819031000151932. [DOI] [PubMed] [Google Scholar]
- 104.Andres TL. Kadin ME. Immunologic markers in the differential diagnosis of small round cell tumors from lymphocytic lymphoma and leukemia. Am J Clin Pathol. 1983;79:546. doi: 10.1093/ajcp/79.5.546. [DOI] [PubMed] [Google Scholar]
- 105.Nakano A. Harada T. Morikawa S, et al. Expression of leukocyte common antigen (CD45) on various human leukemia/lymphoma cell lines. Acta Pathol Jpn. 1990;40:107. doi: 10.1111/j.1440-1827.1990.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 106.Taetle R. Ostergaard H. Smedsrud M, et al. Regulation of CD45 expression in human leukemia cells. Leukemia. 1991;5:309. [PubMed] [Google Scholar]
- 107.Press OW. Howell-Clark J. Anderson S, et al. Retention of B-cell-specific monoclonal antibodies by human lymphoma cells. Blood. 1994;83:1390. [PubMed] [Google Scholar]
- 108.van der Jagt RHC. Badger CC. Appelbaum FR, et al. Localization of radiolabeled antimyeloid antibodies in a human acute leukemia xenograft tumor model. Cancer Res. 1992;52:89. [PubMed] [Google Scholar]
- 109.Matthews DC. Appelbaum FR. Eary JF, et al. Phase I study of (131)I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94:1237. [PubMed] [Google Scholar]
- 110.Glatting G. Muller M. Koop B, et al. Anti-CD45 monoclonal antibody YAML568: A promising radioimmunoconjugate for targeted therapy of acute leukemia. J Nucl Med. 2006;47:1335. [PubMed] [Google Scholar]
- 111.Pagel JM. Appelbaum FR. Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pagel JM. Matthews DC. Kenoyer A, et al. Pretargeted radioimmunotherapy using anti-CD45 monoclonal antibodies to deliver radiation to murine hematolymphoid tissues and human myeloid leukemia. Cancer Res. 2009;69:185. doi: 10.1158/0008-5472.CAN-08-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pagel JM. Hedin N. Drouet L, et al. Eradication of disseminated leukemia in a syngeneic murine leukemia model using pretargeted anti-CD45 radioimmunotherapy. Blood. 2008;111:2261. doi: 10.1182/blood-2007-06-097451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Green DJ. Pagel JM. Nemecek ER, et al. Pretargeting CD45 enhances the selective delivery of radiation to hematolymphoid tissues in nonhuman primates. Blood. 2009;114:1226. doi: 10.1182/blood-2009-03-210344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gautherot E. Bouhou J. Le Doussal JM, et al. Therapy for colon carcinoma xenografts with bispecific antibody-targeted, iodine-131-labeled bivalent hapten. Cancer. 1997;80(suppl):2618. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2618::aid-cncr37>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 116.van Schaijk FG. Oosterwijk E. Soede AC, et al. Pretargeting of carcinoembryonic antigen-expressing tumors with a biologically produced bispecific anticarcinoembryonic antigen X anti-indium-labeled diethylenetriaminepentaacetic acid antibody. Clin Cancer Res. 2005;11(suppl):7130s. doi: 10.1158/1078-0432.CCR-1004-0006. [DOI] [PubMed] [Google Scholar]
- 117.Gautherot E. Rouvier E. Daniel L, et al. Pretargeted radioimmunotherapy of human colorectal xenografts with bispecific antibody and 131I-labeled bivalent hapten. J Nucl Med. 2000;41:480. [PubMed] [Google Scholar]
- 118.Kraeber-Bodéré F. Saï-Maurel C. Campion L, et al. Enhanced antitumor activity of combined pretargeted radioimmunotherapy and paclitaxel in medullary thyroid cancer xenograft. Mol Cancer Ther. 2002;1:267. [PubMed] [Google Scholar]
- 119.Kraeber-Bodéré F. Faivre-Chauvet A. Saï-Maurel C, et al. Toxicity and efficacy of radioimmunotherapy in carcinoembryonic antigen-producing medullary thyroid cancer xenograft: Comparison of iodine 131-labeled F(ab')2 and pretargeted bivalent hapten and evaluation of repeated injections. Clin Cancer Res. 1999;5(suppl):3183s. [PubMed] [Google Scholar]
- 120.Gautherot E. Kraeber-Bodere F. Daniel L, et al. Immunohistology of carcinoembryonic antigen (CEA)-expressing tumors grafted in nude mice after radioimmunotherapy with 131I-labeled bivalent hapten and anti-CEA X antihapten bispecific antibody. Clin Cancer Res. 1999;5(suppl):3177s. [PubMed] [Google Scholar]
- 121.Gestin JF. Loussouarn A. Bardies M, et al. Two-step targeting of xenografted colon carcinoma using a bispecific antibody and 188Re-labeled bivalent hapten: Biodistribution and dosimetry studies. J Nucl Med. 2001;42:146. [PubMed] [Google Scholar]
- 122.Sharkey RM. Karacay H. Richel H, et al. Optimizing bispecific antibody pretargeting for use in radioimmunotherapy. Clin Cancer Res. 2003;9(suppl):3897S. [PubMed] [Google Scholar]
- 123.Sharkey RM. Cardillo TM. Rossi EA, et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11:1250. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 124.Karacay H. Brard PY. Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 125.Li GP. Zhang H. Zhu CM, et al. Avidin-biotin system pretargeting radioimmunoimaging and radioimmunotherapy and its application in mouse model of human colon carcinoma. World J Gastroenterol. 2005;11:6288. doi: 10.3748/wjg.v11.i40.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strnad J. Hamilton AE. Beavers LS, et al. Molecular cloning and characterization of a human adenocarcinoma/epithelial cell surface antigen complementary DNA. Cancer Res. 1989;49:314. [PubMed] [Google Scholar]
- 127.Szala S. Froehlich M. Scollon M, et al. Molecular cloning of cDNA for the carcinoma-associated antigen GA733-2. Proc Natl Acad Sci U S A. 1990;87:3542. doi: 10.1073/pnas.87.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lewis MR. Wang M. Axworthy DB, et al. In vivo evaluation of pretargeted 64Cu for tumor imaging and therapy. J Nucl Med. 2003;44:1284. [PubMed] [Google Scholar]
- 129.Gold DV. Lew K. Maliniak R, et al. Characterization of monoclonal antibody PAM4 reactive with a pancreatic cancer mucin. Int J Cancer. 1994;57:204. doi: 10.1002/ijc.2910570213. [DOI] [PubMed] [Google Scholar]
- 130.Gold DV. Karanjawala Z. Modrak DE, et al. PAM4-reactive MUC1 is a biomarker for early pancreatic adenocarcinoma. Clin Cancer Res. 2007;13:7380. doi: 10.1158/1078-0432.CCR-07-1488. [DOI] [PubMed] [Google Scholar]
- 131.Mariani G. Molea N. Bacciardi D, et al. Initial tumor targeting, biodistribution, and pharmacokinetic evaluation of the monoclonal antibody PAM4 in patients with pancreatic cancer. Cancer Res. 1995;55(suppl):5911s. [PubMed] [Google Scholar]
- 132.Gold DV. Schutsky K. Modrak D, et al. Low-dose radioimmunotherapy ((90)Y-PAM4) combined with gemcitabine for the treatment of experimental pancreatic cancer. Clin Cancer Res. 2003;9:3929S. [PubMed] [Google Scholar]
- 133.Cardillo TM. Karacay H. Goldenberg DM, et al. Improved targeting of pancreatic cancer: Experimental studies of a new bispecific antibody, pretargeting enhancement system for immunoscintigraphy. Clin Cancer Res. 2004;10:3552. doi: 10.1158/1078-0432.CCR-03-0340. [DOI] [PubMed] [Google Scholar]
- 134.Gold DV. Goldenberg DM. Karacay H, et al. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 2008;68:4819. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- 135.Albrecht H. DeNardo GL. DeNardo SJ. Development of anti-MUC1 di-scFvs for molecular targeting of epithelial cancers, such as breast and prostate cancers. Q J Nucl Med Mol Imaging. 2007;51:304. [PubMed] [Google Scholar]
- 136.Graves SS. Dearstyne E. Lin Y, et al. Combination therapy with Pretarget CC49 radioimmunotherapy and gemcitabine prolongs tumor doubling time in a murine xenograft model of colon cancer more effectively than either monotherapy. Clin Cancer Res. 2003;9:3712. [PubMed] [Google Scholar]
- 137.Buchsbaum DJ. Khazaeli MB. Axworthy DB, et al. Intraperitoneal pretarget radioimmunotherapy with CC49 fusion protein. Clin Cancer Res. 2005;11:8180. doi: 10.1158/1078-0432.CCR-05-0607. [DOI] [PubMed] [Google Scholar]
- 138.Förster GJ. Santos EB. Smith-Jones PM, et al. Pretargeted radioimmunotherapy with a single-chain antibody/streptavidin construct and radiolabeled DOTA–biotin: Strategies for reduction of the renal dose. J Nucl Med. 2006;47:140. [PubMed] [Google Scholar]
- 139.Sato N. Hassan R. Axworthy DB, et al. Pretargeted radioimmunotherapy of mesothelin-expressing cancer using a tetravalent single-chain Fv–streptavidin fusion protein. J Nucl Med. 2005;46:1201. [PubMed] [Google Scholar]
- 140.De Santis R. Albertoni C. Petronzelli F, et al. Low and high tenascin-expressing tumors are efficiently targeted by ST2146 monoclonal antibody. Clin Cancer Res. 2006;12:2191. doi: 10.1158/1078-0432.CCR-05-2526. [DOI] [PubMed] [Google Scholar]
- 141.Moosmayer D. Berndorff D. Chang CH, et al. Bispecific antibody pretargeting of tumor neovasculature for improved systemic radiotherapy of solid tumors. Clin Cancer Res. 2006;12:5587. doi: 10.1158/1078-0432.CCR-06-0210. [DOI] [PubMed] [Google Scholar]
- 142.van Schaijk FG. Oosterwijk E. Molkenboer-Kuenen JD, et al. Pretargeting with bispecific anti-renal cell carcinoma X anti-DTPA(In) antibody in 3 RCC models. J Nucl Med. 2005;46:495. [PubMed] [Google Scholar]
- 143.van Schaijk FG. Boerman OC. Soede AC, et al. Comparison of IgG and F(ab′)2 fragments of bispecific anti-RCCxanti-DTIn-1 antibody for pretargeting purposes. Eur J Nucl Med Mol Imaging. 2005;32:1089. doi: 10.1007/s00259-005-1796-x. [DOI] [PubMed] [Google Scholar]
- 144.Oosterwijk E. Ruiter DJ. Hoedemaeker PJ, et al. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38:489. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- 145.Kranenborg MH. Boerman OC. Oosterwijk-Wakka JC, et al. Development and characterization of anti-renal cell carcinoma X antichelate bispecific monoclonal antibodies for two-phase targeting of renal cell carcinoma. Cancer Res. 1995;55(suppl):5864s. [PubMed] [Google Scholar]
- 146.van Schaijk FG. Oosterwijk E. Soede AC, et al. Pretargeting with labeled bivalent peptides allowing the use of four radionuclides: (111)In, (131)I, (99m)Tc, and (188)Re. Clin Cancer Res. 2003;9(suppl):3880S. [PubMed] [Google Scholar]
- 147.van Schaijk FG. Broekema M. Oosterwijk E, et al. Residualizing iodine markedly improved tumor targeting using bispecific antibody-based pretargeting. J Nucl Med. 2005;46:1016. [PubMed] [Google Scholar]
- 148.Pastan I. Lovelace ET. Gallo MG, et al. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991;51:3781. [PubMed] [Google Scholar]
- 149.Yao Z. Zhang M. Axworthy DB, et al. Radioimmunotherapy of A431 xenografted mice with pretargeted B3 antibody–streptavidin and (90)Y-labeled 1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA)-biotin. Cancer Res. 2002;62:5755. [PubMed] [Google Scholar]
- 150.Weiden PL. Breitz HB. Press O, et al. Pretargeted radioimmunotherapy (PRIT) for treatment of non-Hodgkin's lymphoma (NHL): Initial phase I/II study results. Cancer Biother Radiopharm. 2000;15:15. doi: 10.1089/cbr.2000.15.15. [DOI] [PubMed] [Google Scholar]
- 151.Forero A. Weiden PL. Vose JM, et al. Phase 1 trial of a novel anti-CD20 fusion protein in pretargeted radioimmunotherapy for B-cell non-Hodgkin lymphoma. Blood. 2004;104:227. doi: 10.1182/blood-2003-09-3284. [DOI] [PubMed] [Google Scholar]
- 152.Palumbo G. Grana CM. Cocca F, et al. Pretargeted antibody-guided radioimmunotherapy in a child affected by resistant anaplastic large cell lymphoma. Eur J Haematol. 2007;79:258. doi: 10.1111/j.1600-0609.2007.00910.x. [DOI] [PubMed] [Google Scholar]
- 153.Kraeber-Bodéré F. Bardet S. Hoefnagel CA, et al. Radioimmunotherapy in medullary thyroid cancer using bispecific antibody and iodine 131-labeled bivalent hapten: Preliminary results of a phase I/II clinical trial. Clin Cancer Res. 1999;5(suppl):3190s. [PubMed] [Google Scholar]
- 154.Vuillez JP. Kraeber-Bodere F. Moro D, et al. Radioimmunotherapy of small cell lung carcinoma with the two-step method using a bispecific anti-carcinoembryonic antigen/anti-diethylenetriaminepentaacetic acid (DTPA) antibody and iodine-131 Di-DTPA hapten: Results of a phase I/II trial. Clin Cancer Res. 1999;5(suppl):3259s. [PubMed] [Google Scholar]
- 155.Kraeber-Bodéré F. Rousseau C. Bodet-Milin C, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med. 2006;47:247. [PubMed] [Google Scholar]
- 156.Chatal JF. Campion L. Kraeber-Bodere F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic–antigen radioimmunotherapy: A collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24:1705. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 157.Barbet J. Campion L. Kraeber-Bodere F, et al. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:6077. doi: 10.1210/jc.2005-0044. [DOI] [PubMed] [Google Scholar]
- 158.Forero-Torres A. Shen S. Breitz H, et al. Pretargeted radioimmunotherapy (RIT) with a novel anti-TAG-72 fusion protein. Cancer Biother Radiopharm. 2005;20:379. doi: 10.1089/cbr.2005.20.379. [DOI] [PubMed] [Google Scholar]
- 159.Shen S. Forero A. LoBuglio AF, et al. Patient-specific dosimetry of pretargeted radioimmunotherapy using CC49 fusion protein in patients with gastrointestinal malignancies. J Nucl Med. 2005;46:642. [PubMed] [Google Scholar]
- 160.Knox SJ. Goris ML. Tempero M, et al. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin Cancer Res. 2000;6:406. [PubMed] [Google Scholar]
- 161.Breitz HB. Fisher DR. Goris ML, et al. Radiation absorbed dose estimation for 90Y-DOTA-biotin with pretargeted NR-LU-10/streptavidin. Cancer Biother Radiopharm. 1999;14:381. doi: 10.1089/cbr.1999.14.381. [DOI] [PubMed] [Google Scholar]
- 162.Grana C. Chinol M. Robertson C, et al. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: A pilot study. Br J Cancer. 2002;86:207. doi: 10.1038/sj.bjc.6600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Paganelli G. Bartolomei M. Ferrari M, et al. Pre-targeted locoregional radioimmunotherapy with 90Y-biotin in glioma patients: Phase I study and preliminary therapeutic results. Cancer Biother Radiopharm. 2001;16:227. doi: 10.1089/10849780152389410. [DOI] [PubMed] [Google Scholar]