Abstract
PURPOSE
To identify whether histopathologic presence of neovascularization is predictive for allograft failure following penetrating keratoplasty for herpes simplex virus (HSV) keratitis.
METHODS
Retrospective, interventional case series of 62 consecutive patients with HSV keratitis who underwent penetrating keratoplasty at the Kellogg Eye Center between 1990 and 2000. Reviews of the patients’ clinical charts and the histopathologic slides of their excised corneal buttons were performed to identify associations between histopathologic neovascularization and clinical outcomes.
Main Outcome Measure: To determine whether histopathologic presence of neovascularization predicts allograft failure.
RESULTS
Histopathologic presence of neovascularization was present in 31% of corneal specimens and predicted subsequent allograft failure (P = .002) and HSV recurrence (P = .05).
CONCLUSION
Histopathologic presence of neovascularization is a risk factor for corneal allograft failure and HSV recurrence.
Keywords: penetrating keratoplasty, histopathology, herpes simplex
INTRODUCTION
Patients undergoing penetrating keratoplasty (PKP) for sequelae of herpes simplex virus (HSV) keratitis are at higher risk for adverse corneal allograft outcomes when compared to individuals undergoing grafting for conditions such as keratoconus and Fuchs’ corneal dystrophy.1,2 The post-operative course can be complicated by high rates of HSV recurrence, graft rejection, and graft failure.3–6 Our prior histopathologic and immunopathologic evaluation of inflammation and inflammatory biomarkers in host corneal tissue removed at the time of PKP revealed that these features are predictive for allograft rejection.7 In this study, we examined excised host corneal tissue from a series of patients undergoing PKP for visual rehabilitation following active HSV keratitis to identify whether histopathologic neovascularization is predictive for allograft failure.
MATERIALS AND METHODS
Patients
All PKP’s performed for sequelae of HSV keratitis at the University of Michigan from August 1990 to December 2000 were included in this study. A total of 79 allografts were performed on 73 patients. Data were not available for 3 patients. Six patients were grafted twice during this time period and only their first grafts were eligible for inclusion, resulting in a total of 70 allografts. Eight other patients had primary grafts done prior to 1990, and had subsequent grafts done during our study period. These repeat keratoplasties were excluded, leaving 62 primary grafts in this study. All surgeries were performed by corneal subspecialists. Charts were reviewed for the following information: disease-free time before surgery, quadrants of pre-operative host vascularity, HSV recurrence, allograft rejection, allograft failure, and histopathologic presence of inflammation and neovascularization in the excised corneal tissue. This study received Institutional Review Board approval at the University of Michigan Medical Center.
Active HSV keratitis was diagnosed by the presence of dendritic or geographic epithelial keratitis, and/or ulceration. HSV keratouveitis was identified by the presence of keratic precipitates (KP) on both the donor and host endothelium. Graft rejection is often difficult to distinguish clinically from HSV recurrence, but was specifically defined in this study as: an endothelial or epithelial rejection line, subepithelial infiltrates, an anterior chamber reaction with KP on the donor endothelium only, or graft edema with associated KP on the donor endothelium only. Graft failure was defined as irreversible loss of graft clarity. Clinical quiescence of HSV infection was defined as no change on clinical examination for at least 6 months.
Postoperative oral acyclovir prophylaxis was prescribed in 51 (85%) of the 62 patients. The initial dose used was variable, as was the tapering regimen, however, patients were on at least 800mg per day for an average of 6 months, and at least 400mg per day for an average of 17 months. Postoperative topical prednisolone acetate 0.1% eye drops (averaging 4 times daily and subsequently tapered) were used in all patients. Episodes of HSV recurrence were treated with oral acyclovir or trifluridine eye drops (Viroptic, Glaxo Wellcome). Episodes of rejection were treated with prednisolone acetate 1% eye drops, tapered over several weeks.
Pathology
Each specimen removed from all 62 patients was examined grossly for regions of maximal vascularization, opacity, and variations in thickness. The specimen was then bisected along a secant 0.5 mm from and parallel to the diameter demonstrating, in order, maximal vascularization, opacity, or variable thickness. After routine processing, six micron paraffin step sections were obtained at 100 micron intervals for 1mm of the specimen, straddling the diameter of maximal gross pathology. The paraffin sections were stained with hematoxylin and eosin. The sections from each specimen were evaluated and graded in the week subsequent to its removal by an ophthalmic pathologist (VME) who was masked as to all clinical details except for the diagnosis.
Each of the 62 specimens was rendered a pathologic diagnosis and graded for the presence of inflammation (none visible, present) and for the presence of neovascularization (none visible, present).
Statistical Analysis
The clinical and histopathologic data were analyzed using the chi square test, Fisher’s exact test, analysis of variance, Kaplan-Meier survival curves, the log rank test, and Cox regression. Unless otherwise indicated, data are given as mean ± SD. SAS 9.0 statistical software (SAS Institute, Cary, NC) was used for the data analyses and comparisons.
RESULTS
The average patient age at surgery was 55 ± 22 years (range, 5–85 years) and the average disease duration was 19 ± 12 years (range, 0.25 to 72 years). Fifty-three percent of patients were female. The average duration of clinical quiescence before surgery was 50 ± 78 months (range, 3–360 months). Average follow-up was 43 ± 32 months (range, 3–142 months). Indications for surgery were corneal scarring in 60 (97%) patients, descemetocele in 1 (1.5%), and perforation in 1 (1.5%). Nine (15%) patients had an HSV recurrence in their allograft during the study follow-up; 1 patient manifested with keratouveitis, 1 with geographic epithelial keratitis, and the remaining 7 with dendritic epithelial keratitis.
Histopathologic evidence of neovascularization was seen in 19 (31%) of the specimens excised during PKP. The histopathologic presence of neovascularization in the removed host corneal tissue was predictive for graft failure (P = .002, log-rank test) (Figure 1). Of the 19 patients whose corneal specimens exhibited neovascularization, 4 (21%) subsequently had graft failure. In contrast, none of the 43 patients whose corneal tissue did not have neovascularization developed graft failure. Of the 19 patients with histopathologic evidence of neovascularization, 5 (26%) experienced an HSV recurrence. In the remaining 53 patients without histopathologic neovascularization, only 4 (8%) experienced an HSV recurrence. Kaplan-Meier analysis shows that histopathologic evidence of neovascularization was associated with an increased rate of HSV recurrence (P = .05, log-rank test) in the allograft.
Figure 1. Failure of corneal allografts in patients with HSV stromal keratitis.
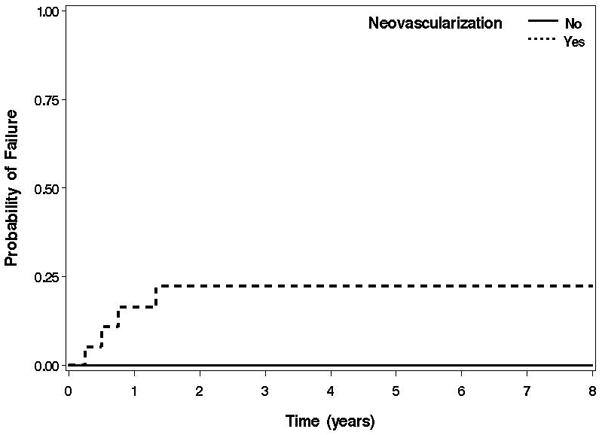
Kaplan-Meier survival curves of allograft failure in patients with and without neovascularization on histopathologic evaluation of their excised corneal tissue.
Corneal neovascularization was clinically present preoperatively in 39 (63%) patients, and was seen histopathologically in 19 (31%) of the specimens excised during PKP. All of the corneal specimens with histopathologic evidence of neovascularization were from patients who had clinical corneal neovascularization preoperatively. There was a statistically significant association between the extent of preoperative neovascularization and the histopathologic presence of inflammation (P = .01, extended Fisher’s exact test) in the excised corneal tissue. Thirty (77%) of the 39 patients with clinical evidence of neovascularization preoperatively were found to have histopathologic evidence of inflammation.
DISCUSSION
Numerous prior studies have shown that the preoperative clinical presence of neovascularization is a risk factor for corneal allograft failure.8–10 Further, allograft rejection episodes have been shown to be more difficult to treat in patients with preoperative clinical neovascularization.11 Very little prior research has been done evaluating the histopathologic features of corneal tissue removed at the time of corneal transplantation. One study published in 2004 by Branco and associates,12 looked at the records of all corneal tissue submitted from 1972 to 2001 to the pathology laboratory at the University of California at San Francisco. There were 4,207 grafts performed, 76 (1.8%) of which were for HSV keratitis. They reported on the pathological findings in corneas with a clinical diagnosis of HSV keratitis including neovascularization in 59%. The authors did not comment on what effect the presence of this finding had on subsequent allograft failure or HSV recurrence.
We found that the histopathologic presence of neovascularization in the corneal tissue is an important predictor of graft failure (P = .002). Its presence within the central corneal tissue that is removed at the time of PKP probably indicates that these patients had severe stromal disease placing them at higher risk for subsequent failure. The histopathologic presence of blood vessels in the corneal tissue indicates that the neovascularization extends to the edge of the host corneal bed and is therefore present at the graft margin at the time of transplantation. In the cohort of patients in this study, the preoperative clinical presence of neovascularization did not correlate with poor allograft outcomes;13 however, the histopathologic presence of neovascularization correlated with increased rates of allograft failure and HSV recurrence. Identifying these features in patients and their excised tissues may be helpful in identifying the patients who are at highest risk for adverse allograft outcomes. These patients would have the most to gain from careful postoperative monitoring. These findings are of practical significance to the clinician in care of patients after PKP.
Corneal neovascularization was present preoperatively in 63% of patients on clinical examination. However, it was only found histopathologically in 31% of the specimens excised during PKP. This discrepancy may have resulted from clinical examinations which documented peripheral preoperative corneal neovascularization in tissue not removed during PKP. Less likely is that histopathologic sectioning did not include an axis involved with neovascularization as the specimens were examined under a dissecting microscope and sectioned along the axis of greatest neovascularization as described above.
There was a statistically significant correspondence between the presence of preoperative clinical corneal neovascularization and the histopathologic presence of inflammation in the same corneal specimens after removal at PKP (P = .01). This suggests that corneal neovascularization is an important preoperative clinical factor indicative of actual inflammation in the diseased cornea. This association between preoperative clinical corneal neovascularization and the histopathologic presence of inflammation in the excised tissue has important pathogenetic and clinical implications. Neovascularization may be essential to the delivery of cellular and serum components of inflammation and host immune responses, setting the stage for graft rejection and failure.14,15
Despite the retrospective nature of this study, the histopathologic features of neovascularization in the removed host corneal tissue were well defined, as were the clinical endpoints of allograft failure and HSV recurrence. This lends confidence that the conclusions drawn from the study are likely to be true and clinically relevant. Moreover, as the histopathologic findings were determined in tissue grossed and processed in the usual fashion for corneal surgical pathology specimens, the observations made are relevant to routine clinical practice. This establishes a role for the pathologist in assisting the clinician in their choice of postoperative patient management.
Acknowledgments
This study was supported by NIH grants EY017885 (RMS) and EY7003 and EY9441 (VME) and a departmental grant from Research to Prevent Blindness (DCM); Dr. Elner is the recipient of a Senior Scientific Award from Research to Prevent Blindness.
This manuscript is based on a presentation at the Annual Meeting of the American Ophthalmological Society and subsequently published in the Transactions of the American Ophthalmological Society in 2008.
References
- 1.Epstein RJ, Seedor JA, Dreizen NG, et al. Penetrating keratoplasty for herpes simplex keratitis and keratoconus. Allograft rejection and survival. Ophthalmology. 1987;94:935–944. doi: 10.1016/s0161-6420(87)33356-1. [DOI] [PubMed] [Google Scholar]
- 2.Thompson RW, Jr, Price MO, Bowers PJ, Price FW., Jr Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 3.Cohen EJ, Laibson PR, Arentsen JJ. Corneal transplantation for herpes simplex keratitis. Am J Ophthalmol. 1983;95:645–650. doi: 10.1016/0002-9394(83)90384-7. [DOI] [PubMed] [Google Scholar]
- 4.Foster CS, Duncan J. Penetrating keratoplasty for herpes simplex keratitis. Am J Ophthalmol. 1981;92:336–343. doi: 10.1016/0002-9394(81)90522-5. [DOI] [PubMed] [Google Scholar]
- 5.Lomholt JA, Baggesen K, Ehlers N. Recurrence and rejection rates following corneal transplantation for herpes simplex keratitis. Acta Ophthalmol Scand. 1995;73:29–32. doi: 10.1111/j.1600-0420.1995.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 6.Ficker LA, Kirkness CM, Rice NS, Steele AD. Long term prognosis for corneal grafting in herpes simplex keratitis. Eye. 1988;2:400–408. doi: 10.1038/eye.1988.73. [DOI] [PubMed] [Google Scholar]
- 7.Shtein RM, Garcia DD, Musch DC, Elner VM. HSV Keratitis: Histopathologic Predictors of Corneal Allograft Complications. Trans Am Ophthalmol Soc. in press. [PMC free article] [PubMed] [Google Scholar]
- 8.Boisjoly HM, Tourigny R, Bazin R, et al. Risk factors of corneal graft failure. Ophthalmology. 1993;100:1728–1735. doi: 10.1016/s0161-6420(93)31409-0. [DOI] [PubMed] [Google Scholar]
- 9.Price FW, Whitson WE, Johns S, Gonzales JS. Risk factors for corneal graft failure. J Refract Surg. 1996;12:134–143. doi: 10.3928/1081-597X-19960101-24. [DOI] [PubMed] [Google Scholar]
- 10.Yamagami S, Suzuki Y, Tsuru T. Risk factors for graft failure in penetrating keratoplasty. Acta Ophthalmol Scand. 1996;74:584–588. doi: 10.1111/j.1600-0420.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 11.Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981;99:599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- 12.Branco BC, Gaudio PA, Margolis TP. Epidemiology and molecular analysis of herpes simplex keratitis requiring primary penetrating keratoplasty. Br J Ophthalmol. 2004;88:1285–1288. doi: 10.1136/bjo.2003.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia DD, Farjo Q, Musch DC, Sugar A. Effect of prophylactic oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea. 2007;26:930–934. doi: 10.1097/ICO.0b013e3180e79b77. [DOI] [PubMed] [Google Scholar]
- 14.Murthy RC, McFarland TJ, Yoken J, et al. Corneal transduction to inhibit angiogenesis and graft failure. Invest Ophthalmol Vis Sci. 2003;44:1837–1842. doi: 10.1167/iovs.02-0853. [DOI] [PubMed] [Google Scholar]
- 15.Gebhardt BM, Shi W. Experimental corneal allograft rejection. Immunol Res. 2002;25:1–26. doi: 10.1385/IR:25:1:01. [DOI] [PubMed] [Google Scholar]


