Abstract
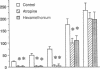
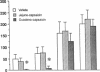
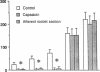
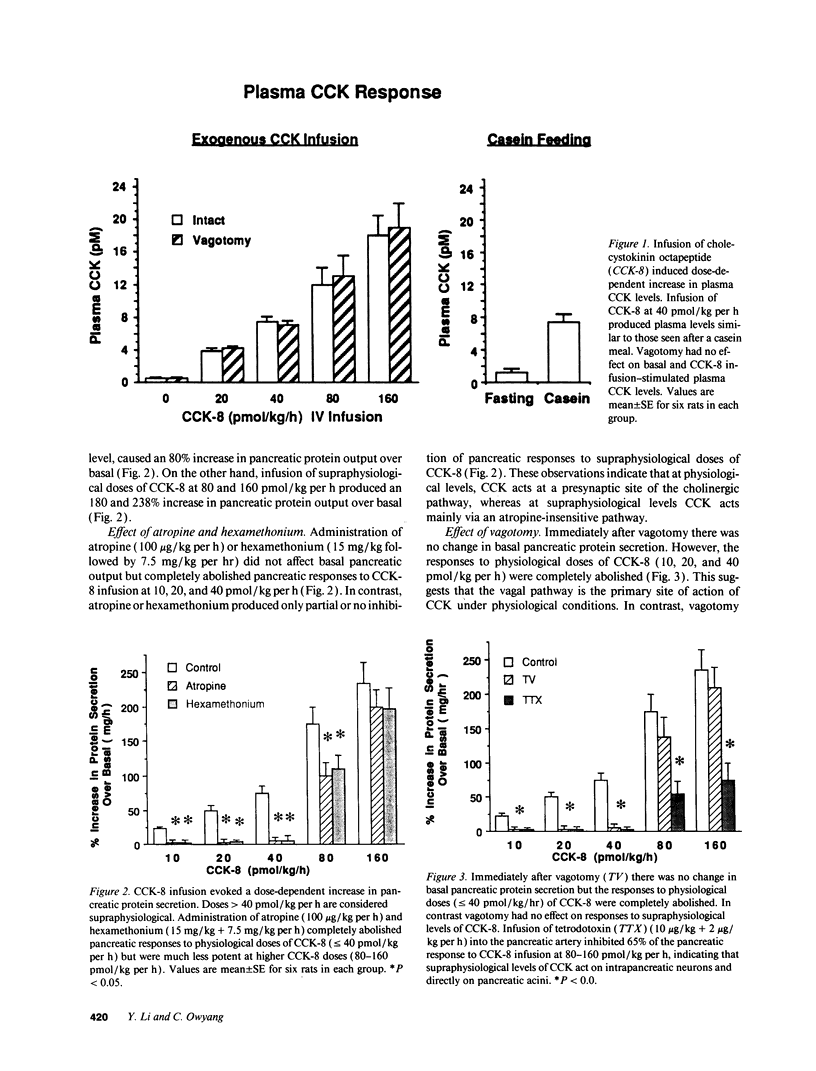
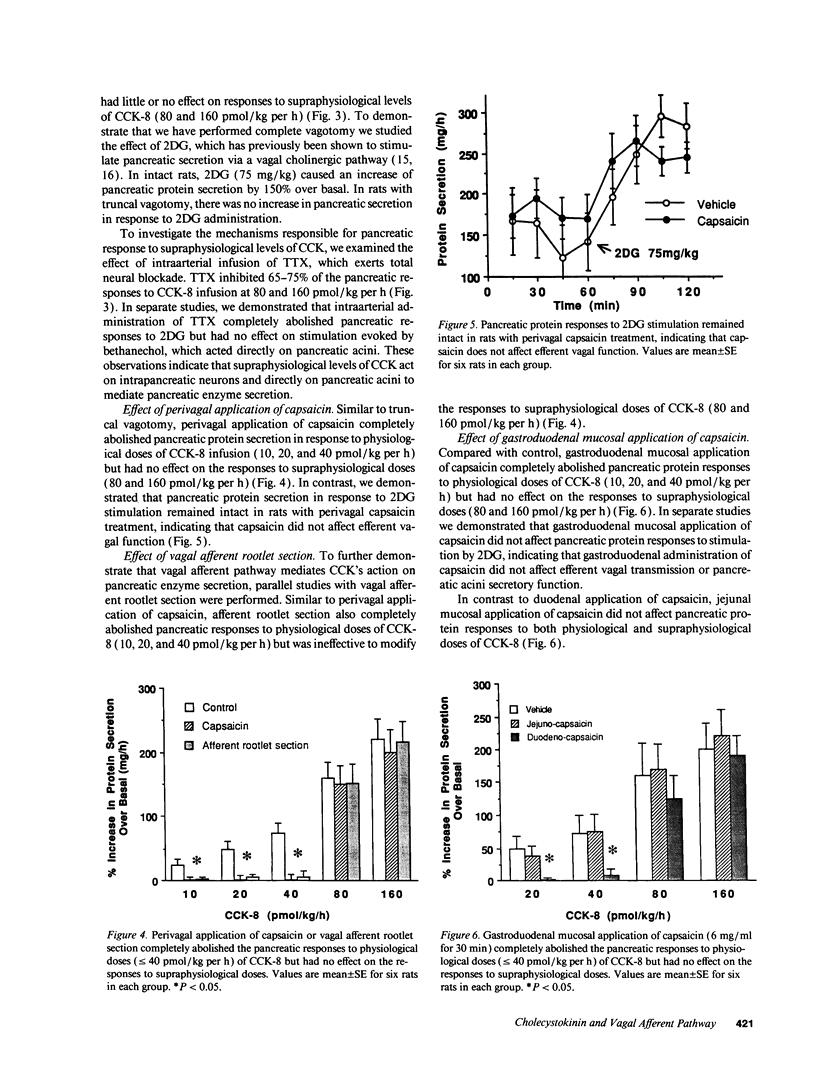
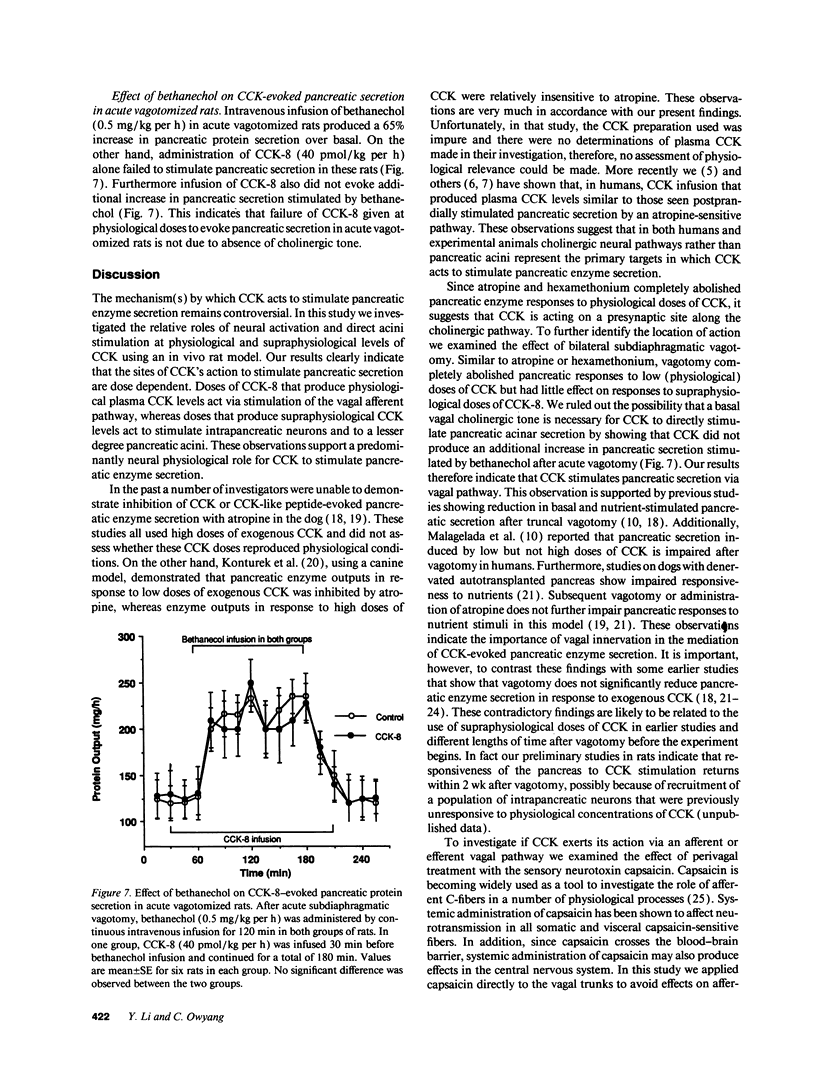
To establish the mechanism(s) and site(s) of action of cholecystokinin (CCK) on pancreatic secretion under physiological conditions, we used an in vivo model using anesthetized rats with pancreaticobiliary cannulas. Infusion of CCK-8 (10-160 pmol/kg per h) produced a dose-dependent increase in plasma CCK levels. CCK-8 infusion at 40 pmol/kg per h produced a plasma CCK level of 7.9 +/- 1.5 pM and an 80% increase in pancreatic protein output over basal. This level was closely approximated by a postprandial peak plasma CCK level by 6.2 +/- 1.1 pM. Pretreatment with atropine or hexamethonium completely abolished pancreatic protein response to low doses of CCK-8 (10-40 pmol/kg per h) but had only partial effect on doses > 40 pmol/kg per h. Bilateral vagotomy also abolished the pancreatic responses to low doses of CCK-8. Similarly perivagal treatment with a sensory neurotoxin, capsaicin, caused a complete inhibition of pancreatic protein secretion in response to CCK-8 infusion. In contrast, pancreatic protein responses to bethanechol were similar in control and capsaicin-treated rats. In separate studies we demonstrated that gastroduodenal but not jejunal application of capsaicin for 30 min abolished pancreatic protein secretion in response to physiological doses of CCK-8. In conclusion, CCK at physiological levels stimulates pancreatic enzyme secretion via a capsaicin-sensitive afferent vagal pathway originating from the gastroduodenal mucosa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Beglinger C., Braun U., Reinshagen M., Koop I., Schafmayer A., Rovati L., Arnold R. Interaction of the cholinergic system and cholecystokinin in the regulation of endogenous and exogenous stimulation of pancreatic secretion in humans. Gastroenterology. 1991 Feb;100(2):537–543. doi: 10.1016/0016-5085(91)90227-c. [DOI] [PubMed] [Google Scholar]
- Bozkurt T., Adler G., Koop I., Arnold R. Effect of atropine on intestinal phase of pancreatic secretion in man. Digestion. 1988;41(2):108–115. doi: 10.1159/000199739. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Debas H. T., Konturek S. J., Grossman M. I. Effect of extragastric and truncal vagotomy on pancreatic secretion in the dog. Am J Physiol. 1975 Apr;228(4):1172–1177. doi: 10.1152/ajplegacy.1975.228.4.1172. [DOI] [PubMed] [Google Scholar]
- Gamse R., Petsche U., Lembeck F., Jancsò G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982 May 13;239(2):447–462. doi: 10.1016/0006-8993(82)90521-2. [DOI] [PubMed] [Google Scholar]
- Henriksen F. W. Effe of vagotomy or atropine on the canine pancreatic response to secretin and pancreozymin. Scand J Gastroenterol. 1969;4(2):137–144. doi: 10.3109/00365526909179921. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Inoue K., Fried G. M., Wiener I., Zhu X. G., Greeley G. H., Jr, Thompson J. C. Colonic inhibition of cholecystokinin release and pancreatic protein secretion in dogs. Surg Gynecol Obstet. 1984 Nov;159(5):423–428. [PubMed] [Google Scholar]
- Konturek S. J., Becker H. D., Thompson J. C. Effect of vagotomy on hormones stimulating pancreatic secretion. Arch Surg. 1974 May;108(5):704–708. doi: 10.1001/archsurg.1974.01350290068011. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Radecki T., Biernat J., Thor P. Effect of vagotomy on pancreatic secretion evoked by endogenous and exogenous cholecystokinin and caerulein. Gastroenterology. 1972 Aug;63(2):273–278. [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Obtulowicz W. Effect of atropine on pancreatic responses endogenous and exogenous cholecystokinin. Am J Dig Dis. 1972 Oct;17(10):911–917. doi: 10.1007/BF02239529. [DOI] [PubMed] [Google Scholar]
- Louie D. S., May D., Miller P., Owyang C. Cholecystokinin mediates feedback regulation of pancreatic enzyme secretion in rats. Am J Physiol. 1986 Feb;250(2 Pt 1):G252–G259. doi: 10.1152/ajpgi.1986.250.2.G252. [DOI] [PubMed] [Google Scholar]
- Louie D. S., Williams J. A., Owyang C. Action of pancreatic polypeptide on rat pancreatic secretion: in vivo and in vitro. Am J Physiol. 1985 Oct;249(4 Pt 1):G489–G495. doi: 10.1152/ajpgi.1985.249.4.G489. [DOI] [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., Summerskill W. H. Altered pancreatic and biliary function after vagotomy and pyloroplasty. Gastroenterology. 1974 Jan;66(1):22–27. [PubMed] [Google Scholar]
- Peikin S. R., Rottman A. J., Batzri S., Gardner J. D. Kinetics of amylase release by dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1978 Dec;235(6):E743–E749. doi: 10.1152/ajpendo.1978.235.6.E743. [DOI] [PubMed] [Google Scholar]
- Putnam W. S., Liddle R. A., Williams J. A. Inhibitory regulation of rat exocrine pancreas by peptide YY and pancreatic polypeptide. Am J Physiol. 1989 Apr;256(4 Pt 1):G698–G703. doi: 10.1152/ajpgi.1989.256.4.G698. [DOI] [PubMed] [Google Scholar]
- Raybould H. E., Taché Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol. 1988 Aug;255(2 Pt 1):G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- Singer M. V. Pancreatic secretory response to intestinal stimulants: a review. Scand J Gastroenterol Suppl. 1987;139:1–13. doi: 10.3109/00365528709089768. [DOI] [PubMed] [Google Scholar]
- Solomon T. E., Grossman M. I. Effect of atropine and vagotomy on response of transplanted pancreas. Am J Physiol. 1979 Feb;236(2):E186–E190. doi: 10.1152/ajpendo.1979.236.2.E186. [DOI] [PubMed] [Google Scholar]
- Soudah H. C., Lu Y., Hasler W. L., Owyang C. Cholecystokinin at physiological levels evokes pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol. 1992 Jul;263(1 Pt 1):G102–G107. doi: 10.1152/ajpgi.1992.263.1.G102. [DOI] [PubMed] [Google Scholar]
- South E. H., Ritter R. C. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides. 1988 May-Jun;9(3):601–612. doi: 10.1016/0196-9781(88)90171-4. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Matsumoto J., Ueshima K., Okabe S. Role of capsaicin-sensitive afferent neurons in alkaline secretory response to luminal acid in the rat duodenum. Gastroenterology. 1991 Oct;101(4):954–961. doi: 10.1016/0016-5085(91)90721-v. [DOI] [PubMed] [Google Scholar]