Abstract
Active discharge of basidiospores in most species of Basidiomycota is powered by the rapid movement of a droplet of fluid, called Buller’s drop, over the spore surface. This paper is concerned with the operation of the launch mechanism in species with the largest and smallest ballistospores. Aleurodiscus gigasporus (Russulales) produces the largest basidiospores on record. The maximum dimensions of the spores, 34 × 28 µm, correspond to a volume of 14 pL and to an estimated mass of 17 ng. The smallest recorded basidiospores are produced by Hyphodontia latitans (Hymenochaetales). Minimum spore dimensions in this species, 3.5 × 0.5 µm, correspond to a volume of 0.5 fL and mass of 0.6 pg. Neither species has been studied using high-speed video microscopy, but this technique was used to examine ballistospore discharge in species with spores of similar sizes (slightly smaller than A. gigasporus and slightly larger than those of H. latitans). Extrapolation of velocity measurements from these fungi provided estimates of discharge distances ranging from a maximum of almost 2 mm in A. gigasporus to a minimum of 4 µm in H. latitans. These are, respectively, the longest and shortest predicted discharge distances for ballistospores. Limitations to the distances traveled by basidiospores are discussed in relation to the mechanics of the discharge process and the types of fruit-bodies from which the spores are released.
Introduction
Spore discharge powered by fluid movement over the spore surface is a defining characteristic of the Basidiomycota and is utilized by the majority of species in the phylum (Webster and Weber 2007). This ballistospore discharge mechanism launches basidiospores from mushroom gills and spines, and from the inner surfaces of tubes in poroid species, and also propels rust, smut, and yeast spores into the open air. Ballistospore discharge does not occur in other fungal phyla.
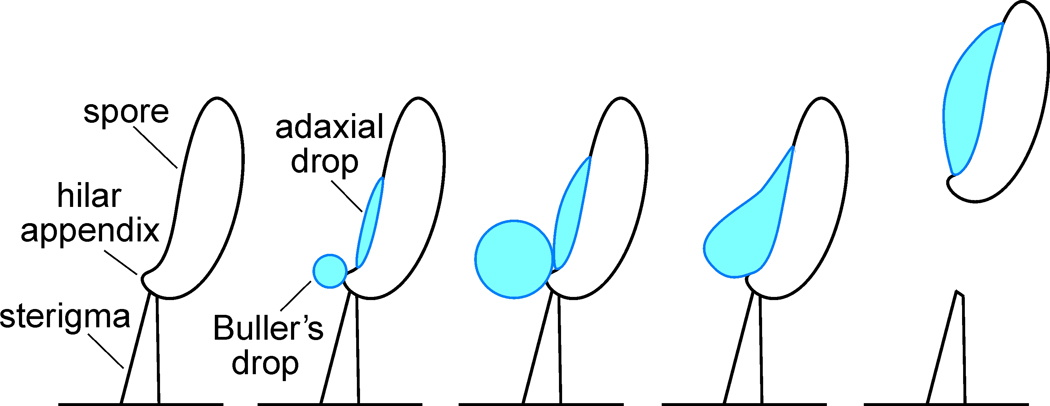
The mature basidiospore is attached to a slender aerial projection called a sterigma (Fig 1). A few seconds before discharge, the connection between the spore and sterigma is loosened, greatly reducing the force required to separate the spore from its perch (Van Neil et al. 1972; Webster et al. 1984; Noblin et al. 2009). The subsequent launch of the spore can be treated as a three-stage process (Fig 1): (i) drop growth, water condenses on a projection from the base of the ballistospore, called the hilar appendix, forming a spherical droplet, called Buller’s drop; at the same time, condensation forms a second discrete drop of fluid, called the adaxial drop, on the spore surface adjacent to Buller’s drop; (ii) drop coalescence, sustained condensation causes the drops of fluid to expand for a few seconds until they make contact and coalesce; (iii) fluid and spore movement, Buller’s drop merges with the adaxial drop, causing a fast redistribution of mass that imparts momentum to the spore and adhering fluid, catapulting the spore from its sterigma (Webster and Chen 1990; Money 1998).
Fig 1.
Schematic showing process of ballistospore discharge. Buller’s drop and the adaxial drop form via condensation of water on the spore surface and their coalescence causes a rapid shift in the center of mass of the spore that is responsible for the launch. From Stolze-Rybczynski et al. (2009).
The details of this process have been studied in a handful of species using high-speed video cameras (Pringle et al. 2005; Stolze-Rybczynski et al. 2009; Noblin et al. 2009). These studies show that basidiospores are launched at speeds varying from 0.1 to 1.8 m s−1 and travel over distances of 0.04 to 1.26 mm (corresponding to between 9- and 63-times the length of the spores). Differences in range reflect variations in morphology and dispersal strategy: spores are launched over short distances in gilled mushrooms avoiding spore loss within the fruit-body; yeast spores are discharged much farther, carrying them through the boundary layer of still air clinging to their growth substrates where they may be dispersed by air currents (Stolze-Rybczynski et al. 2009).
The range of the ballistospore discharge mechanism is dwarfed by other discharge processes in the fungi (Buller 1909; Ingold 1999, 2001a,b). Ascospores can be propelled over distances of many centimeters after their explosive discharge from pressurized asci (Ingold 1971; Trail 2007). A similar mechanism fires the sporangia of Pilobolus over horizontal distances of up to 2.5 m, and the artillery fungus, Sphaerobolus, utilizes a snap-buckling mechanism to eject its spore-filled gleba up to 6 m from the fruit-body (Yafetto et al. 2008; Money and Fischer 2009).
Both spore size and the size of Buller’s drop are strongly correlated with discharge distance, with larger spores with bigger drops being propelled over the longest distances (Stolze-Rybczynski 2009). The taxonomic literature shows that the range of ballistospores sizes examined using high-speed video falls within the extremes of spore dimensions documented for the Basidiomycota. This suggests that the ballistospore discharge mechanism may have been adapted in some species to discharge spores over shorter and longer distances than reported previously. In this paper, we identify the largest and smallest ballistospores in the Basidiomycota and explore the distances that these spores are discharged using a combination of high-speed video imaging and mathematical modeling. This is interesting both from a bioengineering perspective and from an evolutionary viewpoint.
Materials and Methods
Identification of the largest recorded ballistospores
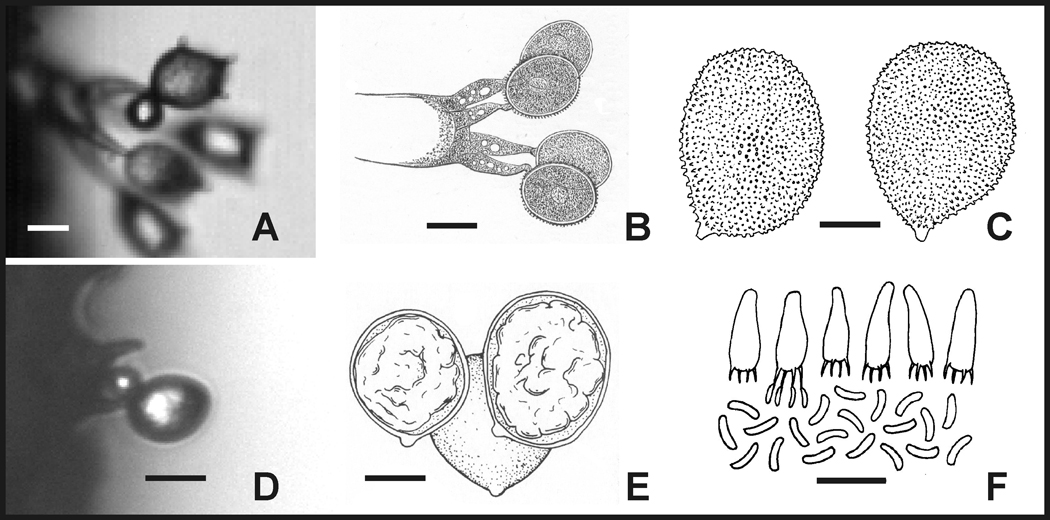
The largest ballistospores are described from species of Aleurodiscus (Russulales) that discharge their spores from the exposed surface of their resupinate basidiomata (Núñez and Ryvarden 1997). A. oakesii is common in North America and causes a disease called smooth patch on the bark of oaks and its basidiomata look like patches of white paint (Tehon and Jacks 1933). Its spores measure 18–27 × 12–14 µm and are decorated with lengthy warts and spines (Fig 2A). The subglobose or broadly ellipsoid spores of A. amorphus, are 24–28 × 18–22 µm in size. The basidia of this species are correspondingly large (100-25 µm) and served as a convenient subject for illustrating basidomycete anatomy for Anton de Bary (1887) in his groundbreaking textbook (Fig 2B). But a species whose description was based on a single collection in China called A. gigasporus produces even larger spores, with a broadly ellipsoidal shape, measuring 29–34 × 22–28 µm (Fig 2C; Ginns and Bandoni 1991; Núñez and Ryvarden 1997). Although spore discharge in A. gigasporus has not been examined (we did not have access to live specimens), the asymmetry of the spores and presence of hilar appendix are reliable morphological characters for discharge involving the formation of Buller’s drop. In addition, spore discharge was studied in its relative, A. oakesii, and conforms to the model described in the introduction to this paper. Based on these observations, we suggest that A. gigasporus produces the largest recorded basidiospore and that this is the largest putative ballistospore. Its maximum dimensions, 34 × 28 µm, correspond to a volume of 1.4 × 10−14 m3 or 14 pL. Assuming a density of 1.2 × 103 kg m−3, this spore has a mass of 17 ng, which is 6-fold heavier than the ballistospores of the rust fungus Gymnosporangium juniperi-virginianae (Stolze-Rybczynski et al. 2009).
Fig 2.
Largest (A–E) and smallest (F) ballistospores. (A), Basidium of Aleurodiscus oakesii with uppermost spore primed for discharge with Buller’s drop; orientation of basidium reflects development of flattened basidiome on tree trunk. (B), Basidium of Aleurodiscus amorphus. (C), Basidiospores of Aleurodiscus gigasporus. (D), Basidium of Xerula radicata with single remaining spore photographed a few milliseconds before discharge with Buller’s drop; orientation reflects position on vertical gill surface. (E), Basidiospores of Xerula australis. (F), Basidia and basidiospores of Hyphodontia latitans; basidia pointing downwards reflecting basidiome that forms on the lower surface of a branch. Scale bars, (A) and (C–F) = 10 µm, B = 20 µm; in (F) scale applies to free spores whose maximum length is 5 µm. Sources: (B), Anton de Bary (1887); (C), adapted from Núñez and Ryvarden (1997); (E), Pegler and Young (1987); (F), Gilbertson and Ryvarden (1986).
To extend this inquiry to the largest spores formed by lamellate basidiomes we have studied spore discharge process in the rooting shank, Xerula radicata. The globose ballistospores of this species measure 17 × 14 µm (Fig 2D). Ballistic data for this species inform our calculations for a related mushroom, Xerula australis, whose spores measure 20–27 × 19–26 µm (Fig 2E; Pegler and Young 1987). Again, we did not have access to fresh specimens of this species.
Identification of the smallest recorded ballistospores
The smallest ballistospores have a 10,000-fold smaller mass than those of A. gigasporus. These are produced by a handful of species including the gilled mushroom Tectella patellaris (Agaricales), 3.7 × 0.7 µm, and the resupinate species Hyphodontia latitans (Hymenochaetales), 3.5–5.0 × 0.5–0.8 µm (Fig 2F; Singer 1944; Ingold 2001b; Gilbertson and Ryvarden 1986). The spores of H. latitans are discharged from the inner surface of angular tubes (1–3 per mm) that form depressions in the surface of the flattened basidome. Spores of H. latitans are roughly cylindrical in shape with an approximate volume of 0.5 µm3 or 0.5 fL and mass of 0.6 pg. We have observed Buller’s drop formation in T. patellaris and confirmed its active discharge mechanism by capturing spore prints from its basidiomata (data not shown). The smallest spores that we have studied using high-speed video include the coral fungus Clavicorona pyxidata (Russulales), and common bracket fungus Trametes versicolor (Poriales). The C. pyxidata measurements were published previously by Stolze-Rybczynski et al. (2009). The predicted launch speed and discharge distance for H. latitans were based on data from these fungi.
Organisms
This study utilized fresh basidiomes of Aleurodiscus oakesii, Xerula radicata, Clavicorona pyxidata, and Trametes versicolor collected from mixed deciduous woodland habitats in Butler County, Ohio. To image spore discharge from these species, thin slices of basidiome (<1 mm) were cut with a scalpel and placed on the surface of distilled water agar in Petri dishes. As noted above, spore discharge was not imaged in Aleurodiscus gigasporus, Xerula australis, and Hyphodontia latitans. Launch speeds and discharge distances for these fungi were extrapolated from data obtained by high-speed imaging of A. oakesii, C. pyxidata, and T. versicolor.
Ultra-high-speed video microscopy
Video recordings were made with FASTCAM-ultima APX and APX-RS cameras (Photron, San Diego, CA) attached to an inverted compound microscope fitted with long-working distance objectives (Olympus, Tokyo). Each video clip was compiled from ≤100 image files extracted from recordings consisting up to 1 million images captured in a few seconds (e.g., 1 million image files captured with 2 µs shutter at 250,000 fps in 4 s). Analysis of digital images was performed using VideoPoint v.2.5 (Lenox Softworks, Lenox MA), Image-Pro Plus 6.2 (Media Cybernetics, Bethesda, MD), and proprietary software from Photron.
Mathematical modeling
Mathematical modeling. For spores moving at the speeds observed, viscous forces dominate over inertial forces. Thus, Stokes’ law, F⃗D = −6πrηv⃗, relates the drag force, F⃗D, to r, the aerodynamic radius of the projectile, η, the viscosity of the air, and v⃗, the particle velocity. Analytical integration of Newton’s second law, F⃗ = ma⃗, yields expressions for the x- and y-positions of the spore as functions of time that can be plotted parametrically to determine the spore trajectory. Spore motion in all of the species examined in this study fell within the laminar flow regime, characterized by Reynolds numbers at or below 1.0. A quasi-empirical interpolation model for viscous drag has been proposed from particles moving through fluids at the onset of turbulence, a regime characterized by Reynolds numbers between 1 and 1,000 (White 1974). The simpler Stokes model for drag was used for analysis in this study, because for Reynolds numbers close to 1.0 the difference between the two drag models is slight. Comparisons between these approaches to modeling the effects of drag will be published in a separate paper, but the validity of the current approach was demonstrated for a range of spore sizes in earlier work by Yafetto et al. (2008) and Stolze-Rybczynski et al. (2009).
Results and Discussion
High-speed video analysis of spore discharge
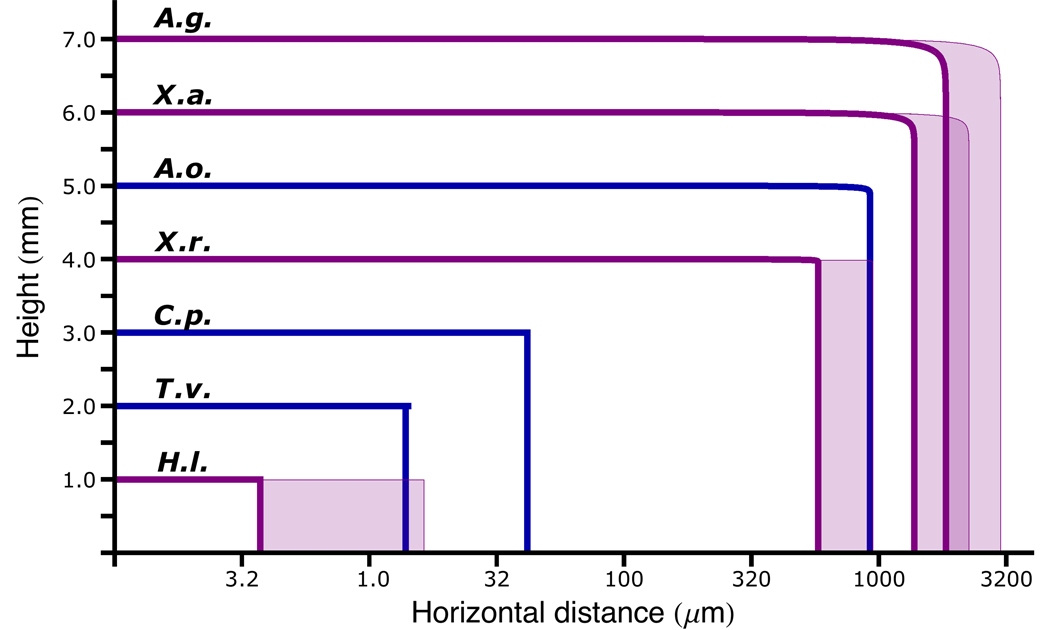
Ballistospores of Aleurodiscus oakesii were launched at a mean velocity of 0.66 ± 0.11 m s−1 (n = 4) and rotated as they left their sterigmata (Fig 3; Supplementary Movie; Table 1). The predicted range of this species, based on spore dimensions and launch speed was 0.92 mm, which represents 39 spore lengths. Calculations show that 9% of the total surface free energy in the Buller’s drop of this species is converted into the kinetic energy of the launch. The radius of Buller’s drop in A. oakesii is 5 µm; applying the same ratio of spore to drop size to A. gigasporus provides a predicted drop radius of 8 µm. If we assume that 9% of the energy in this drop is converted into the kinetic energy of the launch, A. gigasporus will be discharged at 0.53 m s−1 and travel 1.83 mm or 54 times its own length (Fig 4). This is the longest predicted distance of the ballistospore discharge mechanism in the Basidiomycota. Using the measured drop size of Xerula radicata to predict likely drop size in its relative Xerula australis, the same calculations predict a discharge distance of 1.38 mm or 53 spore lengths. This is likely to represent the longest spore launch in a gilled mushroom.
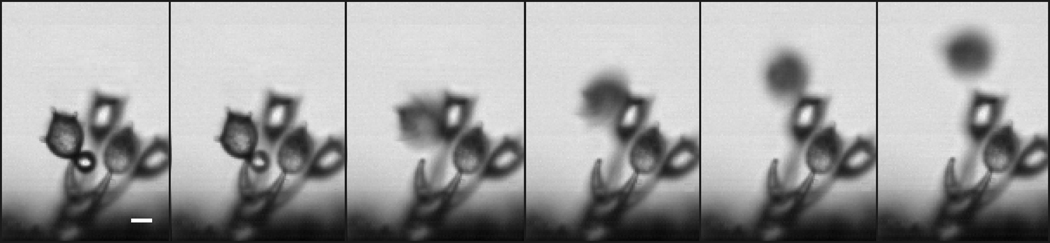
Fig 3.
Successive frames from high-speed video recording of ballistospore discharge in Aleurodiscus oakesii obtained at camera speed of 50,000 frames per second (also see Supplementary Movie). Adaxial drop is not seen easily in this species, but appears as a bulge on the spore surface directly above Buller’s drop in the first and second frames. Spore rotates counterclockwise (in this view) after separation from its sterigma in the third frame and moves beyond the focal plane of microscope. Scale bar = 10 µm.
Table 1.
Spore size, launch speed, and discharge distance for seven species of Basidiomycota.
| Species (Order) | Spore volume (m3) | Buller’s drop radius (µm)1 |
Projectile mass (kg) |
Velocity (m s−1) | Range (mm), spore lengths |
|---|---|---|---|---|---|
| Aleurodiscus gigasporus (Russulales) | 1.4 × 10−14 | 8.3 | 1.9 × 10−11 | 0.53 | 1.83, 54 |
| Xerula australis (Agaricales) | 9.2 × 10−15 | 6.6 | 1.2 × 10−11 | 0.53 | 1.38, 53 |
| Aleurodiscus oakesii (Russulales) | 3.5 × 10−15 | 5.2 | 4.8 × 10−12 | 0.66 ± 0.11 (4) | 0.92, 39 |
| Xerula radicata (Agaricales) | 1.6 × 10−15 | 3.7 | 2.2 × 10−12 | 0.70 | 0.58, 35 |
| Clavicorona pyxidata (Russulales)2 | 3.0 × 10−17 | 1.2 | 4.4 × 10−14 | 0.69 ± 0.06 (5) | 0.042, 9 |
| Trametes versicolor (Poriales) | 6.4 × 10−18 | 0.6 | 8.5 × 10−15 | 0.68 ± 0.07 (6) | 0.014, 3 |
| Hyphodontia latitans (Hymenochaetales) | 4.6 × 10−19 | 0.3 | 6.2 × 10−16 | 1.05 | 0.004, 1 |
Note: Shaded cells in table indicate measurements of Buller’s drop size and velocity (mean ± standard error, sample size) obtained from video recordings. For other species, Buller’s drop size was assumed to scale in proportion to the spore volume: drop size estimates for A. gigasporus, X. australis, and H. latitans were based on A. oakesii, X. radicata, and T. versicolor respectively. The same species comparisons informed the velocity estimates as explained in the text.
Samples sizes for Buller’s drop measurements ranged from n = 3 to 16 and standard errors were all < 0.3 µm.
Data for this species published previously (Stolze-Rybczynski et al. 2009).
Fig 4.
Calculated trajectories of ballistospores based on high-speed video recordings and mathematical modeling. In order to accommodate the nearly 1000-fold span of horizontal ranges, the horizontal distance (in µm) is displayed on a log scale. The spores were launched from arbitrary heights for clarity in this figure. Species: A.g., Aleurodiscus gigasporus; X.a., Xerula australis; A.o., Aleurodiscus oakesii; X.r., Xerula radicata; C.p., Clavicorona pyxidata; T.v., Trametes versicolor; H.l., Hyphodontia latitans. Velocity measurements obtained from video recordings for flights shown in blue (A.o., C.p., T.v.); other trajectories (in purple) based on extrapolation from these measurements. Buller’s drop volume was assumed to scale in proportion to the spore volume; drop size estimates for A.g., X.a., and H.l. were based on measurements from A.o., X.r., and T.v. respectively. The same species comparisons informed estimates of the fraction of surface tension energy that was converted to kinetic energy, but the effect of increasing the energy fractions to 25% are indicated by the shaded regions of the trajectories.
At the other end of the spatial scale, high-speed imaging of two species with tiny spores (Clavicorona pyxidata and Trametes versicolor) was used to inform estimates of launch speed and discharge distance for the minuscule spores of Hyphodontia latitans (Table 1). H. latitans has a predicted launch speed of 1.05 m s−1 and viscous drag limits its discharge distance to as little as 4 µm, which is a single spore length. If the launch of H. latitans is more efficient than C. pyxidata and T. versicolor and 25% of the total free energy in its Buller’s drop was converted into kinetic energy, it might fly as far as 21 µm, equivalent to six times its own length, but longer flights are impossible.
Theoretical limits to the ballistospore discharge mechanism
The use of high-speed imaging of spore discharge coupled with an effective model for the effects of viscous drag on the motion of microscopic particles, predict that the ballistospores of basidiomycetes are discharged over distances ranging from a few micrometers to 2 mm. This establishes the theoretical range of discharge distances for ballistospores. There are a number of reasons why the mechanism of ballistospore discharge is not utilized by fungi, or other microorganisms, to discharge significantly larger or smaller projectiles.
The ballistospore develops by the inflation of the sterigma; once it reaches maximum size the spore is delimited from the sterigma by the formation of a septum at the base of the spore (McLaughlin et al. 1985). The point of contact is called the hilum. Micromanipulation experiments have shown that this connection is weakened a few seconds before the formation of the Buller’s drop, minimizing the force required to break contact at the moment of the launch (Van Neil et al. 1972; Webster et al. 1984). The energy necessary for this separation, or fracture force, was estimated by Noblin et al. (2009) who used glass cantilevers of known spring constant to lift spores from their sterigmata. These investigators were studying the jelly fungus Auricularia auricula and measured separation forces of up to 5 µN falling to <0.4 µN. The average fracture force for the weakly attached spores was 0.15 µN; this may represent the fracture force just prior to spore discharge, but further studies are necessary to confirm this. Noblin et al. (2009) calculated a fracture energy of 1.6 × 10−14 J which represented 3.4% of the energy available from the surface tension in Buller’s drop. A. auricula produces mid-sized ballistospores, but it is unlikely that the fracture force scales in proportion to spore mass in other fungi because the connection always consists of two tiny patches of closely-appressed cell wall material that form an abscission zone. Variations in the contact area will affect the fracture force, but these will have minor physical consequences on launch speed and discharge distance in comparison to differences between the size of the spore and its Buller’s drop. Limited variation in the fracture force may explain why the proportion of the total energy in Buller’s drop converted into the kinetic energy of the launch is highest for larger spores with larger drops (Table 2). This may also place a physical limitation on spore size: spores any smaller than those of H. latitans could not be launched because the motion of their drops would not exert sufficient force to break the connection and get the spore airborne.
Table 2.
Comparison between the energetics of spore discharge in basidiomycete species with the largest (Aleurodiscus gigasporus) and smallest ballistospores (Hyphodontia latitans).
| Species | Aleurodiscus gigasporus | Hyphodontia latitans |
|---|---|---|
| Available surface tension energy1 | 2.9 × 10−11 J | 2.6 × 10−14 J |
| Energy to break connection2 | 1.6 × 10−14 J | 1.6 × 10−14 J |
| Proportion of total energy consumed in fracture | 0.1% | 61.5% |
| Residual energy after fracture | 2.9 × 10−11 J | 1.0 × 10−14 J |
| Kinetic energy of launch3 | 2.7 × 10−12 J | 3.4 × 10−16 J |
| Proportion of residual energy converted to kinetic energy of launch | 9.3% | 3.4% |
Calculation based on the change in surface area of Buller’s drop plus exposed surface of hemispherical adaxial drop of same radius coalescing to form single larger hemispherical drop.
Fracture energy assumed to remain constant for purpose of these calculations, but note that if this decreased by a factor of 10 for H. latitans, separation would consume 6.2% of the total surface tension energy. With the loosening of the connection between the spore and sterigma his, breakage would still remain a greater proportional energetic investment for this species than for fungi with larger ballistospores.
Kinetic energy based on launch speed predicted from model as described in the text.
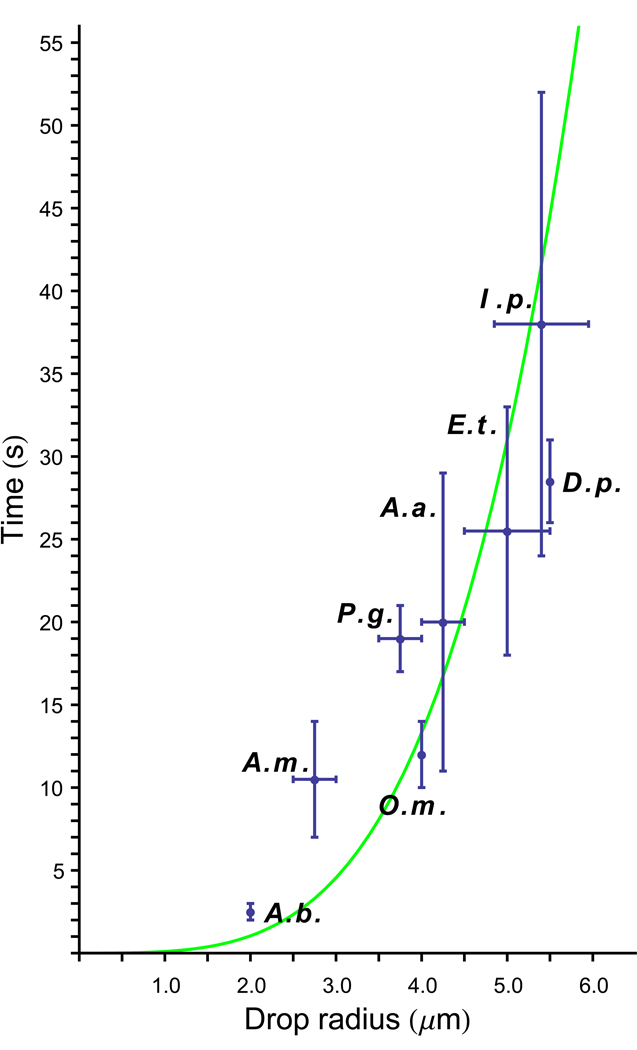
The energy available to large ballistospores easily overcomes the fracture force, but there are other obstacles to launching spores any bigger than those of A. gigasporus. Because the mass of the projectile scales as the cube of its radius, whereas the surface tension energy available for the launch scales as the square of the radii of Buller’s drop and the adaxial drop, the energy to mass ratio decreases for large spores with large drops. Calculations show, however, that the resulting reduction in launch speed is offset by the increased inertia of the larger projectile. The increasing time required for the expansion of larger drops is probably a more important factor that limits the maximum size of ballistospores. The rate of expansion of Buller’s drop follows an asymptotic curve (Webster et al. 1989). This may be due to a reduction in the rate of condensation of water molecules on the drop surface as expansion dilutes the hygroscopic compounds dissolved in the fluid. Sugars and sugar alcohols (mannitol) have been identified in washings from spore deposits and from Buller’s drops collected in micropipets, but their release is not understood (Turner and Webster 1995; Webster et al. 1995; Money 1998; Stolze-Rybczynski et al. 2009). Drop expansion varies from 2–3 s in ballistosporic yeasts, including Sporobolomyces salmonicolor, and the gilled mushroom Agaricus bisporus, to almost 1 min in Itersonilia perplexans (Müller 1954; Webster et al. 1989). Drop radius in I. perplexans varies from 5–6 µm, about the same drop size A. oakesii. A larger drop radius of 8 µm is predicted for A. gigasporus, but this could be significantly smaller and produce a shorter than anticipated flight. Larger drops take longer to form, making them more vulnerable than small drops to changes in environmental humidity (Fig 5). The extreme sensitivity of drop formation to fluctuations in humidity and temperature is reflected in the frequent failure of the mechanism in experiments.
Fig 5.
Relationship between the maximum size of Buller’s drop and time for its formation in variety of Basidiomycota. Data from Webster et al. (1989). Species: A.a., Auricularia auricula; A.b., Agaricus bisporus; A.m., Auricularia mesenterica; D.p., Ditiola peziziformis; E.t., Exidia thuretiana; I.p., Itersonilia perplexans; O.m., Oudemansiella mucida; P.g., Puccinia graminis. Error bars indicate the range of drop sizes and formation times. Data fitted to a quartic function (green curve) showing that the time required for drop formation increases approximately as the fourth power of the drop radius (P < 0.001).
Mission-appropriate mechanisms of spore discharge
Ballistospore discharge is a superbly efficient mechanism for propelling spores over much shorter distances. The velocity of ballistospore discharge and launch distances vary in relation to basidiome morphology. For gilled mushrooms, discharge will be futile if the spores are propelled onto the opposing gill surface, and high-speed imaging shows that spores of these species exhibit shorter ranges than fungi that expel their ballistospores into the airstream directly (Stolze-Rybczynski et al. 2009). The Xerula species examined in the present study show longer ranges but these are matched to the widely-spaced gills of their basidomata. The maximum predicted discharge distance of almost 2 mm for A. gigasporus will propel spores from the surface of the crustose basidiome to access faster moving air beyond the boundary layer of motionless air (Money and Fischer 2009). The short range of H. latitans, and other basidiomycetes with lilliputian spores, is sufficient to separate the spore from its sterigma, but these species must rely upon gravity and air turbulence for long-distance dispersal.
The physiology and mechanics of the ballistospore and ascospore discharge mechanisms are completely different (Ingold 2001a). Ballistospore discharge is driven by the motion of Buller’s drop; ascospore discharge is powered by the hydrostatic pressure within the ascus. But in both cases, the mechanisms are fueled by the accumulation of osmotically-active compounds: ascus pressure is generated by osmolytes in the ascus sap, and the formation of Buller’s drop is driven by the release of the same kinds of compounds onto the surface of the ballistospore. The asci of most Ascomycota function as pressurized squirt guns (Ingold 1971). Species of Dipodascus (Saccharomycetes) extrude their slimy ascospores through the ascus apex, whereas spores of Neurospora and thousands of other Sordariomycetes are shot into the air at very high speeds and travel distances of a few millimeters to centimeters (Trail et al. 2005; Trail 2007; Yafetto et al. 2008). Pezizomycetes and Dothidiomycetes utilize similar mechanisms of spore discharge and some species achieve discharge distances of a few tenths of one meter (Money and Fischer 2009).
Changes in spore and drop size during the evolution of the Basidiomycota have permitted adjustments in discharge distance for ballistosporic basidiomycetes, but the inherent physical limitations of the mechanism identified in this study have made it impossible for these fungi to utilize many of the kinds of dispersal strategies that have evolved among the Ascomycota. Ballistospory has other benefits. It is a highly effective engineering solution for organizing high densities of spore-producing cells in the limited spaces afforded in gilled mushrooms and other types of basidome (Fischer and Money 2010). By discharging spores over a precise and limited range, spore loss within the basidome is limited. This allows the release of vast numbers of spores into the air, which is essential to the reproductive strategies of the mushroom-forming fungi and their relatives. The difficulty in adapting a squirt-gun mechanism for discharging spores into the air over distances <1 mm probably explains why the mushroom-like sporophores that have evolved in the Ascomycota are limited to discharging spores from their exposed surfaces.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Science Foundation (0743074) and the National Institutes of Health/NIEHS (1R15 ES016425, ARRA Supplement). The authors thank Diana Davis for insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material: Supplementary material associated with this article is available in the online version at doi
REFERENCES
- Buller AHR. Researches on Fungi. vol. 1. London: Longmans, Green & Company; 1909. [Google Scholar]
- De Bary A. Comparative Morphology and Taxonomy of the Fungi Mycetozoa and Bacteria (English translation) Oxford: Clarendon Press; 1887. [Google Scholar]
- Fischer MWF, Money NP. Why mushrooms form gills: efficiency of the lamellate morphology. Fungal Biology. 2010;114:57–63. doi: 10.1016/j.mycres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RL, Ryvarden L. North American Polypores. vol. 1. Oslo, Norway: Fungiflora A/S; 1986. [Google Scholar]
- Ginns J, Bandoni RJ. Aleurodiscus gigasporus sp. nov. from China and A. subglobosporus from Japan. Mycologia. 1991;83:548–552. [Google Scholar]
- Ingold CT. Fungal Spores: Their Liberation and Dispersal. Oxford: Clarendon Press; 1971. [Google Scholar]
- Ingold CT. Active liberation of reproductive units in terrestrial fungi. Mycologist. 1999;13:113–116. [Google Scholar]
- Ingold CT. Turgor and spore release in ascus and basidium. Mycologist. 2001a;15:45. [Google Scholar]
- Ingold CT. Range in size and form of basidiospores and ascospores. Mycologist. 2001b;15:165–166. [Google Scholar]
- McLaughlin DJ, Beckett A, Yoon KS. Ultrastructure and evolution of ballistosporic basidiospores. Botanical Journal of the Linnean Society. 1985;91:253–271. [Google Scholar]
- Money NP. More g’s than the Space Shuttle: The mechanism of ballistospore discharge. Mycologia. 1998;90:547–558. [Google Scholar]
- Money NP, Fischer MWF. Biomechanics of spore discharge in phytopathogens. In: Deising H, editor. The Mycota, Volume 5, Plant Relationships. 2nd edition. New York: Springer Verlag; 2009. pp. 115–133. [Google Scholar]
- Müller D. Die Abschleuderung der Sporen von Sporobolomyces-Spiegelhefe-gefilmt. Friesia. 1954;5:65–74. [Google Scholar]
- Noblin X, Yang S, Dumais J. Surface tension propulsion of fungal spores. Journal of Experimental Biology. 2009;212:2835–2843. doi: 10.1242/jeb.029975. [DOI] [PubMed] [Google Scholar]
- Núñez M, Ryvarden L. The genus Aleurodiscus (Basidiomycotina) Oslo: Fungiflora A/S; 1997. [Google Scholar]
- Pegler DN, Young TWK. Classification of Oudemansiella (Basidiomycota: Tricholomataceae), with special reference to spore structure. Transactions of the British Mycological Society. 1987;87:583–602. [Google Scholar]
- Pringle A, Patek SN, Fischer M, Stolze J, Money NP. The captured launch of a ballistospore. Mycologia. 2005;97:866–871. doi: 10.3852/mycologia.97.4.866. [DOI] [PubMed] [Google Scholar]
- Singer R. New genera of fungi. Mycologia. 1944;36:358–368. [PubMed] [Google Scholar]
- Stolze-Rybczynski JL, Cui Y, Stevens MHH, Davis DJ, Fischer MWF, Money NP. Adaptation of the spore discharge mechanism in the Basidiomycota. PLoS ONE. 2009;4(1):e4163. doi: 10.1371/journal.pone.0004163. doi:10.1371/journal.pone.0004163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehon LR, Jacks WR. Smooth patch, a bark lesion of white oak. Journal of Forestry. 1933;31:430–433. [Google Scholar]
- Trail F, Gaffoor I, Vogel S. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fusarium graminearum) Fungal Genetics and Biology. 2005;42:528–533. doi: 10.1016/j.fgb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Trail F. Fungal cannons: explosive spore discharge in the Ascomycota. FEMS Microbiology Letters. 2007;276:12–18. doi: 10.1111/j.1574-6968.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- Turner JCR, Webster J. Mushroom spores--the analysis of Buller’s drop. Chemical Engineering Science. 1995;50:2359–2360. [Google Scholar]
- Van Neil CB, Garner GE, Cohen AL. On the mechanism of ballistospore discharge. Archiv für Mikrobiologie. 1972;84:129–140. doi: 10.1007/BF00412433. [DOI] [PubMed] [Google Scholar]
- Webster J, Chen C-Y. Ballistospore discharge. Transactions of the Mycological Society of Japan. 1990;31:301–315. [Google Scholar]
- Webster J, Weber RWS. Introduction to Fungi. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Webster J, Davey RA, Duller GA, Ingold CT. Ballistospore discharge in Itersonilia perplexans. Transactions of the British Mycological Society. 1984;82:13–29. [Google Scholar]
- Webster J, Davey RA, Turner JCR. Vapor as the source of water in Buller’s drop. Transactions of the British Mycological Society. 1989;93:297–302. [Google Scholar]
- Webster J, Davey RA, Smirnoff N, Fricke W, Hinde P, Tomos D, Turner JCR. Mannitol and hexoses are components of Buller’s drop. Mycological Research. 1995;99:833–838. [Google Scholar]
- White FM. Viscous Fluid Flow. New York: McGraw Hill; 1974. [Google Scholar]
- Yafetto L, Carroll L, Cui Y, Davis DJ, Fischer MWF, Henterly AC, Kessler JD, Kilroy H, Shidler JB, Stolze-Rybczynski JL, Sugawara Z, Money NP. The fastest flights in nature: high-speed spore discharge mechanisms among fungi. PLoS ONE. 2008;3(9):e3237. doi: 10.1371/journal.pone.0003237. doi:10.1371/journal.pone.0003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.