Abstract
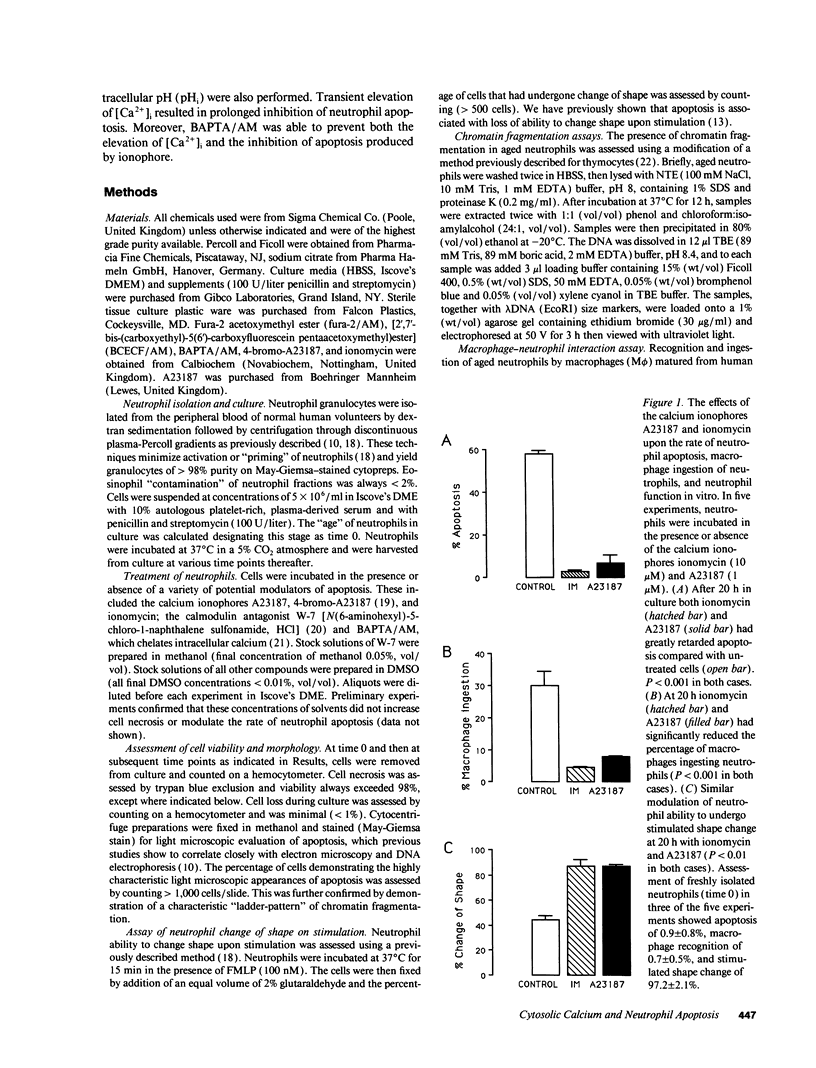
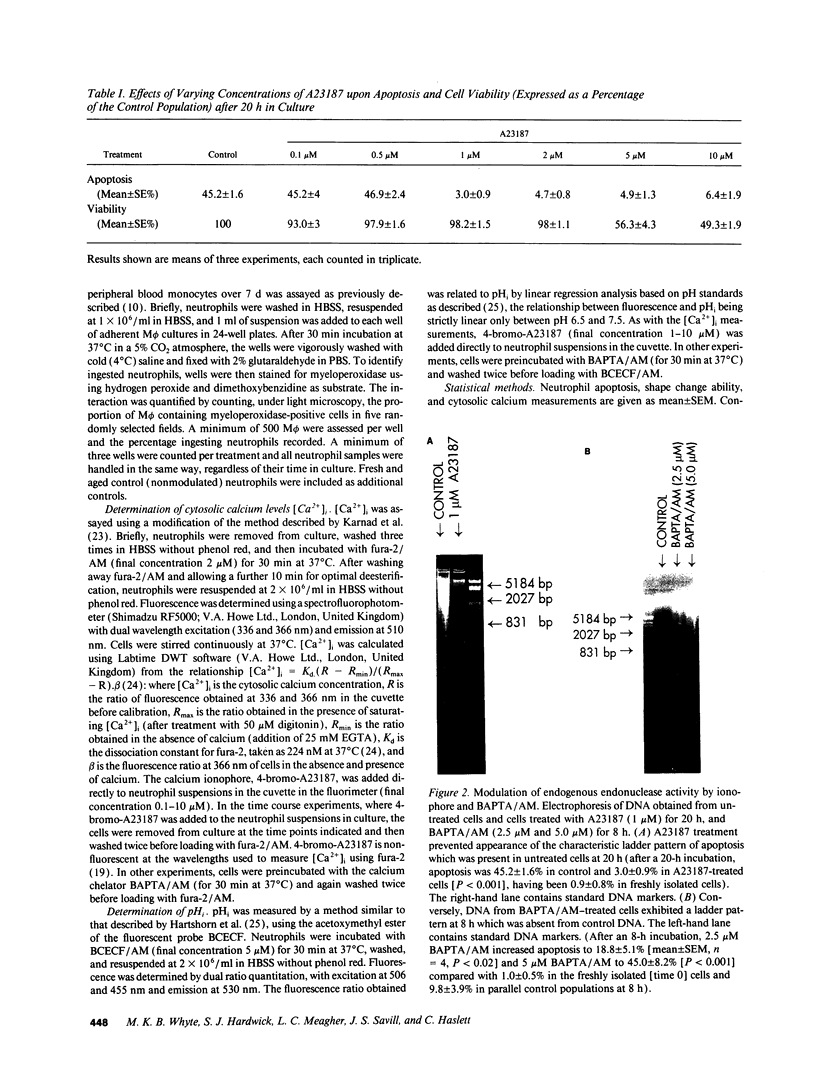
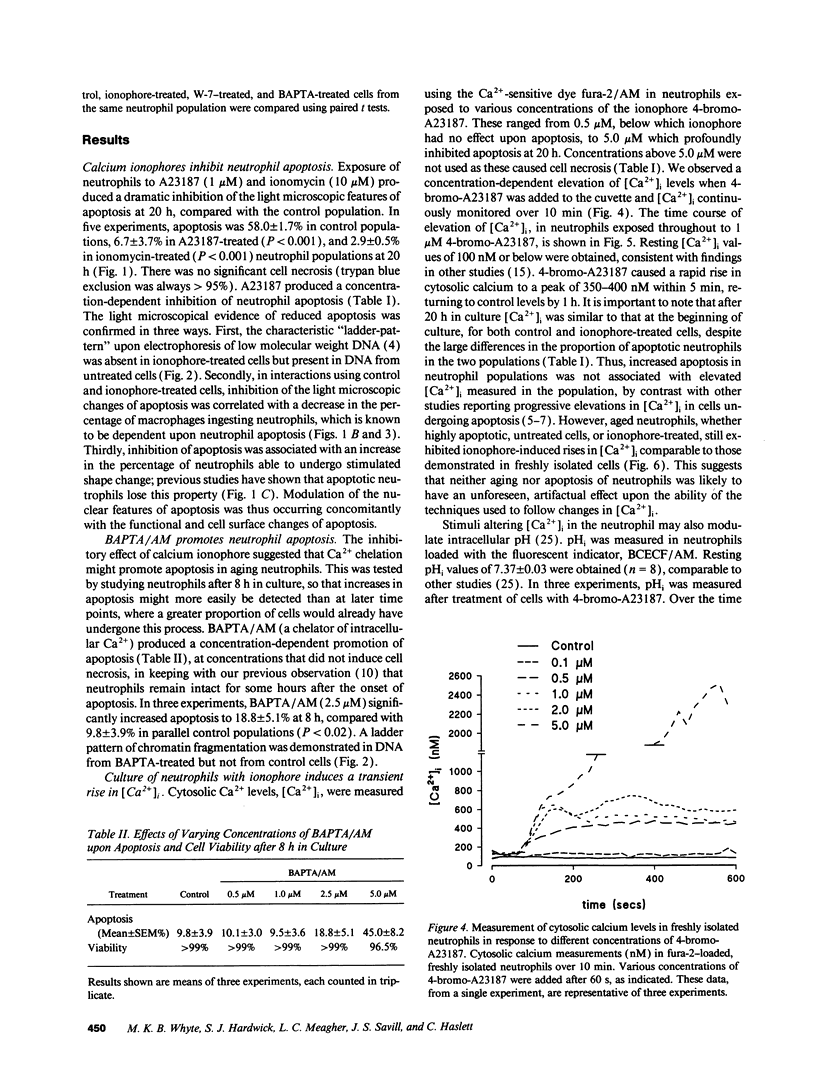
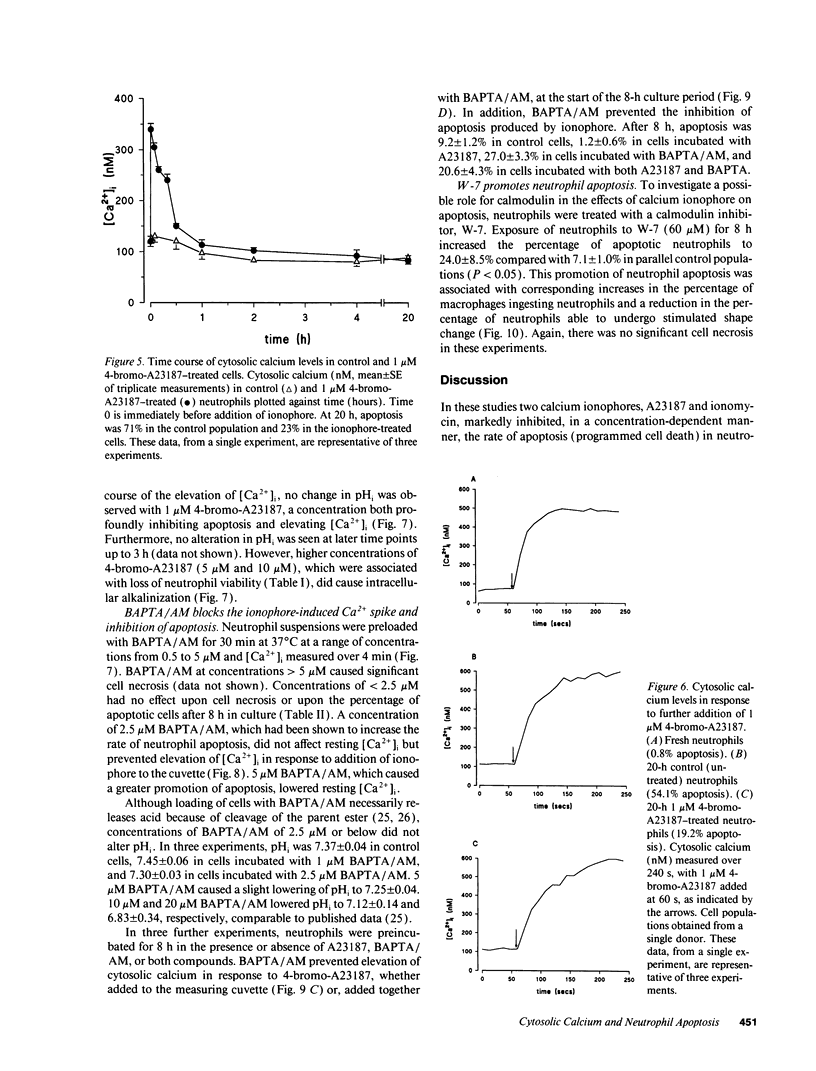
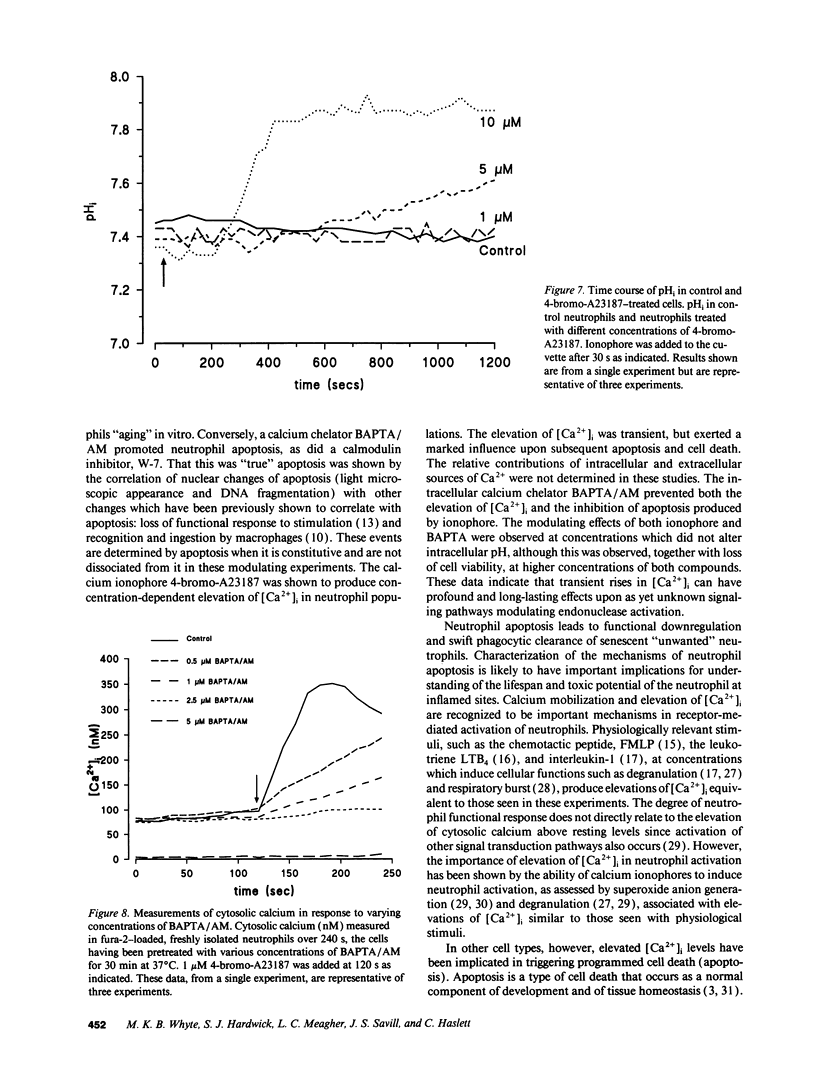
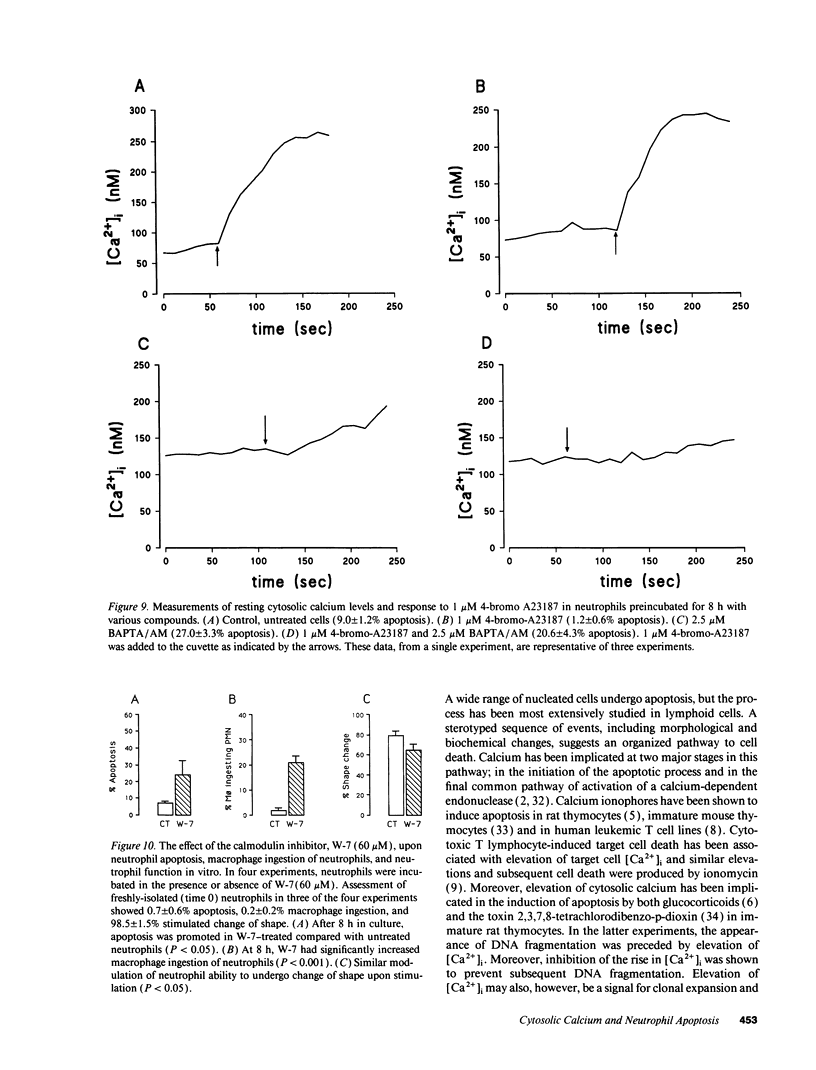
Elevation of cytosolic calcium ([Ca2+]i) has been reported to induce apoptosis in a number of cell types. However, in the neutrophil, which undergoes apoptosis constitutively during aging in vitro, activation by inflammatory mediators elevates [Ca2+]i and prolongs lifespan via inhibition of apoptosis. To examine this paradox, we investigated the effects of modulation of [Ca2+]i upon apoptosis of neutrophils in vitro. Calcium ionophores (A23187, ionomycin) retarded apoptosis in neutrophil populations after 20 h (P < 0.001). Conversely, intracellular Ca(2+)-chelation, using bis-(o-aminophenoxy)-N,N,N'N'-tetraacetic acid (BAPTA) acetoxymethyl ester (AM) promoted apoptosis (P < 0.02). W-7 (an inhibitor of calmodulin) also promoted apoptosis (P < 0.05). Measurements of [Ca2+]i, using fura-2, showed (a) increased apoptosis in neutrophil populations was not associated with elevated [Ca2+]i, (b) neutrophils cultured with ionophore at concentrations inhibiting apoptosis exhibited transient (< 1 h) elevations of [Ca2+]i, to levels previously reported with receptor-mediated stimuli, and (c) BAPTA was able to prevent the elevation of [Ca2+]i and the inhibition of apoptosis produced by ionophore. Modulation of apoptosis occurred without alterations in intracellular pH. Thus, in the neutrophil, unlike lymphoid cells, elevation of [Ca2+]i exerts an inhibitory effect upon apoptosis. Furthermore, these data suggest that transient elevation of [Ca2+]i elicits signaling events leading to prolonged inhibition of apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Verret C. R., Wolley R. C., Eisen H. N. Calcium ion concentrations and DNA fragmentation in target cell destruction by murine cloned cytotoxic T lymphocytes. J Exp Med. 1988 Feb 1;167(2):514–527. doi: 10.1084/jem.167.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Cohen J. J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Tom-Kun J., Mack E., Grinstein S. Bromo-A23187: a nonfluorescent calcium ionophore for use with fluorescent probes. Anal Biochem. 1985 May 1;146(2):349–352. doi: 10.1016/0003-2697(85)90550-0. [DOI] [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd D. R., MacDonald P. N., Komm B. S., Haussler M. R., Miesfeld R. Evidence for early induction of calmodulin gene expression in lymphocytes undergoing glucocorticoid-mediated apoptosis. J Biol Chem. 1991 Oct 5;266(28):18423–18426. [PubMed] [Google Scholar]
- FLIEDNER T. M., CRONKITE E. P., ROBERTSON J. S. GRANULOCYTOPOIESIS. I. SENESCENCE AND RANDOM LOSS OF NEUTROPHILIC GRANULOCYTES IN HUMAN BEINGS. Blood. 1964 Oct;24:402–414. [PubMed] [Google Scholar]
- Golstein P., Ojcius D. M., Young J. D. Cell death mechanisms and the immune system. Immunol Rev. 1991 Jun;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Grigg J. M., Savill J. S., Sarraf C., Haslett C., Silverman M. Neutrophil apoptosis and clearance from neonatal lungs. Lancet. 1991 Sep 21;338(8769):720–722. doi: 10.1016/0140-6736(91)91443-x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hartshorn K. L., Wright J., Collamer M. A., White M. R., Tauber A. I. Human neutrophil stimulation by influenza virus: relationship of cytoplasmic pH changes to cell activation. Am J Physiol. 1990 Jun;258(6 Pt 1):C1070–C1076. doi: 10.1152/ajpcell.1990.258.6.C1070. [DOI] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Asano M., Iwadare S., Matsumoto I., Totsuka T., Aoki N. A novel vascular relaxing agent, N-(6--aminohexyl)-5-chloro-1-naphthalensulfonamide which affects vascular smooth muscle actomyosin. J Pharmacol Exp Ther. 1978 Oct;207(1):8–15. [PubMed] [Google Scholar]
- Jones D. P., McConkey D. J., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation in rat liver nuclei. J Biol Chem. 1989 Apr 15;264(11):6398–6403. [PubMed] [Google Scholar]
- Karnad A. B., Hartshorn K. L., Wright J., Myers J. B., Schwartz J. H., Tauber A. I. Priming of human neutrophils with N-formyl-methionyl-leucyl-phenylalanine by a calcium-independent, pertussis toxin-insensitive pathway. Blood. 1989 Nov 15;74(7):2519–2526. [PubMed] [Google Scholar]
- Korchak H. M., Vosshall L. B., Zagon G., Ljubich P., Rich A. M., Weissmann G. Activation of the neutrophil by calcium-mobilizing ligands. I. A chemotactic peptide and the lectin concanavalin A stimulate superoxide anion generation but elicit different calcium movements and phosphoinositide remodeling. J Biol Chem. 1988 Aug 15;263(23):11090–11097. [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Pozzan T. Role of cytosolic free calcium and phospholipase C in leukotriene-B4-stimulated secretion in human neutrophils. Comparison with the chemotactic peptide formyl-methionyl-leucyl-phenylalanine. Eur J Biochem. 1987 Jan 2;162(1):161–168. doi: 10.1111/j.1432-1033.1987.tb10556.x. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Tsien R. Y., Miner C., Bookchin R. M. Physiological [Ca2+]i level and pump-leak turnover in intact red cells measured using an incorporated Ca chelator. Nature. 1982 Jul 29;298(5873):478–481. doi: 10.1038/298478a0. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Amador-Pérez J. F., Orrenius S., Jondal M. Calcium-dependent killing of immature thymocytes by stimulation via the CD3/T cell receptor complex. J Immunol. 1989 Sep 15;143(6):1801–1806. [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Nicotera P., Hartzell P., Bellomo G., Wyllie A. H., Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989 Feb 15;269(1):365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- Means A. R. Molecular mechanisms of action of calmodulin. Recent Prog Horm Res. 1988;44:223–262. doi: 10.1016/b978-0-12-571144-9.50012-0. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M., López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990 Sep;9(9):2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
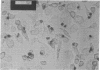
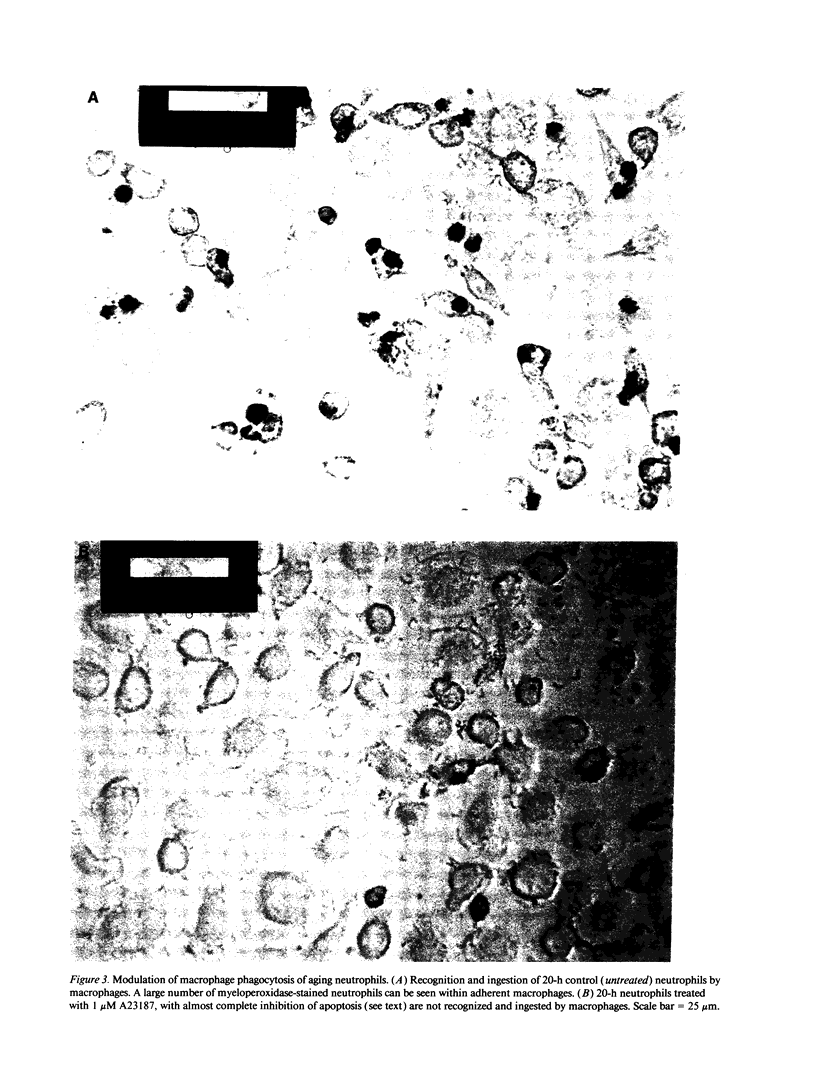
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Epps D. E., Justen J. M., Sam L. M., Wynalda M. A., Fitzpatrick F. A., Yein F. S. Human neutrophil activation with interleukin-1. A role for intracellular calcium and arachidonic acid lipoxygenation. Biochem Pharmacol. 1987 Nov 15;36(22):3851–3858. doi: 10.1016/0006-2952(87)90449-7. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Maecker H. T., Levy R. DNA fragmentation and cell death mediated by T cell antigen receptor/CD3 complex on a leukemia T cell line. Eur J Immunol. 1989 Oct;19(10):1911–1919. doi: 10.1002/eji.1830191023. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ohmura T., Yamakado T., Hidaka H. Two types of calcium-dependent protein phosphorylations modulated by calmodulin antagonists. Naphthalenesulfonamide derivatives. Mol Pharmacol. 1982 Sep;22(2):408–412. [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]