Abstract
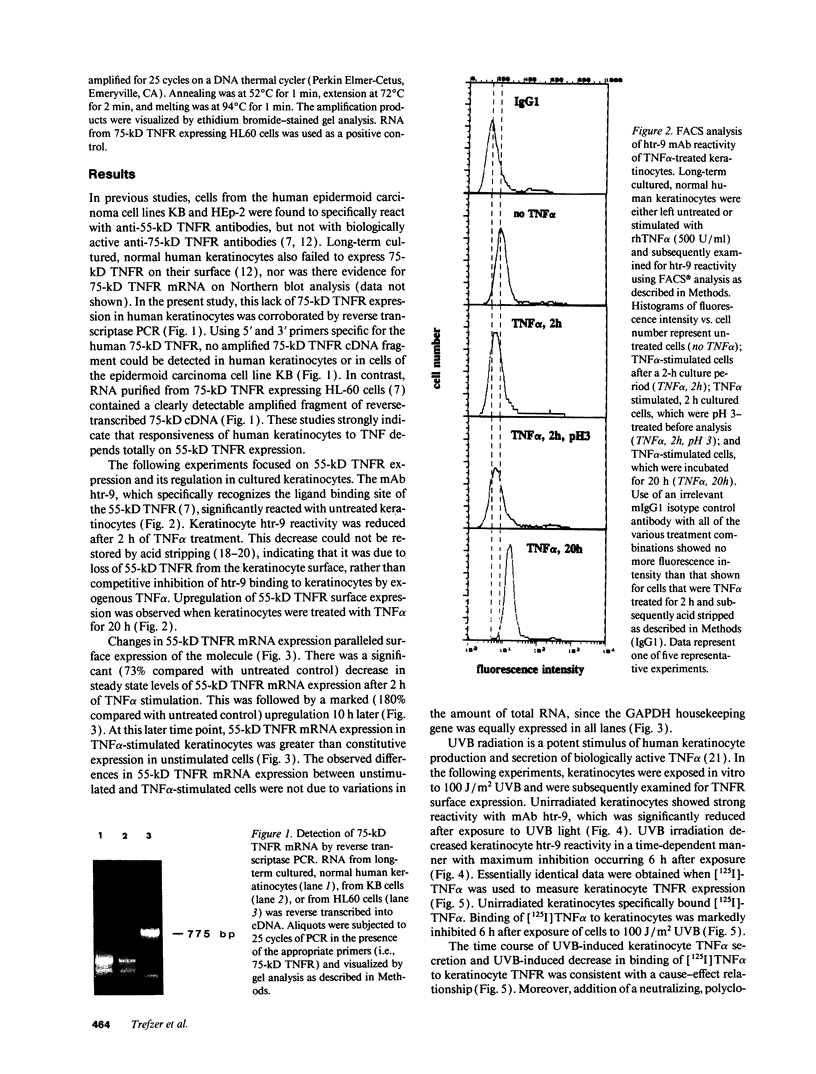
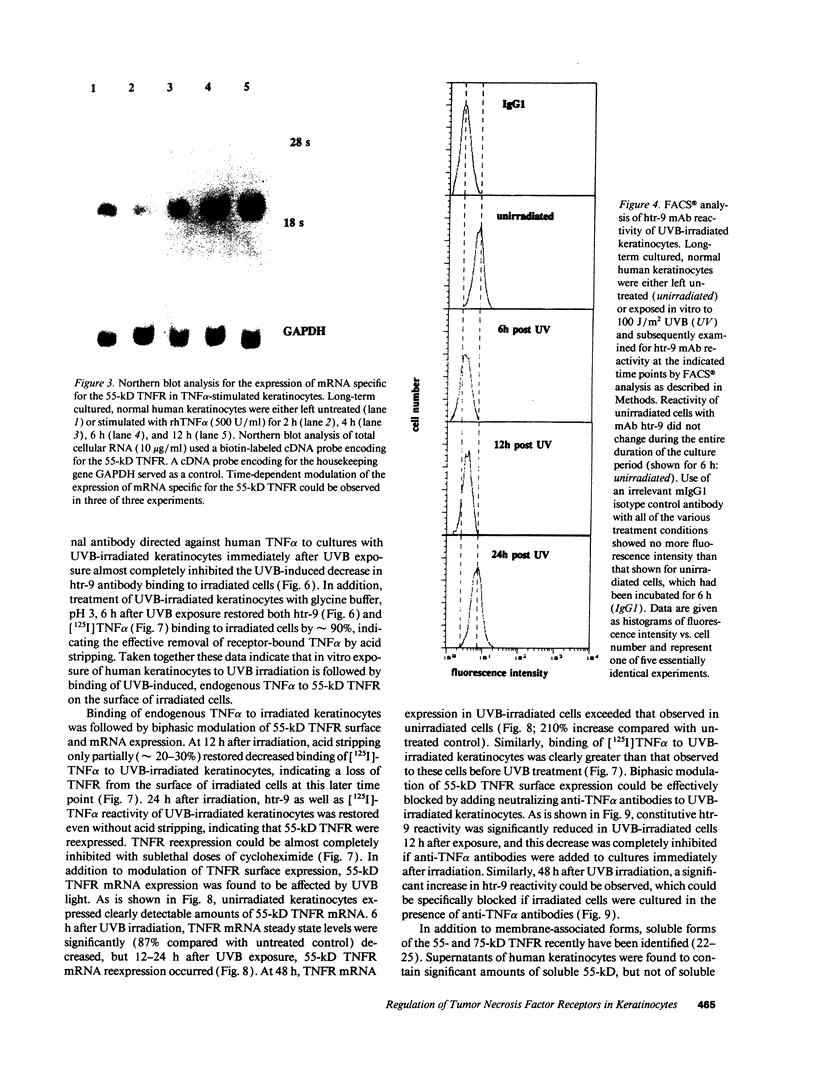
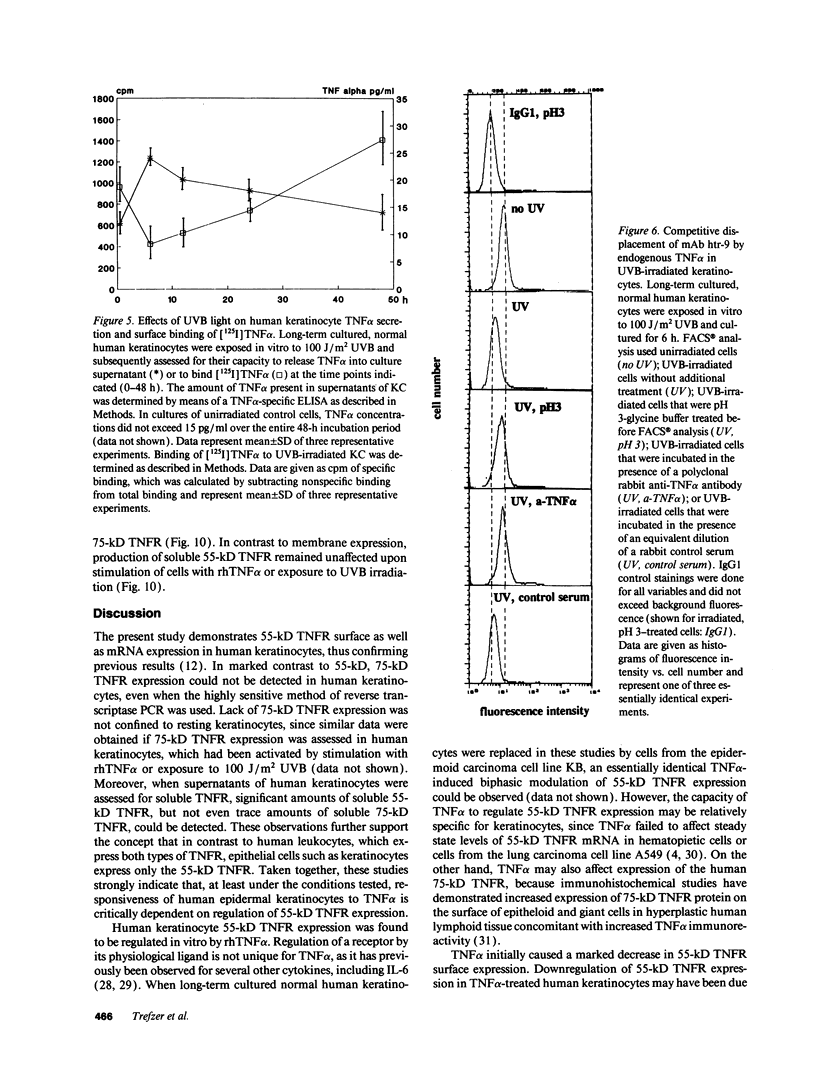
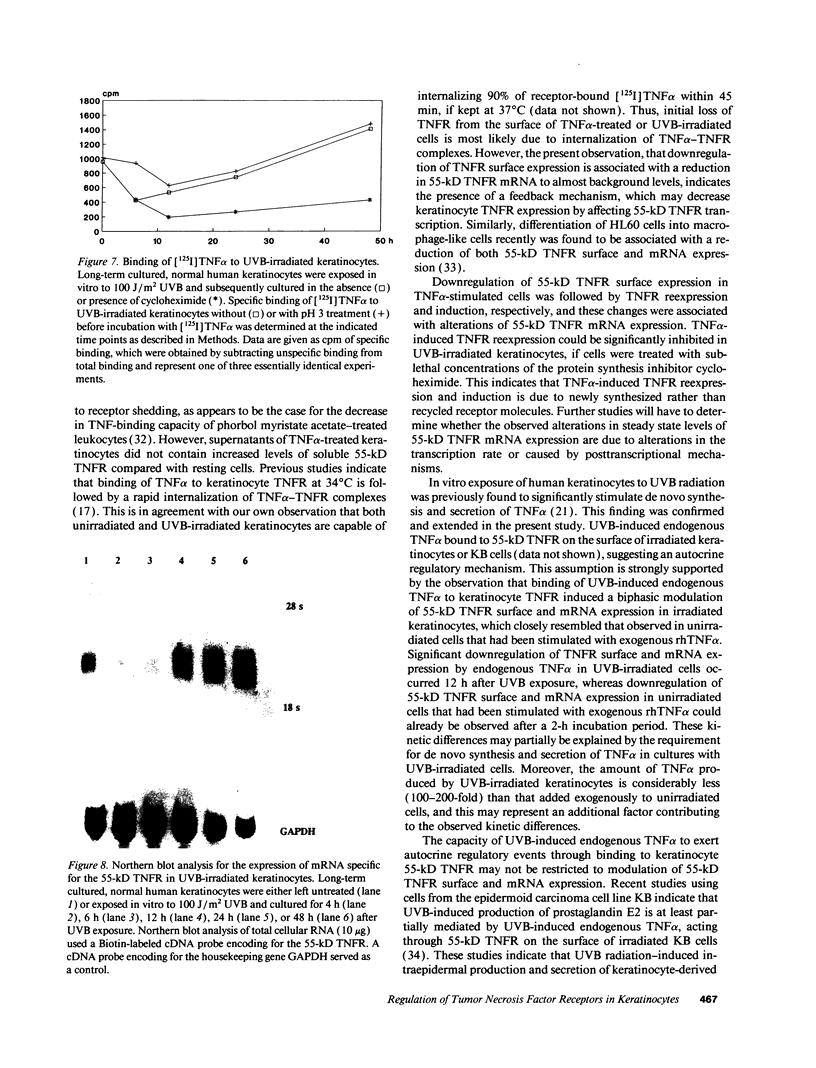
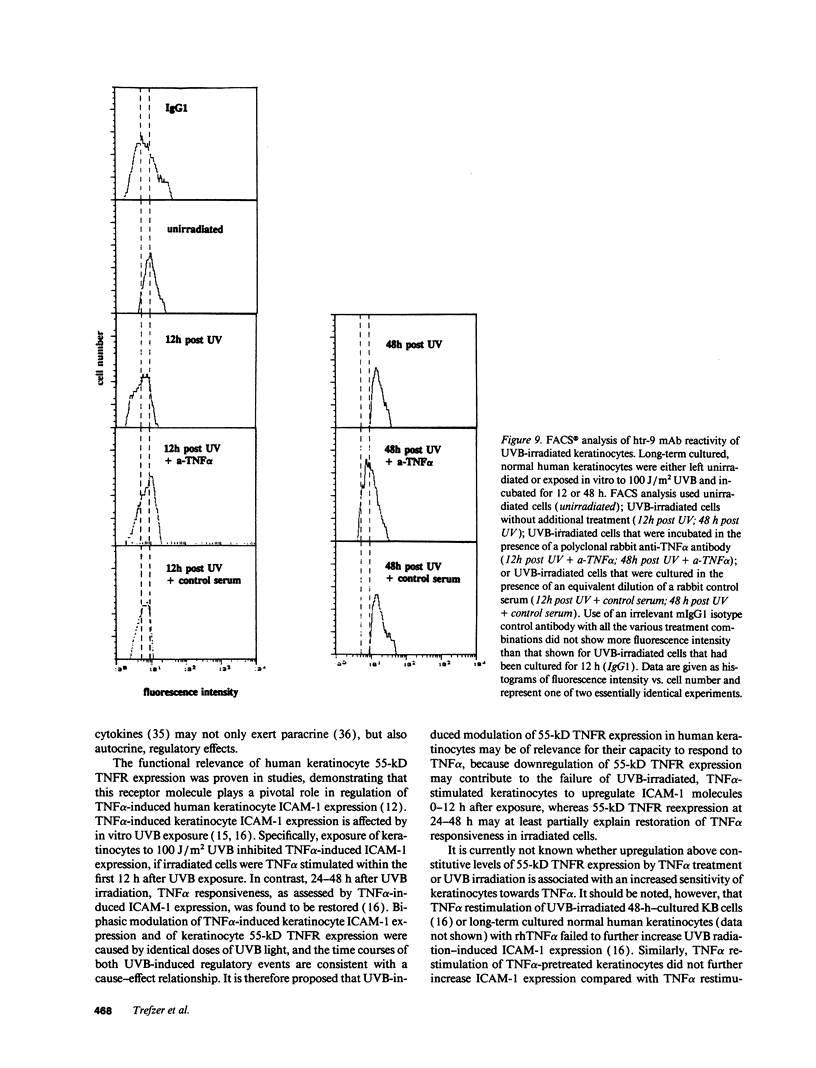
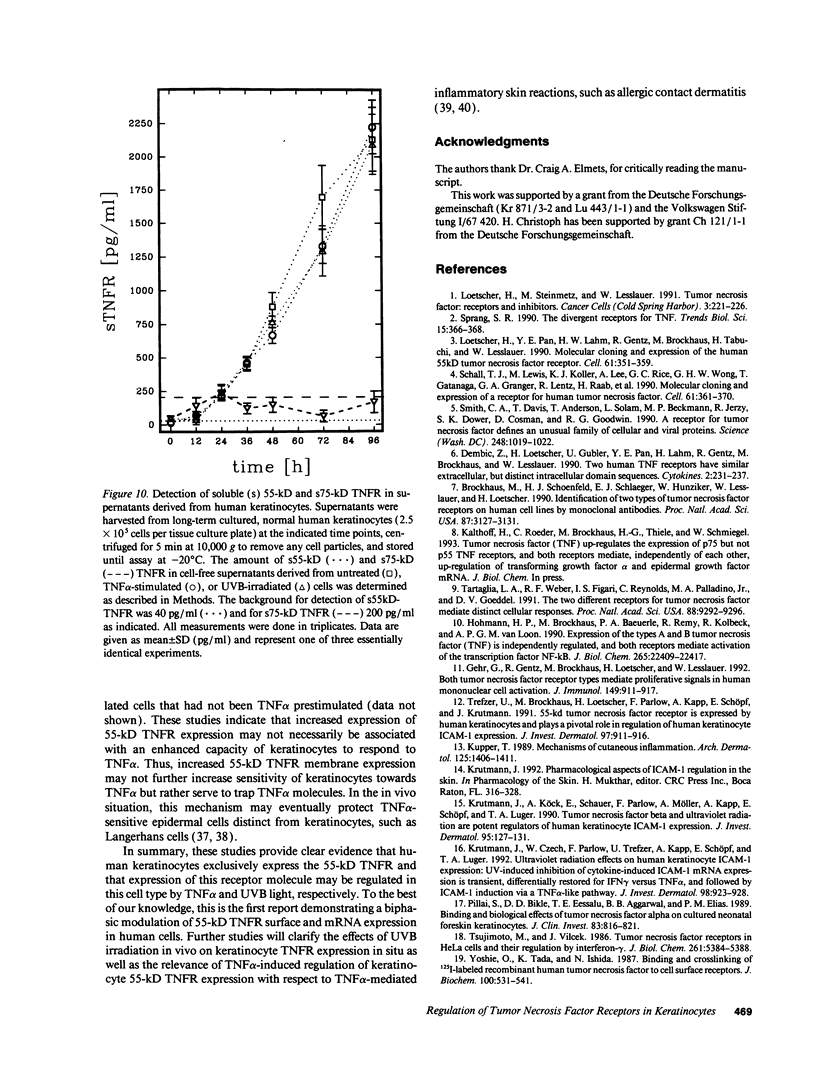
In previous studies we showed that cultured human keratinocytes expressed the 55-kD TNF receptor (TNFR) and that its expression the important for TNF alpha-mediated upregulation of intercellular adhesion molecule-1 (ICAM-1) expression on keratinocytes. Because factors that either reduce or enhance TNFR expression are likely to have a major impact on the biological effects of TNF alpha on keratinocytes, these studies were conducted to determine the factors that regulate its expression on keratinocytes. Using reverse transcriptase polymerase chain reaction, human keratinocytes were shown to lack 75-kD TNFR expression, indicating that TNF responsiveness of human keratinocytes critically depended on regulation of 55-kD TNFR expression. Human keratinocyte 55-kD TNFR surface and mRNA expression was found to be regulated in vitro by recombinant human (rh) TNF alpha. Stimulation of keratinocytes with rhTNF alpha initially decreased, but later increased, 55-kD TNFR surface expression. This biphasic modulation of 55-kD TNFR surface expression was associated with concomitant changes in 55-kD TNFR mRNA expression. Ultraviolet B (UVB) radiation, a well-known inducer of synthesis and secretion of TNF alpha by human keratinocytes, was found to mimic TNF alpha-induced modulation of 55-kD TNFR surface and mRNA expression via a TNF alpha-mediated autocrine regulatory mechanism. Production of soluble 55-kD TNFR by human keratinocytes remained unaffected by TNF alpha stimulation or UVB irradiation. These studies provide clear evidence that membrane expression of the human 55-kD TNFR may be regulated in human keratinocytes by the ligand itself: TNF alpha. Since in previous studies UVB irradiation transiently inhibited TNF alpha-induced human keratinocyte ICAM-1 expression, it is proposed that UVB radiation-induced biphasic modulation of human keratinocyte 55-kD TNFR expression may affect the capacity of these cells to respond to TNF alpha.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer J., Bauer T. M., Kalb T., Taga T., Lengyel G., Hirano T., Kishimoto T., Acs G., Mayer L., Gerok W. Regulation of interleukin 6 receptor expression in human monocytes and monocyte-derived macrophages. Comparison with the expression in human hepatocytes. J Exp Med. 1989 Nov 1;170(5):1537–1549. doi: 10.1084/jem.170.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus M., Schoenfeld H. J., Schlaeger E. J., Hunziker W., Lesslauer W., Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dembic Z., Loetscher H., Gubler U., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Lesslauer W. Two human TNF receptors have similar extracellular, but distinct intracellular, domain sequences. Cytokine. 1990 Jul;2(4):231–237. doi: 10.1016/1043-4666(90)90022-l. [DOI] [PubMed] [Google Scholar]
- Digel W., Porzsolt F., Schmid M., Herrmann F., Lesslauer W., Brockhaus M. High levels of circulating soluble receptors for tumor necrosis factor in hairy cell leukemia and type B chronic lymphocytic leukemia. J Clin Invest. 1992 May;89(5):1690–1693. doi: 10.1172/JCI115769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebeyehu G., Rao P. Y., SooChan P., Simms D. A., Klevan L. Novel biotinylated nucleotide--analogs for labeling and colorimetric detection of DNA. Nucleic Acids Res. 1987 Jun 11;15(11):4513–4534. doi: 10.1093/nar/15.11.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehr G., Gentz R., Brockhaus M., Loetscher H., Lesslauer W. Both tumor necrosis factor receptor types mediate proliferative signals in human mononuclear cell activation. J Immunol. 1992 Aug 1;149(3):911–917. [PubMed] [Google Scholar]
- Hohmann H. P., Brockhaus M., Baeuerle P. A., Remy R., Kolbeck R., van Loon A. P. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-kappa B. TNF alpha is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990 Dec 25;265(36):22409–22417. [PubMed] [Google Scholar]
- Koch F., Heufler C., Kämpgen E., Schneeweiss D., Böck G., Schuler G. Tumor necrosis factor alpha maintains the viability of murine epidermal Langerhans cells in culture, but in contrast to granulocyte/macrophage colony-stimulating factor, without inducing their functional maturation. J Exp Med. 1990 Jan 1;171(1):159–171. doi: 10.1084/jem.171.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutmann J., Czech W., Parlow F., Trefzer U., Kapp A., Schöpf E., Luger T. A. Ultraviolet radiation effects on human keratinocyte ICAM-1 expression: UV-induced inhibition of cytokine-induced ICAM-1 mRNA expression is transient, differentially restored for IFN gamma versus TNF alpha, and followed by ICAM-1 induction via a TNF alpha-like pathway. J Invest Dermatol. 1992 Jun;98(6):923–928. doi: 10.1111/1523-1747.ep12460737. [DOI] [PubMed] [Google Scholar]
- Krutmann J., Köck A., Schauer E., Parlow F., Möller A., Kapp A., Förster E., Schöpf E., Luger T. A. Tumor necrosis factor beta and ultraviolet radiation are potent regulators of human keratinocyte ICAM-1 expression. J Invest Dermatol. 1990 Aug;95(2):127–131. doi: 10.1111/1523-1747.ep12477839. [DOI] [PubMed] [Google Scholar]
- Kupper T. S. Mechanisms of cutaneous inflammation. Interactions between epidermal cytokines, adhesion molecules, and leukocytes. Arch Dermatol. 1989 Oct;125(10):1406–1412. doi: 10.1001/archderm.125.10.1406. [DOI] [PubMed] [Google Scholar]
- Köck A., Schwarz T., Kirnbauer R., Urbanski A., Perry P., Ansel J. C., Luger T. A. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990 Dec 1;172(6):1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall L., Lantz M., Gullberg U., Olsson I. Modulation of the constitutive gene expression of the 55 kD tumor necrosis factor receptor in hematopoietic cells. Biochem Biophys Res Commun. 1990 Oct 30;172(2):557–563. doi: 10.1016/0006-291x(90)90709-v. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Gentz R., Zulauf M., Lustig A., Tabuchi H., Schlaeger E. J., Brockhaus M., Gallati H., Manneberg M., Lesslauer W. Recombinant 55-kDa tumor necrosis factor (TNF) receptor. Stoichiometry of binding to TNF alpha and TNF beta and inhibition of TNF activity. J Biol Chem. 1991 Sep 25;266(27):18324–18329. [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Steinmetz M., Lesslauer W. Tumor necrosis factor: receptors and inhibitors. Cancer Cells. 1991 Jun;3(6):221–226. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Hauser C., Vassalli P. Tumor necrosis factor is a critical mediator in hapten induced irritant and contact hypersensitivity reactions. J Exp Med. 1991 Mar 1;173(3):673–679. doi: 10.1084/jem.173.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Bikle D. D., Eessalu T. E., Aggarwal B. B., Elias P. M. Binding and biological effects of tumor necrosis factor alpha on cultured human neonatal foreskin keratinocytes. J Clin Invest. 1989 Mar;83(3):816–821. doi: 10.1172/JCI113963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteu F., Brockhaus M., Wallach D., Engelmann H., Nathan C. F. Human neutrophil elastase releases a ligand-binding fragment from the 75-kDa tumor necrosis factor (TNF) receptor. Comparison with the proteolytic activity responsible for shedding of TNF receptors from stimulated neutrophils. J Biol Chem. 1991 Oct 5;266(28):18846–18853. [PubMed] [Google Scholar]
- Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990 Aug 1;172(2):599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel B., Brockhaus M., Dürmüller U., Gudat F. Tumor necrosis factor receptors in lymphoid tissues and lymphomas. Source and site of action of tumor necrosis factor alpha. Am J Pathol. 1991 Jul;139(1):7–15. [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Spinas G. A., Keller U., Brockhaus M. Release of soluble receptors for tumor necrosis factor (TNF) in relation to circulating TNF during experimental endotoxinemia. J Clin Invest. 1992 Aug;90(2):533–536. doi: 10.1172/JCI115891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprang S. R. The divergent receptors for TNF. Trends Biochem Sci. 1990 Oct;15(10):366–368. doi: 10.1016/0968-0004(90)90228-4. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A., Jr, Goeddel D. V. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trefzer U., Brockhaus M., Loetscher H., Parlow F., Kapp A., Schöpf E., Krutmann J. 55-kd tumor necrosis factor receptor is expressed by human keratinocytes and plays a pivotal role in regulation of human keratinocyte ICAM-1 expression. J Invest Dermatol. 1991 Nov;97(5):911–916. doi: 10.1111/1523-1747.ep12491668. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Vilcek J. Tumor necrosis factor receptors in HeLa cells and their regulation by interferon-gamma. J Biol Chem. 1986 Apr 25;261(12):5384–5388. [PubMed] [Google Scholar]
- Tsujimoto M., Vilcek J. Tumor necrosis factor-induced downregulation of its receptors in HeLa cells. J Biochem. 1987 Dec;102(6):1571–1577. doi: 10.1093/oxfordjournals.jbchem.a122206. [DOI] [PubMed] [Google Scholar]
- Vermeer M., Streilein J. W. Ultraviolet B light-induced alterations in epidermal Langerhans cells are mediated in part by tumor necrosis factor-alpha. Photodermatol Photoimmunol Photomed. 1990 Dec;7(6):258–265. [PubMed] [Google Scholar]
- Winzen R., Wallach D., Engelmann H., Nophar Y., Brakebusch C., Kemper O., Resch K., Holtmann H. Selective decrease in cell surface expression and mRNA level of the 55-kDa tumor necrosis factor receptor during differentiation of HL-60 cells into macrophage-like but not granulocyte-like cells. J Immunol. 1992 Jun 1;148(11):3454–3460. [PubMed] [Google Scholar]
- Yoshie O., Tada K., Ishida N. Binding and crosslinking of 125I-labeled recombinant human tumor necrosis factor to cell surface receptors. J Biochem. 1986 Sep;100(3):531–541. doi: 10.1093/oxfordjournals.jbchem.a121744. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Streilein J. W. Genetic basis of the effects of ultraviolet light B on cutaneous immunity. Evidence that polymorphism at the Tnfa and Lps loci governs susceptibility. Immunogenetics. 1990;32(6):398–405. doi: 10.1007/BF00241633. [DOI] [PubMed] [Google Scholar]
- Zohlnhöfer D., Graeve L., Rose-John S., Schooltink H., Dittrich E., Heinrich P. C. The hepatic interleukin-6 receptor. Down-regulation of the interleukin-6 binding subunit (gp80) by its ligand. FEBS Lett. 1992 Jul 20;306(2-3):219–222. doi: 10.1016/0014-5793(92)81004-6. [DOI] [PubMed] [Google Scholar]