Abstract
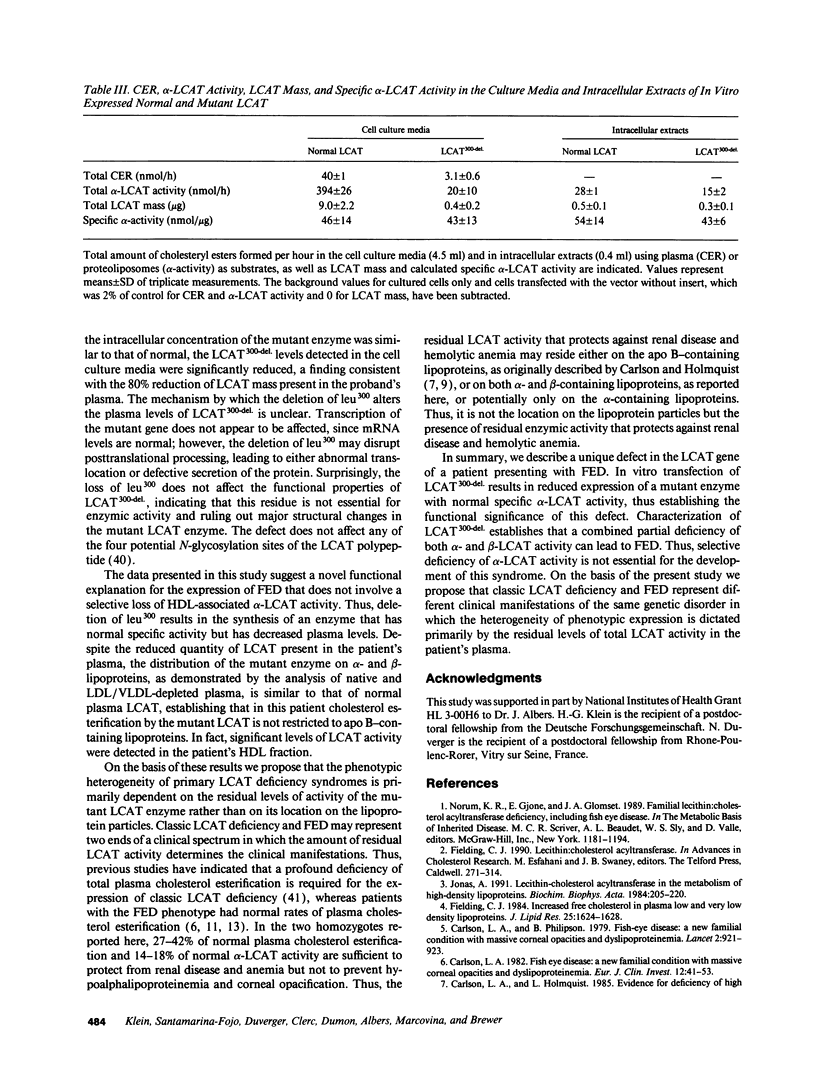
We have identified the molecular defect in two siblings presenting with classical clinical and biochemical features of Fish Eye disease (FED), including corneal opacities, HDL cholesterol < 10 mg/dl, normal plasma cholesteryl esters, and elevated triglycerides. In contrast to previously reported patients with FED who are unable to esterify HDL-associated cholesterol, our patients' plasma lecithin-cholesterol acetyltransferase (alpha-LCAT)-specific activities assayed using an HDL-like proteoliposome substrate were 12.7-25.7 nmol/micrograms (19.5 +/- 1.8 in controls). In addition, significant residual cholesterol esterification was present in VLDL/LDL-depleted plasma, confirming the presence of HDL-associated alpha-LCAT activity. DNA sequence analysis of the proband's LCAT gene identified deletion of the triplet coding for leu300, which resulted in the loss of a restriction site for MlnI. Digestion of PCR-amplified DNA using MlnI established that both siblings are homozygous for this defect. Expression of LCAT300-del. in human embryonic kidney-293 cells revealed normal mRNA and intracellular LCAT concentrations. However, reduced amounts of LCAT300-del., which had a normal specific alpha-LCAT activity, were present in the media. In summary, we report the first case of FED associated with a mutant enzyme that has a normal alpha-LCAT-specific activity. The functional significance of this LCAT gene defect has been established in an in vitro expression system, which demonstrates that very small amounts of this functional LCAT mutant enzyme accumulate in the media. Characterization of LCAT300-del. established that selective alpha-LCAT deficiency is not a prerequisite for the development of FED. On the basis of our combined results, we propose that the residual amounts of total plasma LCAT activity and not its distribution on lipoproteins primarily determines the heterogeneity in phenotypic expression observed in familial LCAT deficiency syndromes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Adolphson J. L., Chen C. H. Radioimmunoassay of human plasma lecithin-cholesterol acyltransferase. J Clin Invest. 1981 Jan;67(1):141–148. doi: 10.1172/JCI110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg O. U., Meng M. S., Skarlatos S. I., Previato L., Brunzell J. D., Brewer H. B., Jr, Fojo S. S. Lipoprotein lipaseBethesda: a single amino acid substitution (Ala-176----Thr) leads to abnormal heparin binding and loss of enzymic activity. Proc Natl Acad Sci U S A. 1990 May;87(9):3474–3478. doi: 10.1073/pnas.87.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson L. A. Fish eye disease: a new familial condition with massive corneal opacities and dyslipoproteinaemia. Eur J Clin Invest. 1982 Feb;12(1):41–53. doi: 10.1111/j.1365-2362.1982.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Evidence for deficiency of high density lipoprotein lecithin: cholesterol acyltransferase activity (alpha-LCAT) in fish eye disease. Acta Med Scand. 1985;218(2):189–196. doi: 10.1111/j.0954-6820.1985.tb08846.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Evidence for the presence in human plasma of lecithin: cholesterol acyltransferase activity (beta-LCAT) specifically esterifying free cholesterol of combined pre-beta- and beta-lipoproteins. Studies of fish eye disease patients and control subjects. Acta Med Scand. 1985;218(2):197–205. doi: 10.1111/j.0954-6820.1985.tb08847.x. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Paradoxical esterification of plasma cholesterol in fish eye disease. Acta Med Scand. 1985;217(5):491–499. doi: 10.1111/j.0954-6820.1985.tb03252.x. [DOI] [PubMed] [Google Scholar]
- Chait A., Iverius P. H., Brunzell J. D. Lipoprotein lipase secretion by human monocyte-derived macrophages. J Clin Invest. 1982 Feb;69(2):490–493. doi: 10.1172/JCI110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Albers J. J. Characterization of proteoliposomes containing apoprotein A-I: a new substrate for the measurement of lecithin: cholesterol acyltransferase activity. J Lipid Res. 1982 Jul;23(5):680–691. [PubMed] [Google Scholar]
- Chen C. H., Albers J. J. Distribution of lecithin-cholesterol acyltransferase (LCAT) in human plasma lipoprotein fractions. Evidence for the association of active LCAT with low density lipoproteins. Biochem Biophys Res Commun. 1982 Aug;107(3):1091–1096. doi: 10.1016/0006-291x(82)90633-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clerc M., Dumon M. F., Sess D., Freneix-Clerc M., Mackness M., Conri C. A 'Fish-eye disease' familial condition with massive corneal opacities and hypoalphalipoproteinaemia: clinical, biochemical and genetic features. Eur J Clin Invest. 1991 Dec;21(6):616–624. doi: 10.1111/j.1365-2362.1991.tb01418.x. [DOI] [PubMed] [Google Scholar]
- Collet X., Fielding C. J. Effects of inhibitors of N-linked oligosaccharide processing on the secretion, stability, and activity of lecithin:cholesterol acyltransferase. Biochemistry. 1991 Apr 2;30(13):3228–3234. doi: 10.1021/bi00227a010. [DOI] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. A one-tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 1988 Oct 25;16(20):9878–9878. doi: 10.1093/nar/16.20.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobiásová M., Schützová M. Cold labelled substrate and estimation of cholesterol esterification rate in lecithin cholesterol acyltransferase radioassay. Physiol Bohemoslov. 1986;35(4):319–327. [PubMed] [Google Scholar]
- Fielding C. J. The origin and properties of free cholesterol potential gradients in plasma, and their relation to atherogenesis. J Lipid Res. 1984 Dec 15;25(13):1624–1628. [PubMed] [Google Scholar]
- Funke H., von Eckardstein A., Pritchard P. H., Albers J. J., Kastelein J. J., Droste C., Assmann G. A molecular defect causing fish eye disease: an amino acid exchange in lecithin-cholesterol acyltransferase (LCAT) leads to the selective loss of alpha-LCAT activity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4855–4859. doi: 10.1073/pnas.88.11.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gascuel O., Golmard J. L. A simple method for predicting the secondary structure of globular proteins: implications and accuracy. Comput Appl Biosci. 1988 Aug;4(3):357–365. doi: 10.1093/bioinformatics/4.3.357. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Holmquist L., Carlson L. A. Alpha-lecithin:cholesterol acyltransferase deficiency. Lack of both phospholipase A2 and acyltransferase activities characteristic of high density lipoprotein lecithin:cholesterol acyltransferase in fish eye disease. Acta Med Scand. 1987;222(1):23–26. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim Biophys Acta. 1991 Jul 30;1084(3):205–220. doi: 10.1016/0005-2760(91)90062-m. [DOI] [PubMed] [Google Scholar]
- Klein H. G., Lohse P., Duverger N., Albers J. J., Rader D. J., Zech L. A., Santamarina-Fojo S., Brewer H. B., Jr Two different allelic mutations in the lecithin:cholesterol acyltransferase (LCAT) gene resulting in classic LCAT deficiency: LCAT (tyr83-->stop) and LCAT (tyr156-->asn). J Lipid Res. 1993 Jan;34(1):49–58. [PubMed] [Google Scholar]
- Klein H. G., Lohse P., Pritchard P. H., Bojanovski D., Schmidt H., Brewer H. B., Jr Two different allelic mutations in the lecithin-cholesterol acyltransferase gene associated with the fish eye syndrome. Lecithin-cholesterol acyltransferase (Thr123----Ile) and lecithin-cholesterol acyltransferase (Thr347----Met). J Clin Invest. 1992 Feb;89(2):499–506. doi: 10.1172/JCI115612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J., Wion K., Drayna D., Fielding C., Lawn R. Human lecithin-cholesterol acyltransferase gene: complete gene sequence and sites of expression. Nucleic Acids Res. 1986 Dec 9;14(23):9397–9406. doi: 10.1093/nar/14.23.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G., Malgaretti N., Magnaghi P., Taramelli R. Nucleotide sequence of the cDNA for lecithin-cholesterol acyl transferase (LCAT) from the rat. Nucleic Acids Res. 1990 Sep 11;18(17):5308–5308. doi: 10.1093/nar/18.17.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Skretting G., Prydz H. An amino acid exchange in exon I of the human lecithin: cholesterol acyltransferase (LCAT) gene is associated with fish eye disease. Biochem Biophys Res Commun. 1992 Jan 31;182(2):583–587. doi: 10.1016/0006-291x(92)91772-i. [DOI] [PubMed] [Google Scholar]
- Stokke K. T., Norum K. R. Determination of lecithin: cholesterol acyltransfer in human blood plasma. Scand J Clin Lab Invest. 1971 Feb;27(1):21–27. doi: 10.3109/00365517109080184. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Langner C. A., Gordon J. I., Taylor B. A., McLean J. W., Lusis A. J. Tissue-specific expression, developmental regulation, and chromosomal mapping of the lecithin: cholesterol acyltransferase gene. Evidence for expression in brain and testes as well as liver. J Biol Chem. 1989 Dec 25;264(36):21573–21581. [PubMed] [Google Scholar]




