Abstract
The endogenous opioid system has been implicated in sexual behavior, palatable intake, fear, and anxiety. The present study examined whether ovariectomized female transgenic preproenkephalin-knockout (PPEKO) mice and their wild-type and heterozygous controls displayed alterations in fear and anxiety paradigms, sucrose intake, and lordotic behavior. To examine stability of responding, three squads of the genotypes were tested across seasons over a 20-month period. In a fear-conditioning paradigm, PPEKO mice significantly increased freezing to both fear and fear + shock stimuli relative to controls. In the open field, PPEKO mice spent significantly less time and traversed significantly less distance in the center of an open field than wild-type controls. Further, PPEKO mice spent significantly less time and tended to be less active on the light side of a dark–light chamber than controls, indicating that deletion of the enkephalin gene resulted in exaggerated responses to fear or anxiety-provoking environments. These selective deficits were observed consistently across testing squads spanning 20 months and different seasons. In contrast, PPEKO mice failed to differ from corresponding controls in sucrose, chow, or water intake across a range (0.0001–20%) of sucrose concentrations and failed to differ in either lordotic or female approach to male behaviors when primed with estradiol and progesterone, thereby arguing strongly for the selectivity of a fear and anxiety deficit which was not caused by generalized and nonspecific debilitation. These transgenic data strongly suggest that opioids, and particularly enkephalin gene products, are acting naturally to inhibit fear and anxiety.
Endogenous opioid systems are involved intimately in fear-related behaviors. For example, the work of Fanselow and coworkers has established an opioidergic modulation of freezing responses in rodents, which is a basic species-specific response to threatening stimuli (1–5). It would be hypothesized then that gene deletion of an endogenous enkephalin opioid peptide gene should alter the ability of the animal to respond appropriately to fear- or threat-inducing stimuli as evidenced in paradigms ostensibly measuring these responses including the open-field, dark–light transition, and conditioned shock paradigms.
Opioid agonists have been shown also to potently stimulate intake of sweet solutions (6–12), ostensibly by increasing the hedonic value of the solution. It then would be hypothesized that loss of an endogenous enkephalin opioid peptide should blunt the proingestive actions of sucrose over a range of sucrose concentrations.
Finally, previous molecular data lead to a prediction about possible effects of an enkephalin gene knockout on estrogen-dependent lordosis behavior. Estrogen administration rapidly induces high levels of enkephalin mRNA in the ventromedial nucleus of the hypothalamus (VMH), a nerve-cell group essential for lordosis behavior (13, 14). This induction includes a transcriptional facilitation by estradiol (15–17) and is greater in females than in males (18). That the enkephalin induction might be related causally to female reproductive behavior is likely; antisense DNA directed against enkephalin mRNA and microinjected into the VMH significantly reduced lordosis behavior (19). Therefore, we predicted that female mice bearing enkephalin gene deletions would show lower levels of lordosis behavior compared with their wild-type (WT) controls.
Materials and Methods
Female transgenic preproenkephalin-knockout (PPEKO) mice and their WT and heterozygous (HZ) littermates were used. The mice were bred in mixed-background C57BL/6J and 129 strains. The preproenkephalin gene was targeted by probing a murine Sw129/ReJ genomic library and isolating the enkephalin gene. The target vector was produced by subcloning two genomic DNA fragments into a pBluescript II SK-based vector (obtained from S. Potter, University of Cincinnati, Cincinnati) containing the neo and herpes simplex virus (HSV)-tk genes, both driven by the HSV thymidine kinase promoter. First, a 2.1-kb XbaI fragment, containing the 3′ part of exon 3 and part of intron 3, was filled in and subcloned into a blunted HindIII site of the cassette. The resulting construct was digested with NotI, filled in, and then ligated to a blunted 6-kb SalI–BglII fragment containing exon 1, intron 1, exon 2, and a part of intron 2.
At 5–7 weeks of age, mice were grouped by genotype and shipped from the University of Medicine and Dentistry of New Jersey in three different shipments to The Rockefeller University over a 20-month test period. These three different squads of animals began testing in the Winter of 1997, Summer of 1998, and Spring of 1999, because questions have been raised recently about the consistency of behavioral data in gene-knockout studies (20). After arrival at The Rockefeller University, all mice were housed individually in plastic cages (30 × 20 × 15 cm) throughout the entire study and maintained on a 12/12-h light–dark cycle (light off at 11 a.m.) at a constant temperature of 22°C. Mouse chow and water were available ad libitum. After approximately 10 days of acclimation, all mice were anesthetized and ovariectomized, and each was s.c. implanted with a silastic capsule (1.5-cm long) containing 17 β-estradiol benzoate (50 μg in 0.1 ml of sesame oil total volume). Then, 5 h before testing, female mice were primed with a s.c. injection of progesterone (500 μg in 0.1 ml sesame oil) to ensure high sexual receptivity.
By using the χ2 test, a significant effect of mortality was noted between PPEKO and WT mice (χ2 = 6.23, P < 0.05, df = 2). For example, in the first two squads of mice (n = 22 of each genotype), 12 PPEKO, 16 HZ, and 21 WT survived surgery. Similar differences in postsurgical recovery rates also were noted (data not shown), such that PPEKO took longer to heal after surgery than either WT or HZ mice. Testing began 3 weeks after the ovariectomized mice were implanted with 17 β-estradiol benzoate.
Sex-Behavior Methods.
The ovariectomized females were tested for sexual behavior with sexually experienced and vigorous males (Swiss–Webster, 25–30 g) between 2 and 3 h into the dark portion of the light–dark cycle over 6 consecutive days. Testing occurred in the males' home cage in the females' home room. Each test lasted for 15 min or until the male mounted the female 17 times, whichever occurred first. Female mice were assigned randomly to a male. By using both on-site observation and videotape review, a behavioral observer blind to the genotype of the females recorded lordotic responses of the female to male mounting, intromission, and ejaculation. Female responses to male mounts or intromissions were scored as (i) totally unreceptive, kicking, rearing, boxing, or fleeing (score 0); (ii) proceptive-still posture with extension of legs (score 0.5); and (iii) receptive-lordosis posture with dorsiflexion of the vertebral column (score 1–3 using 0.5 intervals, depending on the angle of the degree of female dorsiflexion). Female responses that scored 1 or higher were considered lordotic responders and were included for the calculation of the lordosis quotient [LQ = the total number of lordosis responses/total number of mounts multiplied by 100 (L/M × 100)]. After completion of sex-behavior testing, the silastic capsule containing 17 β-estradiol benzoate was removed under light anesthesia, and the animals were allowed to recover for 2 weeks.
Ingestive-Behavior Methods.
After this recovery period, mice were tested in the ingestive-behavior paradigm. Because all three squads had been separated previously and grouped by genotype, each group was divided randomly in half. The first half of each group then was assigned to a series of conditions that included 24-h daily exposure to nine ascending series of sucrose concentrations: 0% (2 days), 0.0001, 0.001, 0.01, 0.1, 1, 5, 10, 20, and then 0% (2 days) again. The second half of each group received identical conditions except they were exposed to a descending series of sucrose concentrations. In this paradigm, all animals were provided with two preweighed bottles: one containing the particular concentration of the sucrose solution and the other containing water. Mice were trained initially with both bottles filled with water for 4–5 days before data acquisition. The positions of the bottle placements were counterbalanced across animals and were varied within animals according to an ABBA design. Fresh preweighed mouse chow was provided to the mice at the same time as they were given their water and sucrose bottles. Chow intake, adjusted for spillage, sucrose intake, and water intake were each calculated daily.
Open-Field Methods.
Two weeks elapsed after the ingestive-behavior paradigm to minimize interactions between paradigms. All female mice were tested starting between 2 and 3 h into their dark cycle for 10 min each day on 3 consecutive days in an open-field apparatus (50-cm long × 50-cm wide × 35-cm high, clear plastic wall), which was illuminated by a 100-W white bulb suspended 1 m directly overhead. Testing occurred in their home room and a thick light-opaque curtain was used to maintain darkness and block any sounds from escaping the test area. Animals were removed directly from their cages and gently placed nose first into a specified corner of the open-field apparatus (Digiscan model RXYCM16TAO, Omnitech Electronics, Columbus, Ohio), and after release, data acquisition began. A Digiscan analyzer (DCM-8) and DIGISCAN software were used to analyze and store horizontal-activity data, which was monitored automatically by infra-red beams. Total moving time, total moving distance, moving time in the center, moving distance in the center, moving time in the margins, and moving distance in the margins were recorded for each mouse.
Dark–Light Transition Methods.
Two weeks after the open-field testing, each of the female mice were tested starting between 2 and 3 h into their dark cycle for 10 min each day on 3 consecutive days in a dark–light apparatus (50-cm long × 50-cm wide × 35-cm high, clear plastic wall), with a black (light opaque) 50-cm long × 25-cm wide × 25-cm high covered-plastic box on one side (the dark side) that had an open 2-cm high × 5-cm long doorway that led to the light side of the apparatus, which was illuminated by a 100-W white bulb suspended 1 m directly overhead. Testing occurred in their home room, and a thick light-opaque curtain was used to maintain darkness and block any sounds from escaping the test area. Animals were removed directly from their cages and gently placed nose first into the doorway of the dark side of the dark–light apparatus, and after release data acquisition began. A Digiscan analyzer and DIGISCAN software were used to analyze and store horizontal-activity data, which was monitored automatically by infra-red beams. Each mouse had the following measurements taken: total moving time and total moving distance for each compartment, and total time in dark, total time in light, total distance traveled in dark and in light, and the latency to move from the dark to the light compartment.
Fear-Conditioning Methods.
Two weeks after the dark–light transition paradigm, conditioned-fear behavior (freezing responses) was assessed to measure aversive emotional learning in the three genotypes. Each of the female mice were tested starting between 2 and 3 h into their dark cycle. Freezing behavior was measured on an initial-acquisition day and then 24 h thereafter to measure fear responses after shock. Conditioning took place in a Plexiglas sound-attenuated chamber with a metal grid floor. Each mouse was placed in the test chamber and allowed to explore freely for 2 min. After this exploration, a white-noise conditioned stimulus (CS) (75-dB piezo buzzer, Radio Shack) was presented for 30 sec and coterminated with a mild (2-sec, 0.5 mA) foot shock. The mouse was removed from the chamber 1.5 min later and returned to its home cage. Then 24 h later, the mouse was placed back into the test chamber and exposed to identical testing conditions. The presence of freezing behavior was recorded during the 30 sec of auditory stimuli on each of the two days and during the 30 sec after foot shock on each of the two days. The first day of auditory stimulation served as a neutral stimulus, and the first day of shock served as the shock stimulus. The second day of auditory stimulation, acting as a CS for shock, served as a measure of fear conditioning, whereas the second day of shock served as the fear + shock condition. Then 9 days later, the CS was presented alone.
Three-way randomized block ANOVA was performed on each of the dependent variables in each of the paradigms that examined the main effects and interactions of the three experimental groups (PPEKO, HZ, and WT), the three testing squads (batches 1, 2, and 3), and the levels of the independent variable under study (e.g., sucrose concentrations in the sucrose drinking paradigm). If significant main or interaction effects were observed, Tukey-corrected post hoc comparisons were performed then to ascertain specific significant experimental effects relative to corresponding control data.
Results
Fear Conditioning.
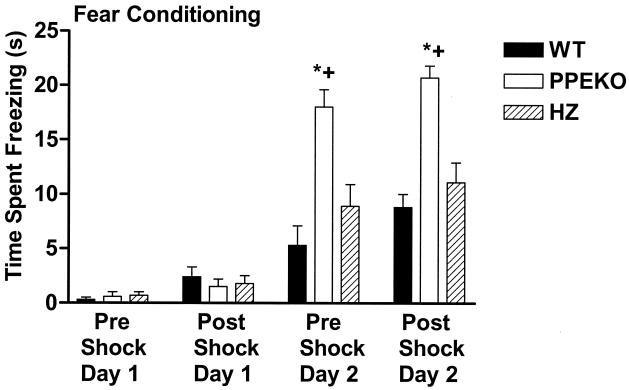
In Fig. 1, it is clear that all three genotypes froze (became motionless) in response to the auditory stimulus acting as the CS for shock on day 2, as compared with day 1. Likewise, their postshock freezing was significantly greater on day 2 than on day 1. Animals did not freeze substantially to the context before the presentation of the CS on any of the test days. Notably, the PPEKO mice froze significantly more to the CS before shock on day 2 than either the WT or HZ controls (Fig. 1). PPEKO mice also displayed a significant increase in freezing after shock on day 2, both relative to freezing levels on day 1 and as compared with their WT and HZ controls (Fig. 1). These differences among groups persisted as nonsignificant trends 9 days later without intervening training (data not shown). Notably, these comparisons among experimental genotype groups were consistent across each of the three testing squads; therefore, their data were combined. Thus, in response to a signal for fear, the PPEKO animals were significantly more reactive than the control groups.
Figure 1.
Alterations in fear conditioning in WT mice, PPEKO mice with both alleles eliminated, and preproenkephalin HZ knockout mice with only one allele eliminated (HZ). The amount of time in a 30-sec period in which the animal exhibited freezing behavior (mean ± SEM) was assessed in animals during an auditory stimulus before (Pre Shock) and after (Post Shock) foot shock on 2 consecutive days (Days 1 and 2). Freezing behavior observed during the first day of auditory stimulation that served as the neutral stimulus (Pre Shock, Day 1) did not differ from the first day of shock, which served as the shock stimulus (Post Shock, Day 1). The PPEKO and HZ groups displayed significantly greater freezing responses during the second day of auditory stimulation, which served as a measure of fear conditioning (Pre Shock, Day 2), and all three groups displayed significantly greater freezing responses during the second day of shock, which served as the fear + shock condition (Post Shock, Day 2) (data not shown). PPEKO mice displayed significantly greater freezing responses during the second day of auditory stimulation both before (Pre Shock, Day 2, fear condition) and after (Post Shock, Day 2, fear + shock condition) shock relative to either WT (*) or HZ (✚) mice. Significant differences were observed among genotypes [ANOVA F(2,28) = 22.32; Tukey's t test, P < 0.0001], across conditions [F(3,18) = 184.08, P < 0.0001], and for the interaction between genotypes and conditions [F(6,84) = 12.54, P < 0.0001]. Variability as a function of squads of testing failed to account for any significant results.
Open-Field Assay.
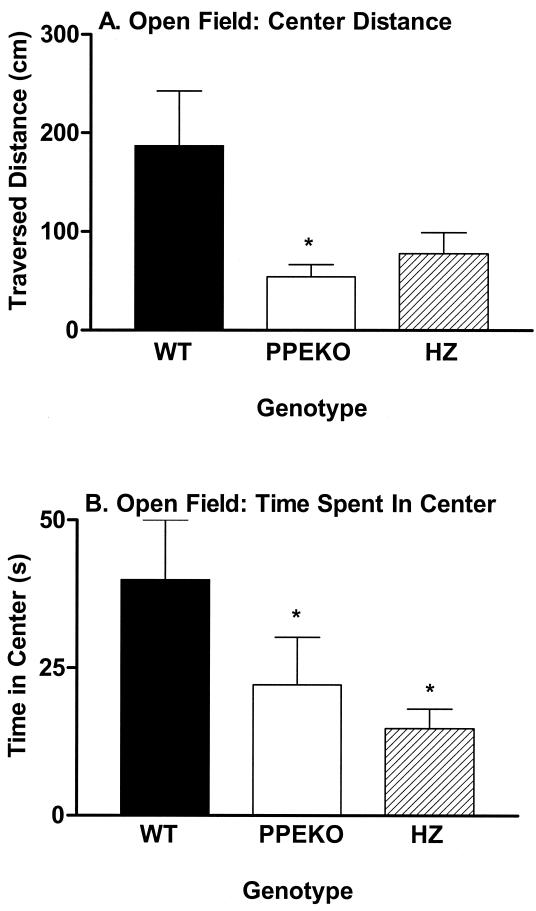
The mouse's activity in specific parts of the “open-field” chamber comprises an assay of its response to a novel anxiety-provoking situation. A mouse that is anxious in response to novelty, by definition, locomotes less, and when it does move it tends to hug the margins of the open-field chamber. In contrast, a mouse that is not anxious spends more time in the center of the apparatus (away from the walls) and traverses more distance in the center of the apparatus. PPEKO mice traversed significantly less distance in the center squares of the open field (Fig. 2A) and spent significantly less time in the center of the open field (Fig. 2B) than corresponding WT controls. These differences were observed consistently in all three testing squads of the study, especially for the first and the third squads. Thus, in concert with the fear-conditioning results, the enkephalin-knockout animals appeared to display an exaggerated response to an anxiety-provoking situation.
Figure 2.
Alterations (mean ± SEM) in either the amount of distance traversed (A) or the amount of time spent (B) in the center of an open field by WT, PPEKO, and HZ mice. PPEKO mice traversed significantly less distance [ANOVA, F(2,28) = 5.52; Tukey's t test, P < 0.01] and spent significantly less time (F = 3.49, P < 0.044) in the center of the open field than WT mice (*). HZ mice also spent significantly less time in the center of the open field than WT mice. Although the second squad of animals displayed significant increases in the traversed distances [F(2,12) = 6.88, P < 0.01] and elapsed time [F(2,12) = 14.98, P < 0.0005] in the center of the open field than the first and third squads, these effects occurred consistently over all genotypes and failed to account for any significant genotype results.
Dark–Light Transition Assay.
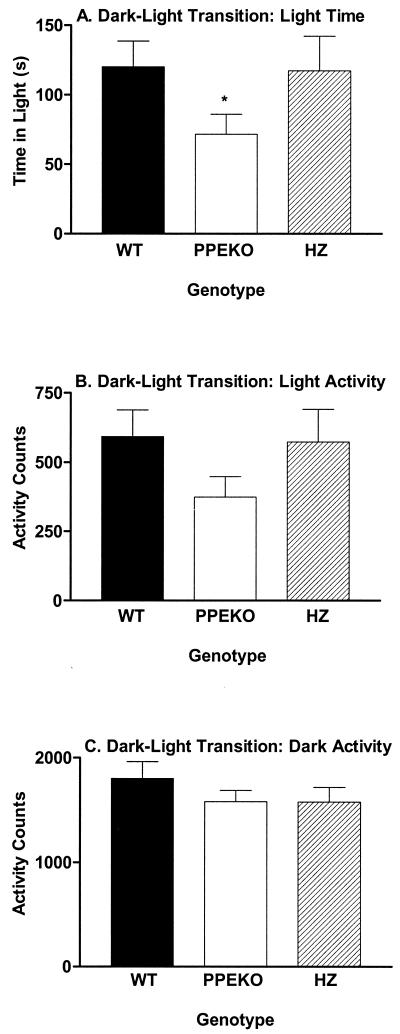
In this test, the questions “if a mouse is placed in the dark side of a standard test chamber, will it come out into the light?” and “How much time does it spend there?” are asked. A more anxious animal by definition spends more time in the dark and less time in the light. PPEKO mice spent significantly less time in the light side than either WT or HZ controls (Fig. 3A), and the knockout mice also tended to be less active once in the light side of the compartment (Fig. 3B). This latter result was not caused simply by a locomotor deficit, because activity levels of PPEKO mice on the dark side of the chamber were equivalent to the two control groups (Fig. 3C). This finding was confirmed in a separate assay of locomotor capacity by using standard running wheels in the home cage that failed to show differences among genotypes (data not shown). Again, these genotype differences in the dark–light transition assay consistently occurred across all three testing squads with no significant changes observed as a function of the testing squad. Thus, as with the fear conditioning and open-field assays, the enkephalin-knockout mice appeared to show an exaggerated response to an anxiety-provoking situation in dark–light transition testing.
Figure 3.
Alterations (mean ± SEM) in either the amount of time spent in the light compartment (A) or the amount of activity occurring in the light (B) and dark (C) compartments in WT, PPEKO, and HZ mice. PPEKO mice spent signicantly less time [ANOVA, F(2,28) = 3.89; Tukey's t test, P < 0.032] in the light compartment than either WT or HZ mice (*). The activity of the three groups in either the light (F = 2.50, P < 0.10) or dark [F = 0.44, not significant (n.s.)] compartments failed to differ significantly from each other. Significant differences were not observed either among squads [light time, F(2,12) = 2.97, n.s.; light activity, F = 1.99, n.s.; dark activity, F = 0.28, n.s.] or for the interaction between genotypes and squads [light time, F(4,24) = 2.12, n.s.; light activity, F = 2.21, n.s.; dark activity, F = 1.80, n.s.].
Sucrose Intake.
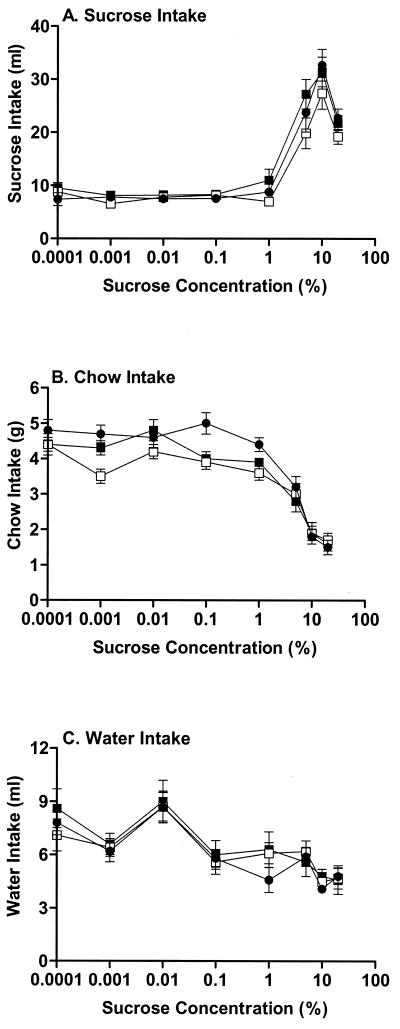
When one follows the amount of sucrose intake as a function of sucrose concentration, crucial differences typically occur at the breakpoint at which sucrose intake dramatically increases. Contrary to our expectation of substantial decreases in sucrose intake across concentrations in PPEKO mice, only small and nonsignificant reductions were observed in these animals at these critical 1, 5, and 10% sucrose concentrations (Fig. 4A). Moreover, the systematic decreases in chow (Fig. 4B) and water (Fig. 4C) intakes across sucrose concentrations were similar for all three genotypes. The similar pattern of these effects was noted consistently for each testing squad, despite overall decreases in sucrose intake noted in mice in the second squad relative to the first and third testing squads (data not shown). The consistency of intakes of sucrose, water, and chow in the three groups of mice suggest that nonspecific behavioral deficits were not responsible for the exaggerated responses observed in PPEKO mice in the previous three paradigms.
Figure 4.
Alterations (mean ± SEM) in sucrose (A), chow (B), or water (C) intake across a range (0.0001–20%) of sucrose concentrations in WT (dark circles), PPEKO (open squares), and HZ (closed squares) mice. As expected, significant increases in sucrose intake [ANOVA, F(8,48) = 334.38; Tukey's t test, P < 0.0001] and significant decreases in chow (F = 319.57, P < 0.0001) and water (F = 47.47, P < 0.0001) intakes were observed as a function of sucrose concentration. However, the patterns of each of the three types of intake across the sucrose concentrations failed to differ among genotypes [sucrose, F(2,30) = 2.76, P < 0.07; chow, F = 2.71, P < 0.083; water, F = 2.44, n.s.]. Although the second squad of animals consumed significantly more sucrose [F(2,12) = 29.42, P < 0.0001] and chow (F = 4.12, P < 0.044) and drank significantly less water (F = 27.71, P < 0.0001) than the first and third squads, these effects occurred consistently over all genotypes and failed to alter the genotype results.
Female Mating Behavior.
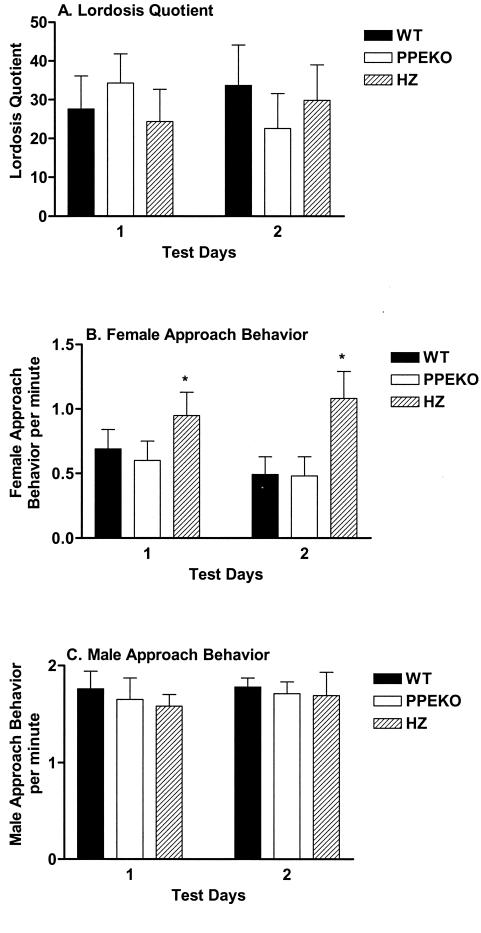
When measuring the lordosis quotient, significant differences failed to be observed among genotypes (Fig. 5A). Surprisingly, female HZ mice approached males more frequently than either WT or PPEKO mice (Fig. 5B). The behavior of the females apparently was not influenced by differential treatment of particular females by the male, because male approach behaviors were equivalent among genotypes (Fig. 5C). Notably, the equivalence among genotypes with respect to the lordosis quotient and male approach behavior were stable across all testing squads.
Figure 5.
Alterations (mean ± SEM) in the lordosis quotient (A) and approach behaviors of female (B) and male (C) mice in sexual encounters involving WT, PPEKO, and HZ female mice. Although the three groups failed to differ in lordotic behavior [F(2,30) = 0.07, n.s.], HZ mice produced significantly more approach behaviors (*) than the other two groups (F = 4.66, P < 0.017). In contrast, male approach behavior failed to differ as a function of female genotype (F = 0.39, n.s.). Although the third squad of animals displayed significantly less lordotic behavior [ANOVA: F(2,12) = 22.76, Tukey's t test: P < 0.0001] and the first squad of animals displayed significantly more female approach behavior (F = 18.86, P < 0.0002) relative to the other squads, these effects occurred consistently over all genotypes and failed to alter the genotype results.
Discussion
Across three different kinds of assays, fear conditioning, open-field activity, and dark–light transition, the enkephalin-knockout mice showed exaggerated responses to fear or anxiety-provoking environments compared with their WT and HZ controls. Surprisingly, these PPEKO mice may comprise an animal model of both heightened fear and exaggerated anxiety. These two types of behavioral responses would not necessarily co-vary, because such aspects of emotionality can be shown to have a multidimensional character (21). Exactly how and where in the central nervous system the preproenkephalin gene influences these behaviors cannot be determined from the present data. However, because viral vector-mediated preproenkephalin overexpression in the amygdala can be antinociceptive (22) and can potentiate the anxiolytic effects of benzodiazepines (23), amygdalar function may have been altered importantly by this preproenkephalin-gene knockout.
The strength of the selective deficits reported above is emphasized by the consistency of these observations over three testing squads of animals, spanning both 20 months of testing and different seasons of the year. Moreover, while this manuscript was being reviewed, we became aware of parallel experiments with δ opioid-receptor knockout mice (24) which, as would be predicted, gave results in total agreement with the present report. Because of these results and many further examples of other reliable bodies of data quoted in a forthcoming discussion (unpublished data) section, we believe that these data illustrating specific effects of a particular gene on a cluster of behaviors demonstrate the precision and reliability achievable with behavioral testing.
In future work, PPEKO mice conceivably could be used to ascertain whether the inhibitory actions of these opioid peptides are acting as a stimulus filter on the characteristics of open-field, dark–light, or fear-conditioning stimuli, an inhibitor of the δ opioid-receptor-mediated substrates of fear and anxiety, and/or an inhibitory response modulator of responses to the open-field, dark–light transition, and fear-conditioning paradigms.
Compared with these predicted findings, we were surprised that the enkephalin-knockout animals failed to display either substantial decreases in sucrose consumption at critical intake concentrations (1, 5, and 10%) or reductions in lordosis behavior. However, the stable ingestive and sexual behaviors displayed by the enkephalin-knockout animals argue strongly that the anxiety differences noted were caused by a selective deficit and not by some generalized and nonspecific debilitation. Combining the two sets of positive and negative results, we now hypothesize that testing PPEKO mice for sucrose intake and for mating behavior under various stressful situations might yield important insights into how the opioid system might be engaged in modulating these motivational behaviors. Finally, the postsurgical mortality and recovery differences between PPEKO and WT mice may reflect an impaired immune system in the PPEKO animals that, when stressed, renders them particularly susceptible to challenges to their immune system and thereby may account for their increased death rate and increased recovery time.
These data provide some caveats about generalizations made in using potential gene-altering procedures. Although an antisense DNA manipulation using the antisense moiety against the preproenkephalin mRNA significantly reduced lordosis behavior if and only if it was microinjected among cells of the ventromedial nucleus of the hypothalamus (19), an effect consistent with pharmacological studies (25); a significant effect on lordosis behavior in PPEKO mice was not observed in the current study. In these and other circumstances, the differences between a site-specific and temporally specific antisense DNA manipulation, on the one hand, and a gene-knockout manipulation, on the other, show that the effect of a gene deletion can depend on exactly when and/or where the manipulation takes place.
Acknowledgments
This work was supported by a National Institutes of Health Grant HD-05751 to D.W.P.
Abbreviations
- WT
wild type
- PPEKO
preproenkephalin-knockout
- HZ
heterozygous
- CS
conditioned stimulus
- n.s.
not significant
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041598498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041598498
References
- 1.Fanselow M S. Learn Motiv. 1981;12:398–419. [Google Scholar]
- 2.Fanselow M S, Bolles R C. J Comp Physiol Psychol. 1979;94:736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- 3.Lester L S, Fanselow M S. Physiol Psychol. 1986;14:5–10. [Google Scholar]
- 4.Fanselow M S, Sigmundi R A. J Exp Psychol Anim Behav Processes. 1986;12:301–309. [PubMed] [Google Scholar]
- 5.Fanselow M S, Calcagnetti D J, Helmstetter F J. Brain Res. 1988;444:147–152. doi: 10.1016/0006-8993(88)90921-3. [DOI] [PubMed] [Google Scholar]
- 6.Calcagnetti D J, Reid L D. Pharmacol Biochem Behav. 1983;18:567–569. doi: 10.1016/0091-3057(83)90282-4. [DOI] [PubMed] [Google Scholar]
- 7.Czirr S A, Reid L D. Brain Res Bull. 1986;17:639–642. doi: 10.1016/0361-9230(86)90195-4. [DOI] [PubMed] [Google Scholar]
- 8.Gosnell B A, Majchrzak M J. Pharmacol Biochem Behav. 1989;33:805–810. doi: 10.1016/0091-3057(89)90474-7. [DOI] [PubMed] [Google Scholar]
- 9.Gosnell B A, Patel C K. Pharmacol Biochem Behav. 1993;45:979–982. doi: 10.1016/0091-3057(93)90151-i. [DOI] [PubMed] [Google Scholar]
- 10.Lynch W C. Pharmacol Biochem Behav. 1986;24:833–836. doi: 10.1016/0091-3057(86)90420-x. [DOI] [PubMed] [Google Scholar]
- 11.Milano W C, Wild K D, Hui Y, Hubbell C L, Reid L D. Pharmacol Biochem Behav. 1989;31:893–897. doi: 10.1016/0091-3057(88)90401-7. [DOI] [PubMed] [Google Scholar]
- 12.Ruegg H, Yu W-Z, Bodnar R J. Physiol Behav. 1997;62:121–128. doi: 10.1016/s0031-9384(97)00151-0. [DOI] [PubMed] [Google Scholar]
- 13.Romano G J, Harlan R E, Shivers B D, Howells R D, Pfaff D W. Mol Endocrinol. 1988;2:1320–1328. doi: 10.1210/mend-2-12-1320. [DOI] [PubMed] [Google Scholar]
- 14.Romano G J, Mobbs C B, Pfaff D W. Mol Brain Res. 1989;5:51–58. doi: 10.1016/0169-328x(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 15.Vasudevan, N., Zhu, Y., Daniel, S., Koibuchi, N., Chin, W. W. & Pfaff, D. W. (2001) Endocrinology, in press. [DOI] [PubMed]
- 16.Zhu Y F, Pfaff D W. Neuroendocrinology. 1995;62:454–466. doi: 10.1159/000127035. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y S, Yen P M, Chin W W, Pfaff D W. Proc Natl Acad Sci USA. 1996;93:12587–12592. doi: 10.1073/pnas.93.22.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano G J, Mobbs C V, Lauber A, Howells R D, Pfaff D W. Mol Brain Res. 1990;536:63–68. doi: 10.1016/0006-8993(90)90009-z. [DOI] [PubMed] [Google Scholar]
- 19.Nicot A, Ogawa S, Berman Y, Carr K D, Pfaff D W. Brain Res. 1997;777:60–68. doi: 10.1016/s0006-8993(97)00967-0. [DOI] [PubMed] [Google Scholar]
- 20.Crabbe J C, Wahlsten D, Dudek B C. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 21.Ramos A, Mormède P. Neurosci Biobehav Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 22.Kang W, Wilson M A, Bender M A, Glorioso J C, Wilson S P. Brain Res. 1998;792:133–135. doi: 10.1016/s0006-8993(98)00194-2. [DOI] [PubMed] [Google Scholar]
- 23.Kang W, Wilson S P, Wilson M A. Neuropsychopharmacology. 1999;22:77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 24.Filliol D, Ghozland S, Chluba J, Martin M, Matthes H W D, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 25.Pfaus J G, Pfaff D W. Horm Behav. 1992;26:457–473. doi: 10.1016/0018-506x(92)90014-m. [DOI] [PubMed] [Google Scholar]