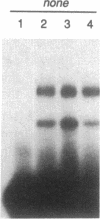
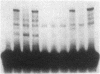
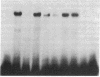
Abstract
Chronically culturing HIT-T15 cells in media containing high glucose concentrations leads to decreased insulin mRNA levels, insulin content, and insulin secretion. These changes can be prevented by culturing the cells in media containing lower glucose levels (Robertson, R. P., H.-J. Zhang, K. L. Pyzdrowski, and T. F. Walseth. 1992. J. Clin. Invest. 90:320-325). The mechanism of this seemingly paradoxical phenomenon was examined by transiently transfecting HIT cells with a chloramphenicol acetyl transferase (CAT) reporter gene controlled by the 5'-regulatory domain of the human insulin gene (INSCAT). Early passages of HIT cells readily expressed INSCAT, whereas late passages of cells chronically cultured in 11.1 mM glucose expressed only 28.7 +/- 2.3% (mean +/- SEM) of the CAT activity expressed in early passages. In contrast, late passages of HIT cells chronically cultured in 0.8 mM glucose retained the ability to express the INSCAT reporter gene to 69.6 +/- 10.0% of the CAT activity observed in early passages. The decrease in INSCAT expression in late passages of cells serially cultured in 11.1 mM glucose was associated with the inability to form a specific nuclear protein-DNA complex with the CT motifs of the human insulin promoter. Formation of this specific protein-DNA complex was preserved in late passages of HIT cells when serially cultured in 0.8 mM glucose. Mutations of the CT motifs caused markedly diminished CAT activity in all passages examined. These data indicate that chronic exposure of the beta cell to high glucose concentrations can paradoxically decrease insulin gene transcription, in part, by altering the ability of a regulatory protein (GSTF) to interact with the insulin gene promoter. This provides a potential mechanism for glucotoxic effects on the beta cell at the level of the insulin gene.
Full text
PDF

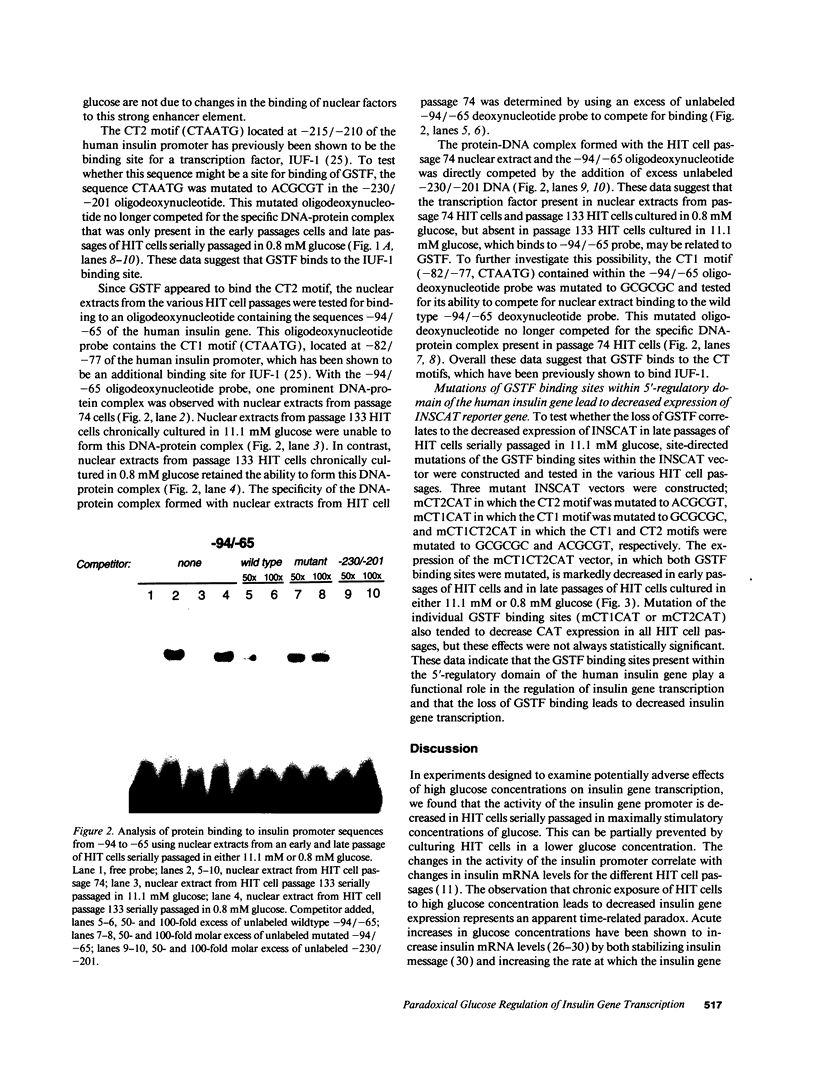
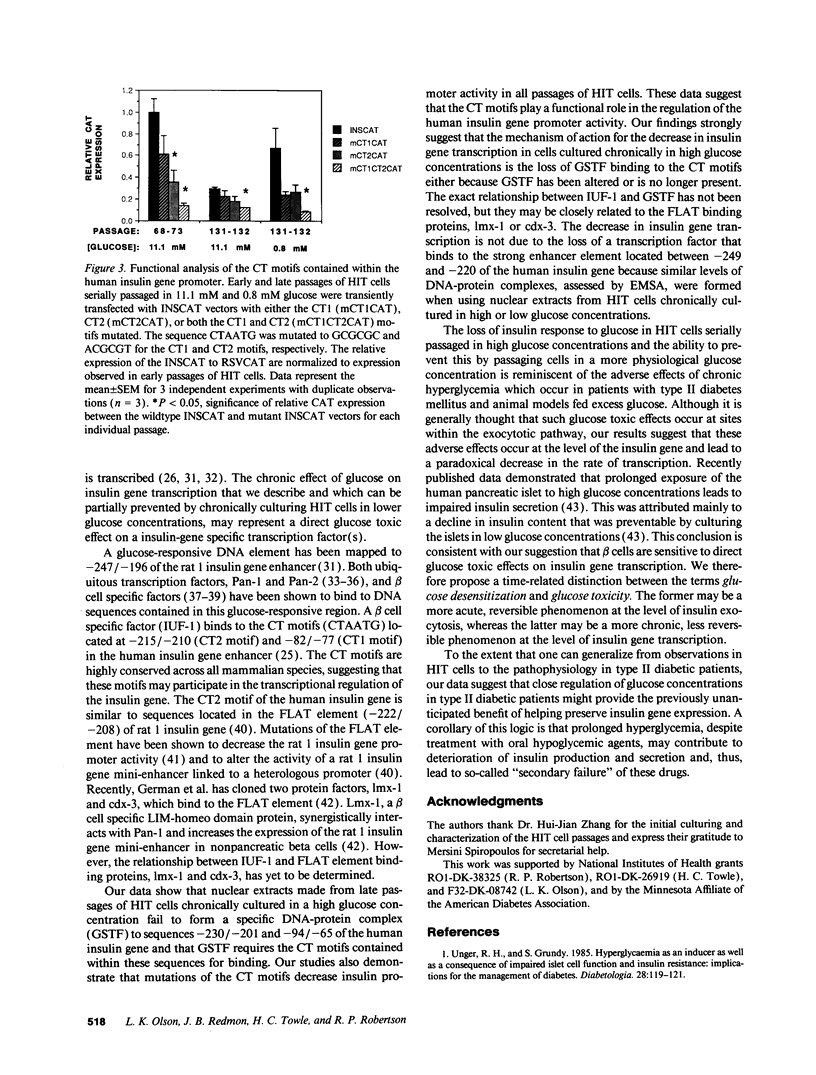

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Hammonds P., Harrison D. E. Insulin secretory responses of a clonal cell line of simian virus 40-transformed B cells. Diabetologia. 1986 Oct;29(10):727–733. doi: 10.1007/BF00870283. [DOI] [PubMed] [Google Scholar]
- Boam D. S., Clark A. R., Docherty K. Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J Biol Chem. 1990 May 15;265(14):8285–8296. [PubMed] [Google Scholar]
- Boam D. S., Docherty K. A tissue-specific nuclear factor binds to multiple sites in the human insulin-gene enhancer. Biochem J. 1989 Nov 15;264(1):233–239. doi: 10.1042/bj2640233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstedt J., Chan S. J. Direct effect of glucose on the preproinsulin mRNA level in isolated pancreatic islets. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1383–1389. doi: 10.1016/0006-291x(82)91267-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Surana M., Fleischer N. Glucose induces insulin gene transcription in a murine pancreatic beta-cell line. J Biol Chem. 1991 Jun 15;266(17):11141–11143. [PubMed] [Google Scholar]
- Eizirik D. L., Korbutt G. S., Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992 Oct;90(4):1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German M. S., Blanar M. A., Nelson C., Moss L. G., Rutter W. J. Two related helix-loop-helix proteins participate in separate cell-specific complexes that bind the insulin enhancer. Mol Endocrinol. 1991 Feb;5(2):292–299. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- German M. S., Moss L. G., Rutter W. J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem. 1990 Dec 25;265(36):22063–22066. [PubMed] [Google Scholar]
- German M. S., Moss L. G., Wang J., Rutter W. J. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical beta-cell nuclear complexes. Mol Cell Biol. 1992 Apr;12(4):1777–1788. doi: 10.1128/mcb.12.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German M. S., Wang J., Chadwick R. B., Rutter W. J. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev. 1992 Nov;6(11):2165–2176. doi: 10.1101/gad.6.11.2165. [DOI] [PubMed] [Google Scholar]
- Giddings S. J., Chirgwin J., Permutt M. A. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes. 1982 Jul;31(7):624–629. doi: 10.2337/diab.31.7.624. [DOI] [PubMed] [Google Scholar]
- Hammonds P., Schofield P. N., Ashcroft S. J. Glucose regulates preproinsulin messenger RNA levels in a clonal cell line of simian virus 40-transformed B cells. FEBS Lett. 1987 Mar 9;213(1):149–154. doi: 10.1016/0014-5793(87)81481-3. [DOI] [PubMed] [Google Scholar]
- Hammonds P., Schofield P. N., Ashcroft S. J., Sutton R., Gray D. W. Regulation and specificity of glucose-stimulated insulin gene expression in human islets of Langerhans. FEBS Lett. 1987 Oct 19;223(1):131–137. doi: 10.1016/0014-5793(87)80523-9. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Nagulesparan M., Klimes I., Clark R., Sasaki H., Aronoff S. L., Vasquez B., Rubenstein A. H., Unger R. H. Improvement of insulin secretion but not insulin resistance after short term control of plasma glucose in obese type II diabetics. J Clin Endocrinol Metab. 1982 Feb;54(2):217–222. doi: 10.1210/jcem-54-2-217. [DOI] [PubMed] [Google Scholar]
- Hill R. S., Boyd A. E., 3rd Perifusion of a clonal cell line of Simian virus 40-transformed beta cells. Insulin secretory dynamics in response to glucose, 3-isobutyl-1-methylxanthine, and potassium. Diabetes. 1985 Feb;34(2):115–120. doi: 10.2337/diab.34.2.115. [DOI] [PubMed] [Google Scholar]
- Imamura T., Koffler M., Helderman J. H., Prince D., Thirlby R., Inman L., Unger R. H. Severe diabetes induced in subtotally depancreatized dogs by sustained hyperglycemia. Diabetes. 1988 May;37(5):600–609. doi: 10.2337/diab.37.5.600. [DOI] [PubMed] [Google Scholar]
- Jacoby D. B., Zilz N. D., Towle H. C. Sequences within the 5'-flanking region of the S14 gene confer responsiveness to glucose in primary hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17623–17626. [PubMed] [Google Scholar]
- Karlsson O., Walker M. D., Rutter W. J., Edlund T. Individual protein-binding domains of the insulin gene enhancer positively activate beta-cell-specific transcription. Mol Cell Biol. 1989 Feb;9(2):823–827. doi: 10.1128/mcb.9.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K., Kuzuya T., Akanuma Y., Hagura R. Increase in insulin response after treatment of overt maturity-onset diabetes is independent of the mode of treatment. Diabetologia. 1980 Jan;18(1):23–28. doi: 10.1007/BF01228297. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Hughes K., Atkins T. W. Insulin release from a cloned hamster B-cell line (HIT-T15). The effects of glucose, amino acids, sulphonylureas and colchicine. Biochem Biophys Res Commun. 1986 Oct 30;140(2):616–625. doi: 10.1016/0006-291x(86)90776-x. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Bonner-Weir S., Weir G. C. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest. 1988 May;81(5):1407–1414. doi: 10.1172/JCI113470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meglasson M. D., Manning C. D., Najafi H., Matschinsky F. M. Fuel-stimulated insulin secretion by clonal hamster beta-cell line HIT T-15. Diabetes. 1987 Apr;36(4):477–484. doi: 10.2337/diab.36.4.477. [DOI] [PubMed] [Google Scholar]
- Moss L. G., Moss J. B., Rutter W. J. Systematic binding analysis of the insulin gene transcription control region: insulin and immunoglobulin enhancers utilize similar transactivators. Mol Cell Biol. 1988 Jun;8(6):2620–2627. doi: 10.1128/mcb.8.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Shen L. P., Meister A., Fodor E., Rutter W. J. Pan: a transcriptional regulator that binds chymotrypsin, insulin, and AP-4 enhancer motifs. Genes Dev. 1990 Jun;4(6):1035–1043. doi: 10.1101/gad.4.6.1035. [DOI] [PubMed] [Google Scholar]
- Ohlsson H., Edlund T. Sequence-specific interactions of nuclear factors with the insulin gene enhancer. Cell. 1986 Apr 11;45(1):35–44. doi: 10.1016/0092-8674(86)90535-0. [DOI] [PubMed] [Google Scholar]
- Ohlsson H., Karlsson O., Edlund T. A beta-cell-specific protein binds to the two major regulatory sequences of the insulin gene enhancer. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4228–4231. doi: 10.1073/pnas.85.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Tsai P., Little S. A., Zhang H. J., Walseth T. F. Receptor-mediated adenylate cyclase-coupled mechanism for PGE2 inhibition of insulin secretion in HIT cells. Diabetes. 1987 Sep;36(9):1047–1053. doi: 10.2337/diab.36.9.1047. [DOI] [PubMed] [Google Scholar]
- Robertson R. P. Type II diabetes, glucose "non-sense," and islet desensitization. Diabetes. 1989 Dec;38(12):1501–1505. doi: 10.2337/diab.38.12.1501. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Zhang H. J., Pyzdrowski K. L., Walseth T. F. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest. 1992 Aug;90(2):320–325. doi: 10.1172/JCI115865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Shulman G. I., Zawalich W., DeFronzo R. A. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest. 1987 Oct;80(4):1037–1044. doi: 10.1172/JCI113157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre R. F., Cook R. A., Crisel R. M., Sharp J. D., Schmidt R. J., Williams D. C., Wilson C. P. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeltz R., Wendorff H. J., Field J. B. Effect of control of blood glucose on plasma insulin responses to various stimuli in secondary failures to oral hypoglycemic agents and in newly diagnosed, maturity onset, ketosis-resistant diabetics. J Clin Endocrinol Metab. 1978 Apr;46(4):519–527. doi: 10.1210/jcem-46-4-519. [DOI] [PubMed] [Google Scholar]
- Shibasaki Y., Sakura H., Takaku F., Kasuga M. Insulin enhancer binding protein has helix-loop-helix structure. Biochem Biophys Res Commun. 1990 Jul 16;170(1):314–321. doi: 10.1016/0006-291x(90)91276-x. [DOI] [PubMed] [Google Scholar]
- Shih H. M., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J Biol Chem. 1992 Jul 5;267(19):13222–13228. [PubMed] [Google Scholar]
- Turner R. C., McCarthy S. T., Holman R. R., Harris E. Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J. 1976 May 22;1(6020):1252–1254. doi: 10.1136/bmj.1.6020.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985 Mar;28(3):119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- Vague P., Moulin J. P. The defective glucose sensitivity of the B cell in non insulin dependent diabetes. Improvement after twenty hours of normoglycaemia. Metabolism. 1982 Feb;31(2):139–142. doi: 10.1016/0026-0495(82)90125-1. [DOI] [PubMed] [Google Scholar]
- Welsh M., Nielsen D. A., MacKrell A. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985 Nov 5;260(25):13590–13594. [PubMed] [Google Scholar]
- Zhang H. J., Walseth T. F., Robertson R. P. Insulin secretion and cAMP metabolism in HIT cells. Reciprocal and serial passage-dependent relationships. Diabetes. 1989 Jan;38(1):44–48. doi: 10.2337/diab.38.1.44. [DOI] [PubMed] [Google Scholar]