Abstract
Low-density lipoprotein receptor-related protein-1 (LRP) on brain capillaries clears amyloid β-peptide (Aβ) from brain. Here, we show that soluble circulating LRP (sLRP) provides key endogenous peripheral ‘sink’ activity for Aβ in humans. Recombinant LRP cluster IV (LRP-IV) bound Aβ in plasma in mice and in Alzheimer’s disease-affected humans with compromised sLRP-mediated Aβ binding, and reduced Aβ-related pathology and dysfunction in a mouse model of Alzheimer mice, suggesting LRP-IV can effectively replace native sLRP and clear Aβ.
LRP binds the Alzheimer’s disease neurotoxin, Aβ, at the abluminal side of the blood-brain barrier (BBB), which initiates Aβ clearance from brain to blood via transcytosis across the BBB1–4. In the liver, LRP mediates systemic clearance of Aβ5. β-secretase cleaves the N-terminus extracellular domain of LRP6, which releases soluble LRP (sLRP). sLRP normally circulates in plasma7.
Two major binding domains of LRP, cluster II and cluster IV8, bind Aβ in vitro with high affinity: i.e., Aβ40 > Aβ42 (ref. 2). We hypothesized that LRP recombinant cluster IV (LRP-IV) retains its high-affinity binding for Aβ in vivo, and that this binding alters Aβ transport at the BBB, which is dominated by the cell-surface LRP1–4 and the receptor for advanced glycation end-products (RAGE)9, resulting in Aβ efflux from the brain. We also hypothesized that plasma sLRP binds Aβ in vivo, which regulates Aβ metabolism and clearance under physiological conditions and in Alzheimer’s disease.
We found that the relative in vitro ligand-binding affinities for LRP-IV were, in descending order (referring in all cases to the human proteins), Aβ40 > Aβ42 ≫ apolipoprotein E3 (apoE3) > apoE4 ≫ tissue plasminogen activator (tPA), as indicated by their respective binding constants, Kd, of 1.9, 5.1, 22.3, 29.9 and 172 nM (Supplementary Fig. 1a–c,e online). Kd values for mouse apoE and tPA were 16.9 and 21.2 nM, respectively (Supplementary Fig. 1d,e). Thus, LRP-IV preferentially bound Aβ peptides as compared to other LRP ligands.
By using an in situ arterial brain perfusion technique in mice9, we showed that LRP-IV completely blocked transport of circulating 125I-Aβ40 across the BBB, and itself was not transported across the BBB (Supplementary Fig. 2a–c online). As determined from the 24-h plasma profile of intact 125I-LRP-IV after an intravenous injection (Supplementary Fig. 2d), the half-time of LRP-IV elimination, et1/2, was 11.8 h, and its mean residence time of LRP-IV in the circulation was 14 h. We also found that 125I-LRP-IV and/or 125I-LRP-IV-Aβ complexes were removed by liver, kidney and spleen, but were not taken up by brain within 24 h (Supplementary Fig. 2e,f).
Short-term LRP-IV treatment reduced mouse endogenous brain Aβ40 and Aβ42 by 51% and 27%, respectively (Fig. 1a), and increased plasma Aβ40 and Aβ42 (Fig. 1b). After LRP-IV treatment, plasma Aβ40 and Aβ42 were associated mainly with LRP-IV, as shown by analysis of Aβ in pellets of sLRP-depleted plasmas precipitated with receptor-associated protein10,11 (Supplementary Fig. 3a–d, Supplementary Methods online). These results were consistent with the 4-5-fold lower affinity of Aβ for mouse endogenous sLRP (Supplementary Fig. 3e) as compared to LRP-IV (Supplementary Fig. 1a). We did not detect either sLRP or LRP-IV in the cerebrospinal fluid (CSF). (Supplementary Fig. 4a online).
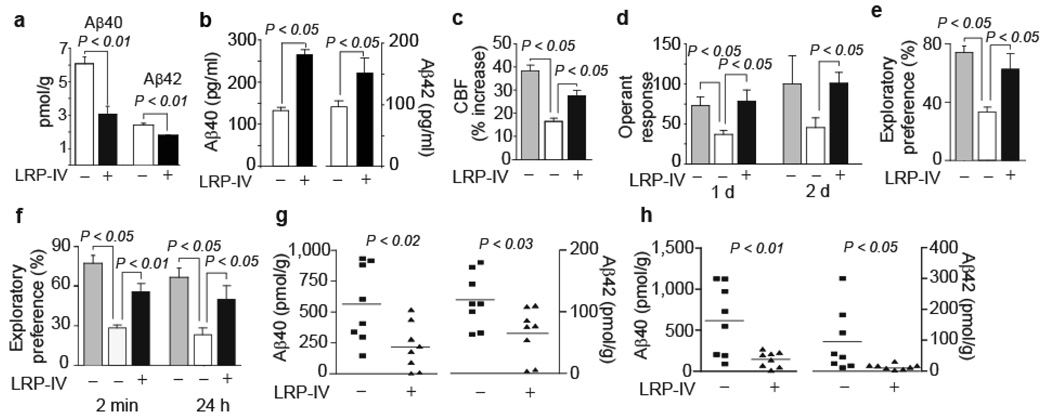
Figure 1.
LRP-IV clears mouse endogenous brain Aβ improves function and clears human brain Aβ in APP+/sw mice. (a,b) Brain levels (a) and total plasma levels (b) of mouse endogenous Aβ40 and Aβ42 in vehicle-treated (white) and LRP-IV-treated (intravenously for 5 d, 20 µg/day) mice (black) Values are means ± s.e.m., n = 3–5 mice per group. (c) Percent increase in cerebral blood flow (CBF) in response to whisker stimulation in littermate controls (gray) and APP+/sw mice treated with vehicle (white) or LRP-IV (black). (d–f) Operant learning at day 1 and 2 (d), novel object location (e) and novel object recognition (f) expressed as percentage exploratory preference in littermate controls (gray) and APP+/sw mice treated with vehicle (white) or LRP-IV (black). (g,h) Aβ40 and Aβ42 levels in hippocampus (g) and cortex (h) of APP+/sw mice treated with vehicle or LRP-IV. In c–h, LRP-IV (40 µg per kg body weight per day) was administered intraperitioneally for 3 months beginning at age 6 months. Values are means ± s.e.m., n = 8 mice per group. All procedures with wild-type mice and APP+/sw mice were according to US NIH guidelines approved by the University Committee on Animal Resources, University of Rochester.
Chronic treatment of mice heterozygous for the sw mutation of APP, the gene encoding Aβ precursor protein (APP+/sw, also called APPsw+/−, mice), with low-dose LRP-IV beginning at 6 months of age increased cerebral blood flow responses to whisker stimulation by 65% (Fig. 1c) and improved operant learning (Fig. 1d) and spatial (Fig. 1e) and recognition (Fig. 1f) memory almost to their levels in nontransgenic controls. We did not find differences in behavior in wild-type mice after LRP-IV or vehicle treatment (data not shown).
In LRP IV-treated vs. vehicle-treated APP+/sw mice Aβ40 and Aβ42 levels in hippocampus and cortex were reduced by 72% and 61% and by >80% and 90%, respectively (Fig. 1g,h). LRP-IV substantially reduced total Aβ load (Fig. 2a), amyloid vascular and parenchymal load (Fig. 2b), and Aβ parenchymal and vascular load (Supplementary Fig. 5a–e online). The ability of LRP-IV to remove vascular Aβ and vascular amyloid is critical, because in mouse models of Alzheimer's disease, dense plaques develop initially on blood vessels2,12.
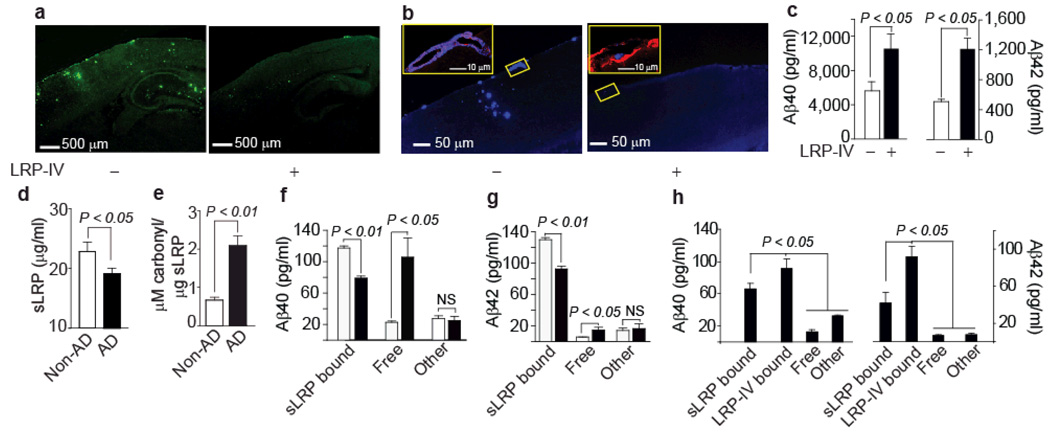
Figure 2.
LRP-IV controls Aβ pathology in APP+/sw mice and sLRP and LRP-IV regulate peripheral Aβ in Alzheimer’s disease. (a) Aβ immunostaining in APP+/sw mice treated with vehicle or LRP-IV. (b) X-34 staining for amyloid (blue) and CD31 immunostaning for vascular endothelium (red) in APP+/sw mice treated with vehicle or LRP-IV. Insets: left, amyloid in the pial vessel on the surface of the brain in vehicle-treated mice; right, absence of amyloid in the pial vessel in LRP-IV treated mice. (c) Total plasma Aβ40 and Aβ42 levels in vehicle-treated (white bars) and LRP-IV-treated (black bars) mice. In a–c, LRP-IV (40 µg per kg body weight per day) was administered intraperitoneally for 3 months beginning at the age of 6 months. Values are means ± s.e.m., n = 8 mice per group. (d) Plasma sLRP levels in individuals without (non-AD; white) and with (AD; black) Alzheimer’s disease. Means ± s.e.m., from 36 non-AD and 44 AD individuals. (e) Oxidized sLRP (carbonyl content) in plasma of non-AD (white) and AD (black) individuals. Means ± s.e.m. from 12 non-AD and 16 AD individuals. (f,g) sLRP-bound Aβ40 and Aβ42, free Aβ40 and Aβ42 (<30 kDa), and Aβ40 and Aβ42 associated with other plasma proteins (>30 kDa) in non-AD controls (white) and AD individuals (black). Means ± s.e.m., n = 18 per group. (h) Effect of LRP-IV on redistribution of different Aβ40 and Aβ42 pools in AD plasma. Means ± s.e.m., n = 6. All procedures with AD mice were according to the NIH guidelines approved by the University Committee on Animal Resources, University of Rochester. The experiments involving human subjects have been approved by the Institutional Review Board Committee of the Washington University School of Medicine through the Alzheimer’s Disease Research Center. Informed consent was obtained from all subjects.
LRP-IV increased plasma Aβ40 and Aβ42 in APP+/sw mice (Fig. 2c), sequestered 52–55% of total Aβ and reduced free Aβ42 and Aβ40 (Supplementary Fig. 3f,g). We did not find LRP-IV or sLRP in the CSF of APP+/sw mice (Supplementary Fig. 4b).
LRP-IV moderately reduced plasma cholesterol in APP+/sw mice, but did not alter plasma levels of major LRP ligands, such as apoE, tPA or pro-matrix metalloproteinase-9 (Supplementary Fig. 6a–d online). After LRP-IV treatment, we did not find changes in the expression of lipoprotein receptors in brain, kidney or liver (Supplementary Fig. 6e–h online).
To test whether Aβ-lowering effect of LRP-IV remains when treatment is initiated at a time point when APP+/sw mice develop prominent cerebral amyloid angiopathy and parenchymal plaques13,14, we treated 11-month-old APP+/sw mice for 6 weeks with LRP-IV or vehicle. Again, LRP-IV reduced Aβ pathology and vascular and brain amyloid deposition by >90% (Supplementary Fig. 7 online) without causing microhemorrhages, as demonstrated by negative Prussian blue staining (data not shown).
In individuals with Alzheimer’s disease, sLRP plasma levels were 30% lower than in non-demented controls (Fig. 2d). There was a 280 % increase in oxidized sLRP (Fig. 2e), which showed extremely low affinity for Aβ and Aβ40 (Supplementary Fig. 3h). The cohort of individuals with Alzheimer’s disease we studied did not show evidence of polymorphism in the LRP gene (Supplementary Table 1 online).
We next showed that native sLRP is a major binding protein for Aβ in human plasma (Fig. 2f–g). After coimmunoprecipitation of sLRP-bound Aβ, we found that about 70% of Aβ40 and about 90% of Aβ42 were normally bound to plasma sLRP in controls. In individuals with Alzheimer’s disease, there was a 30–35% drop in sLRP-bound Aβ40 and Aβ42 associated with a 300–400 % increase in free, protein-unbound Aβ40 and Aβ42 (Fig. 2f,g). A minor fraction of Aβ (>30 kDa) was associated with non-sLRP carriers, such as apoJ (data not shown), in both controls and affected individuals. Incubation of plasma from individuals with Alzheimer’s disease with low dose LRP-IV eliminated free Aβ40 and Aβ42 and significantly decreased (P < 0.05) sLRP-bound Aβ42 and Aβ40 (Fig. 2h).
Our data suggest that recombinant LRP-IV does not penetrate the BBB and CSF and lowers brain Aβ through peripheral binding with no direct central actions. Compared to non-immune Aβ-binding agents, LRP-IV was effective at substantially lower levels. For example, in transgenic mice overexpressing a mutant form of human APP encoded by a minigene with several substitutions found in familial Alzheimer’s disease (V717F, K670M and N671L) under the control of the PDGF β-chain promoter (PD-hAPP) soluble RAGE exerted an Aβ-lowering effect at a daily molar dose ~50-fold greater9 than LRP-IV. Gelsolin and ganglioside M1 lowered brain Aβ in transgenic mice overexpressing a mutant form of human presenilin-1 gene (M146L) and APP+/sw (PS1/APP+/sw mice)15 at a molar daily dose an order of magnitude greater than LRP-IV.
We have demonstrated that native sLRP normally controls 70–90% of circulating Aβ in humans through peripheral binding. This sLRP function is compromised in Alzheimer’s disease, which may contribute to elevated brain Aβ. LRP-IV effectively sequestered Aβ in Alzheimer’s disease-derived plasma and in APP+/sw mice, in the latter case resulting in Aβ efflux from mouse brain. Thus, LRP-IV could serve as a Aβ clearance and sLRP replacement therapy for Alzheimer’s disease with an enhanced peripheral ‘sink’ activity for Aβ compared to endogenous sLRP.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the US National Institutes of Health (NIH) grants R37 AG023084 and R37 NS34467 to B.V.Z. and Washington University St. Louis Alzheimer’s Disease Research Center grant P50 AG05681.
Footnotes
AUTHOR CONTRIBUTIONS
A.S. conducted and performed in vivo studies in wild-type and APP+/sw (APPsw+/−) mice. R.D. conducted and performed pharmacokinetic and cerebral blood flow studies and work on the human study protocol. R.D.B conducted and performed in vitro ligand binding assays and Aβ immunostaining studies. B.J. performed oxidized sLRP assay and sLRP ELISA assay. K.H. performed in vivo studies in wild-type mice. R.P. purified recombinant LRP-IV. A.M. performed cholesterol and apoE assays. P.J.L. supervised R.P. and provided LRP-IV. Z.W. conducted and performed memory tests in mice. T.Z. performed approach and operant learning test in mice. D.P. performed active tPA and pro-MMP-9 assays. A.G. and K.M. performed LRP polymorphism studies. M.C. performed immunoprecipitation of oxidized proteins. Z.Z. performed Prussian blue staining. B.V.Z designed the entire study, supervised all segments of the study and wrote the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/.
References
- 1.Shibata M, et al. J. Clin. Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deane R, et al. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Tanzi RE, Moir RD, Wagner SL. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Bell RD, et al. J. Cereb. Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamaki C, et al. Pharm. Res. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 6.von Arnim CAF, et al. J. Biol. Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- 7.Quinn KA, et al. J. Biol. Chem. 1997;272:23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- 8.Westein E, Denis CV, Bouma BN, Lenting PJ. J. Biol. Chem. 2002;277:2511–2516. doi: 10.1074/jbc.M102293200. [DOI] [PubMed] [Google Scholar]
- 9.Deane Rq, et al. Nat. Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 10.Zerbinatti CV, Bu GJ. Rev. Neurosci. 2005;16:123–135. doi: 10.1515/revneuro.2005.16.2.123. [DOI] [PubMed] [Google Scholar]
- 11.Jensen GA, et al. J. Mol. Biol. 2006;362:700–716. doi: 10.1016/j.jmb.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Kumar-Singh S, et al. Am. J. Pathol. 2005;167:527–543. doi: 10.1016/S0002-9440(10)62995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao K, et al. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 14.Fryer JD, et al. J. Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuoka Y, et al. J. Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.