Abstract
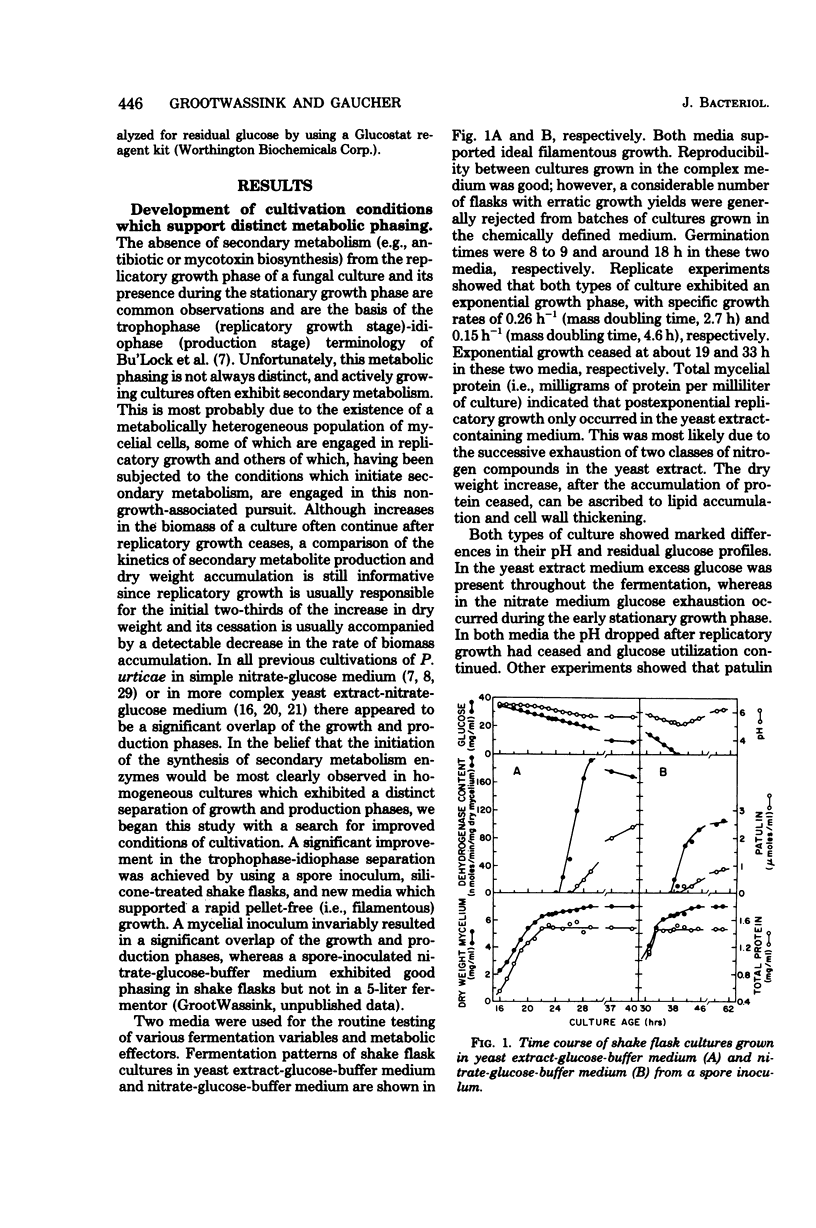
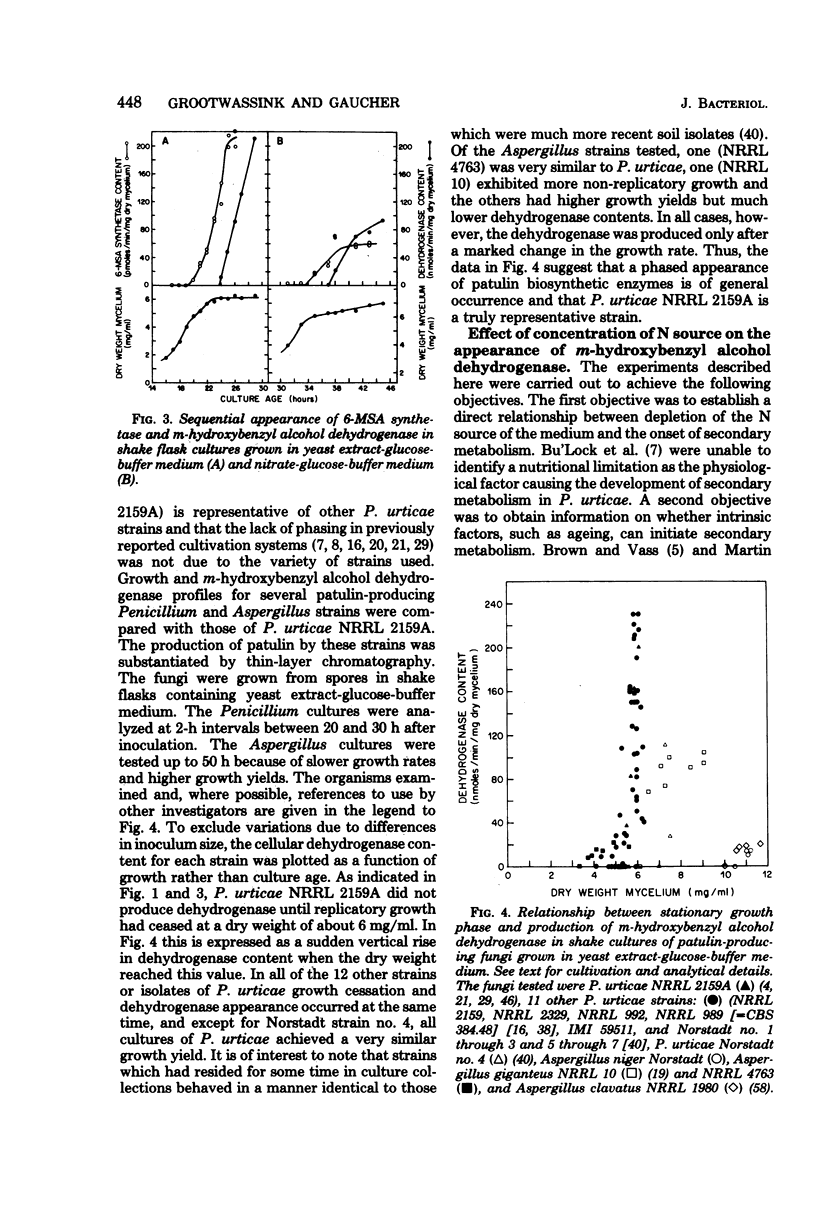
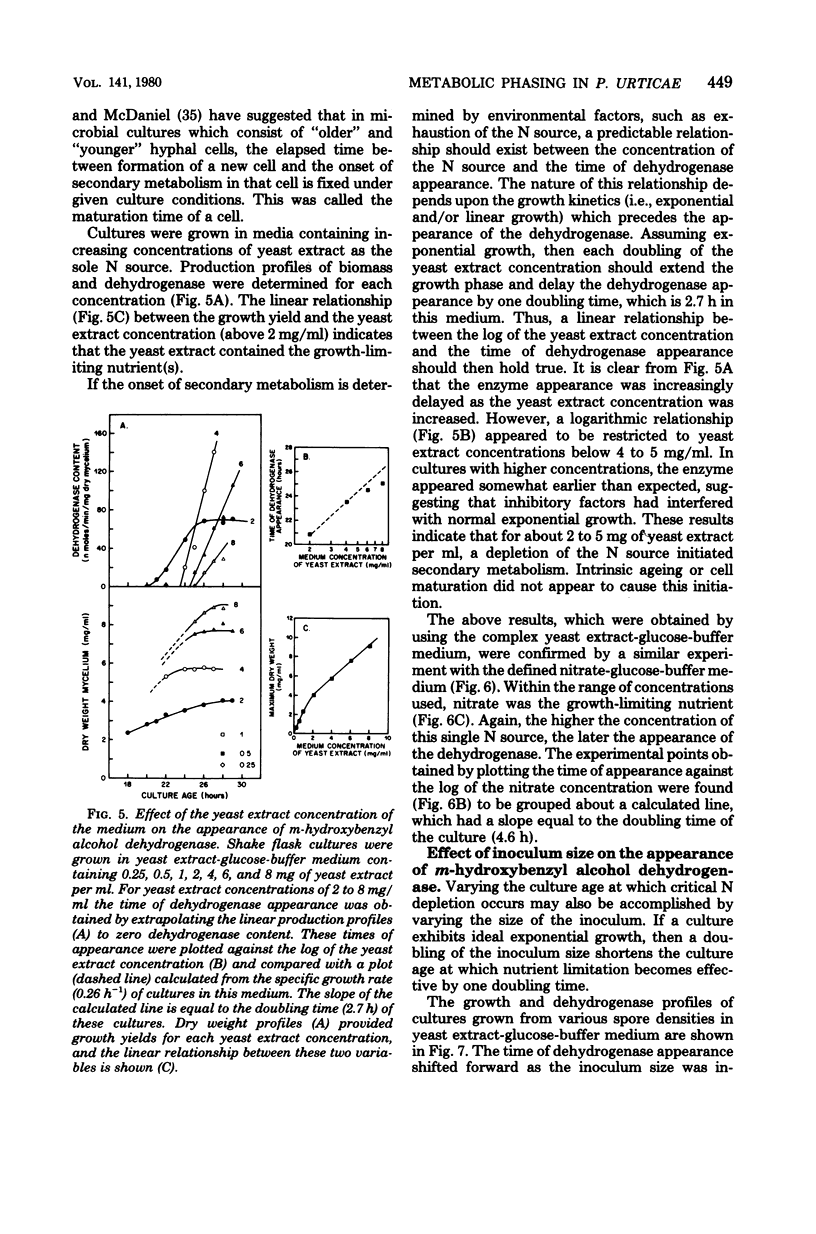
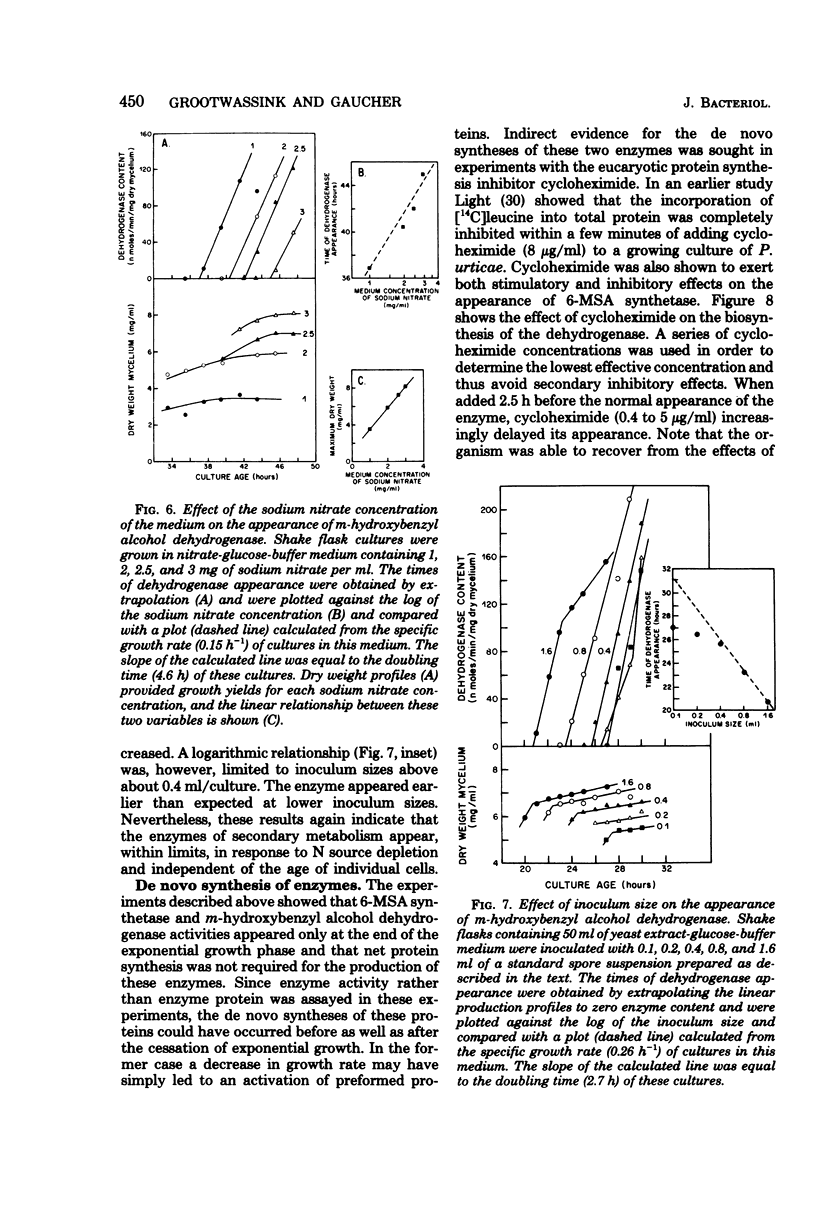
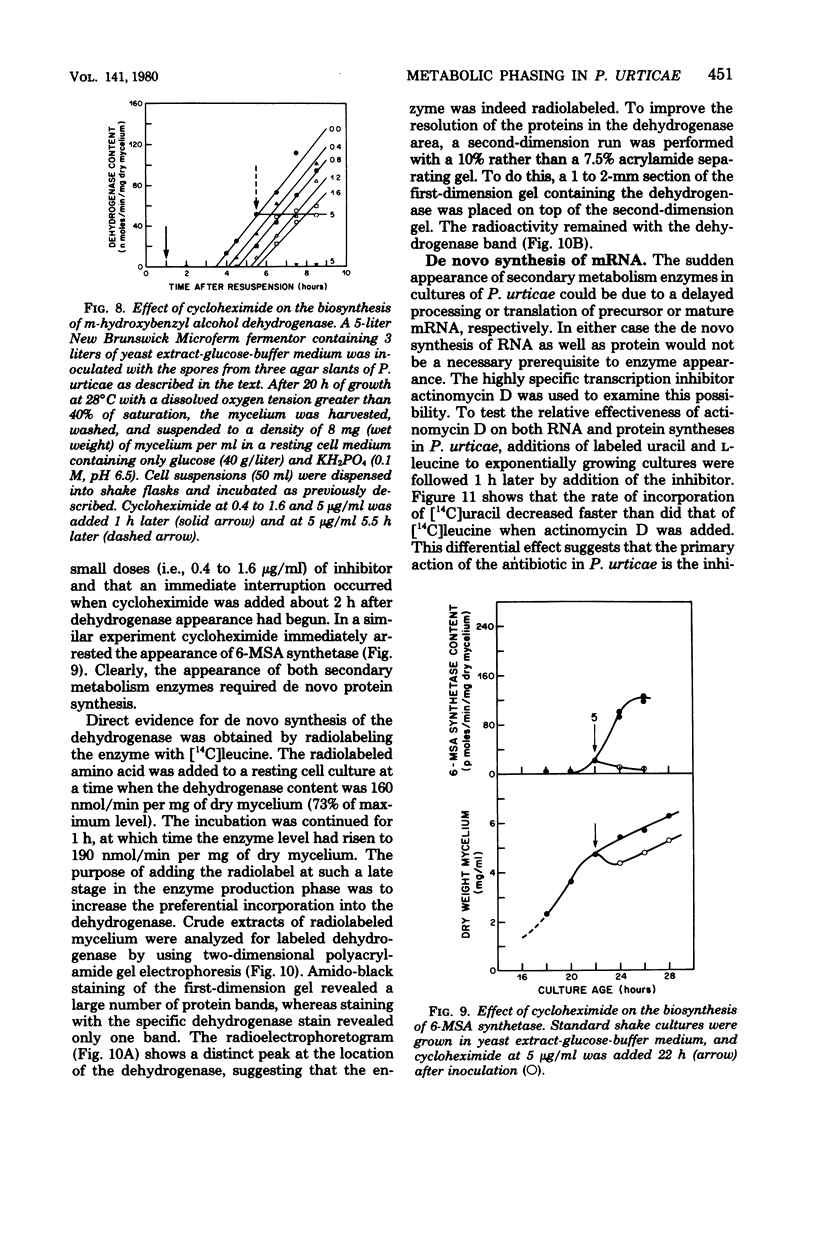
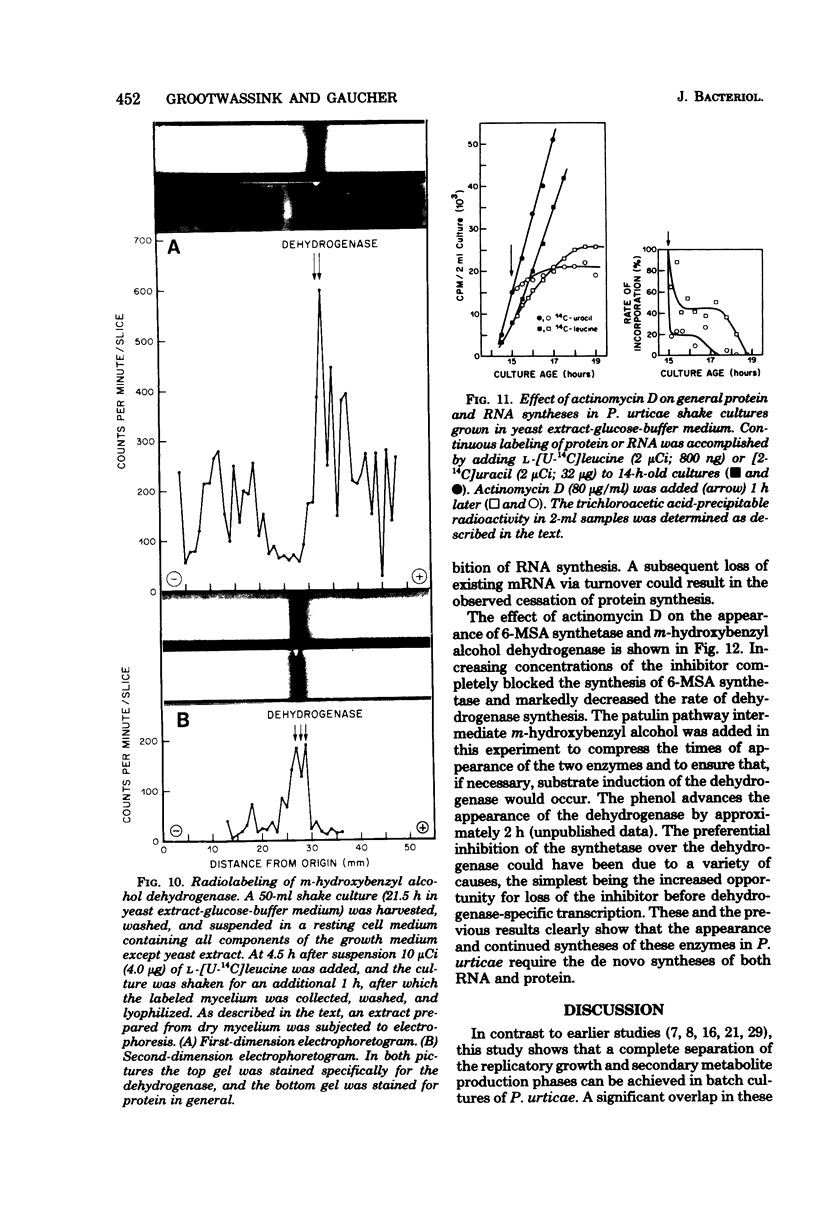
The initiation of patulin biosynthesis in submerged batch cultures of Penicillium urticae NRRL 2159A was investigated at the enzyme level. In contrast to earlier studies, this study achieved a clear temporal separation of growing cells devoid of secondary metabolism-specific enzymes from nongrowing cells, which rapidly produce these enzymes. A spore inoculum, silicone-treated flasks, and two new media which supported a rapid, pellet-free, filamentous type of growth were used. In yeast extract-glucose-buffer medium, a marked drop in the specific growth rate (approximately equal to 0.26 h-1) coincided with the appearance of the first pathway-specific enzyme, 6-methylsalicylic acid synthetase, at about 19 h after inoculation. About 3 h later, when replicatory growth had ceased entirely, the sparsely branched mycelia (length, approximately equal to 550 microns) began the rapid synthesis of a later pathway enzyme, m-hydroxybenzyl alcohol dehydrogenase. A similar sequence of events occurred in a defined nitrate-glucose-buffer medium; 12 other strains or isolates of P. urticae, as well as some patulin-producing aspergilli, behaved in a similar manner. The age at which a culture produced m-hydroxybenzyl alcohol dehydrogenase was increased by increasing the nutrient nitrogen content of the medium or by decreasing the size of the spore inoculum. In each instance the appearance of enzyme was determined by the nutritional status of the culture and not by its age. A similar appearance of patulin pathway enzymes occurred when a growing culture was resuspended in a nitrogen-free 4% glucose solution with or without 0.1 M phosphate (pH 6.5). The appearance of both the synthetase and the dehydrogenase was arrested by the addition of cycloheximide (0.4 to 5 micrograms/ml) or actinomycin D (20 to 80 micrograms/ml). This requirement for de novo protein and ribonucleic acid syntheses was confirmed by the incorporation of labeled leucine into the dehydrogenase, and the possibility that latent or preformed proteins were being activated was eliminated.
Full text
PDF



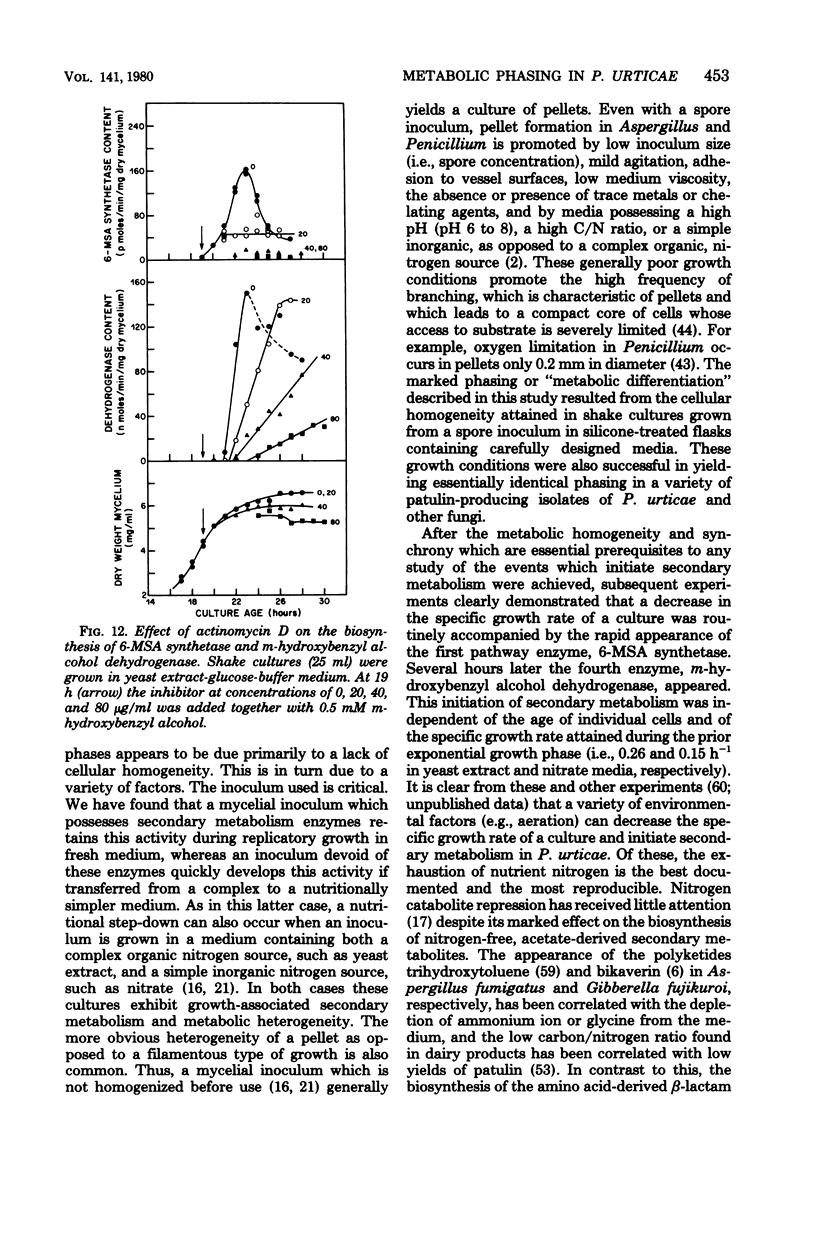


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Demain A. L. Nitrogen nutrition and regulation of cephalosporin production in Streptomyces clavuligerus. Can J Microbiol. 1979 Jan;25(1):61–67. doi: 10.1139/m79-010. [DOI] [PubMed] [Google Scholar]
- Axberg K., Gatenbeck S. The enzymic formation of penicillic acid. FEBS Lett. 1975 Jun 1;54(1):18–20. doi: 10.1016/0014-5793(75)81058-1. [DOI] [PubMed] [Google Scholar]
- BASSETT E. W., TANENBAUM S. W. The biosynthesis of patulin. II. The general physiology of several strains of Penicillium patulum. Biochim Biophys Acta. 1958 May;28(2):247–260. doi: 10.1016/0006-3002(58)90470-0. [DOI] [PubMed] [Google Scholar]
- Bu'Lock J. D., Hamilton D., Hulme M. A., Powell A. J., Smalley H. M., Shepherd D., Smith G. N. Metabolic development and secondary biosynthesis in Penicillium urticae. Can J Microbiol. 1965 Oct;11(5):765–778. doi: 10.1139/m65-104. [DOI] [PubMed] [Google Scholar]
- Bu'Lock J. D., Shepherd D., Winstanley D. J. Regulation of 6-methylsalicylate and patulin synthesis in Penicillium urticae. Can J Microbiol. 1969 Mar;15(3):279–285. doi: 10.1139/m69-051. [DOI] [PubMed] [Google Scholar]
- Cook A. M., Beggs J. D., Fewson C. A. Regulation of growth of Acinetobacter calcoaceticus NCIB8250 on L-mandelate in batch culture. J Gen Microbiol. 1975 Dec;91(2):325–337. doi: 10.1099/00221287-91-2-325. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Demain A. L., Inamine E. Biochemistry and regulation of streptomycin and mannosidostreptomycinase (alpha-D-mannosidase) formation. Bacteriol Rev. 1970 Mar;34(1):1–19. doi: 10.1128/br.34.1.1-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P., Ringelmann E., Lynen F. 6-Methylsalicylic acid synthetase from Penicillium patulum. Some catalytic properties of the enzyme and its relation to fatty acid synthetase. Eur J Biochem. 1976 Sep 15;68(2):591–596. doi: 10.1111/j.1432-1033.1976.tb10847.x. [DOI] [PubMed] [Google Scholar]
- Dimroth P., Walter H., Lynen F. Biosynthese von 6-Methylsalicylsäure. Eur J Biochem. 1970 Mar 1;13(1):98–110. doi: 10.1111/j.1432-1033.1970.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Drew S. W., Demain A. L. Effect of primary metabolites on secondary metabolism. Annu Rev Microbiol. 1977;31:343–356. doi: 10.1146/annurev.mi.31.100177.002015. [DOI] [PubMed] [Google Scholar]
- Elnaghy M. A., Megalla S. E. Gluconic acid production by Penicillium puberulum. Folia Microbiol (Praha) 1975;20(6):504–508. doi: 10.1007/BF02891710. [DOI] [PubMed] [Google Scholar]
- Forrester P. I., Gaucher G. M. Conversion of 6-methylsalicylic acid into patulin by Penicillium urticae. Biochemistry. 1972 Mar 14;11(6):1102–1107. doi: 10.1021/bi00756a025. [DOI] [PubMed] [Google Scholar]
- Forrester P. I., Gaucher G. M. m-Hydroxybenzyl alcohol dehydrogenase from Penicillium urticae. Biochemistry. 1972 Mar 14;11(6):1108–1114. doi: 10.1021/bi00756a026. [DOI] [PubMed] [Google Scholar]
- Gallo M., Katz E. Regulation of secondary metabolite biosynthesis: catabolite repression of phenoxazinone synthase and actinomycin formation by glucose. J Bacteriol. 1972 Feb;109(2):659–667. doi: 10.1128/jb.109.2.659-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher G. M. m-Hydroxybenzyl-alcohol dehydrogenase. Methods Enzymol. 1975;43:540–548. doi: 10.1016/0076-6879(75)43116-0. [DOI] [PubMed] [Google Scholar]
- Jones A., Francis M. M., Vining L. C. Biosynthesis of chloramphenicol in Streptomyces sp. 3022a. Properties of an aminotransferase accepting p-aminophenylalanine as a substrate. Can J Microbiol. 1978 Mar;24(3):238–244. doi: 10.1139/m78-042. [DOI] [PubMed] [Google Scholar]
- Jones A., Westlake D. W. Regulation of chloramphenicol synthesis in Streptomyces sp. 3022a. Properties of arylamine synthetase, an enzyme involved in antibiotic biosynthesis. Can J Microbiol. 1974 Nov;20(11):1599–1611. doi: 10.1139/m74-247. [DOI] [PubMed] [Google Scholar]
- Krupinski V. M., Robbers J. E., Floss H. G. Physiological study of ergot: induction of alkaloid synthesis by tryptophan at the enzymatic level. J Bacteriol. 1976 Jan;125(1):158–165. doi: 10.1128/jb.125.1.158-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Light R. J. 6-methylsalicylic acid decarboxylase from Penicillium patulum. Biochim Biophys Acta. 1969 Nov 4;191(2):430–438. doi: 10.1016/0005-2744(69)90262-9. [DOI] [PubMed] [Google Scholar]
- Light R. J. Effects of cycloheximide and amino acid analogues on biosynthesis of 6-methylsalicylic acid in Penicillium patulum. Arch Biochem Biophys. 1967 Nov;122(2):494–500. doi: 10.1016/0003-9861(67)90224-x. [DOI] [PubMed] [Google Scholar]
- Light R. J. The biosynthesis of 6-methylsalicylic acid. Crude enzyme systems from early and late producing strains of Penicillium patulum. J Biol Chem. 1967 Apr 25;242(8):1880–1886. [PubMed] [Google Scholar]
- Light R. J., Vogel G. 6-Methylsalicylic acid (2,6-cresotic acid) decarboxylase. Methods Enzymol. 1975;43:530–540. doi: 10.1016/0076-6879(75)43115-9. [DOI] [PubMed] [Google Scholar]
- Martin J. F., McDaniel L. E. Production of polyene macrolide antibiotics. Adv Appl Microbiol. 1977;21:1–52. doi: 10.1016/s0065-2164(08)70037-6. [DOI] [PubMed] [Google Scholar]
- Murphy G., Lynen F. Patulin biosynthesis: the metabolism of m-hydroxybenzyl alcohol and m-hydroxybenzaldehyde by particulate preparations from Penicillium patulum. Eur J Biochem. 1975 Oct 15;58(2):467–475. doi: 10.1111/j.1432-1033.1975.tb02394.x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Vogel G., Krippahl G., Lynen F. Patulin biosynthesis: the role of mixed-function oxidases in the hydroxylation of m-cresol. Eur J Biochem. 1974 Nov 15;49(2):443–455. doi: 10.1111/j.1432-1033.1974.tb03849.x. [DOI] [PubMed] [Google Scholar]
- Muth W. L., Nash C. H., 3rd Biosynthesis of mycophenolic acid: purification and characterization of S-adenosyl-L-methionine: demethylmycophenolic acid O-methyltransferase. Antimicrob Agents Chemother. 1975 Sep;8(3):321–327. doi: 10.1128/aac.8.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M. Conidiogenesis and secondary metabolism in Penicillium urticae. Appl Environ Microbiol. 1977 Jan;33(1):147–158. doi: 10.1128/aem.33.1.147-158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M. Identification of phyllostine as an intermediate of the patulin pathway in Penicillium urticae. Biochemistry. 1978 May 2;17(9):1785–1791. doi: 10.1021/bi00602a033. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M. Isoepoxydon, a new metabolite of the patulin pathway in Penicillium urticae. Biochem J. 1979 Aug 15;182(2):445–453. doi: 10.1042/bj1820445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi J., Gaucher G. M. Patulin biosynthesis: the metabolism of phyllostine and isoepoxydon by cell-free preparations from Pencillium urticae. Can J Microbiol. 1979 Aug;25(8):881–887. doi: 10.1139/m79-131. [DOI] [PubMed] [Google Scholar]
- Singh R., Hsieh D. P. Enzymatic conversion of sterigmatocystin into aflatoxin B1 by cell-free extracts of Aspergillus parasiticus. Appl Environ Microbiol. 1976 May;31(5):743–745. doi: 10.1128/aem.31.5.743-745.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott W. T., Bullerman L. B. Influence of carbohydrate and nitrogen source on patulin production by Penicillium patulum. Appl Microbiol. 1975 Nov;30(5):850–854. doi: 10.1128/am.30.5.850-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Ojima N., Ogura K., Seto S. Purification and characterization of dimethylallyl pyrophosphate: aspulvinone dimethylallyltransferase from Aspergillus terreus. Biochemistry. 1978 Jun 27;17(13):2696–2702. doi: 10.1021/bi00606a037. [DOI] [PubMed] [Google Scholar]
- Waksman S. A., Horning E. S., Spencer E. L. Two Antagonistic Fungi, Aspergillus fumigatus and Aspergillus clavatus, and Their Antibiotic Substances. J Bacteriol. 1943 Mar;45(3):233–248. doi: 10.1128/jb.45.3.233-248.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. C., Packter N. M. Relationship between fatty-acid and phenol synthesis in Aspergillus fumigatus. Eur J Biochem. 1974 Jul 15;46(2):323–333. doi: 10.1111/j.1432-1033.1974.tb03624.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto L. A., Segel I. H. The inorganic sulfate transport system of Penicillium chrysogenum. Arch Biochem Biophys. 1966 Jun;114(3):523–538. doi: 10.1016/0003-9861(66)90376-6. [DOI] [PubMed] [Google Scholar]
- Zocher R., Kleinkauf H. Biosynthesis of enniatin B: partial purification and characterization of the synthesizing enzyme and studies of the biosynthesis. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1162–1167. doi: 10.1016/0006-291x(78)91258-5. [DOI] [PubMed] [Google Scholar]