A challenge of personalized medicine is the limited clinical evidence for many personalized medicine technologies. Here, the strategies private payers use to develop coverage policy for personalized medicine are described using the example of the 21-gene assay in breast cancer.
Abstract
Purpose:
Personalized medicine is changing oncology practice and challenging decision making. A key challenge is the limited clinical evidence for many personalized medicine technologies. We describe the strategies private payers employed to develop coverage policy for personalized medicine using the example of the 21-gene assay in breast cancer.
Methods:
We examined the coverage policies of six private payers for the 21-gene assay. We then interviewed senior executives (n = 7) from these payers to elucidate factors informing coverage decisions. We additionally focused on the timing of payer decisions compared with the timing of evidence development, measured by publication of primary studies and relevant clinical guidelines.
Results:
The 21-gene assay became commercially available in 2004. The interviewed payers granted coverage between 2005 and 2008. Their policies varied in structure (eg, whether prior authorization was required). All payers reported clinical evidence as the most important factor in decision making, but all used some health care system factors (eg, physician adoption or medical society endorsement) to inform decision making as well. Payers had different perceptions about the strength of clinical evidence at the time of the coverage decision.
Conclusion:
Coverage of the 21-gene assay is currently widespread, but policies differ in timing and structure. A key approach private payers use to develop coverage policies for novel technologies is considering both clinical evidence and health care system factors. Policy variation may emerge from the range of factors used and perception of the evidence. Future research should examine the role of health care system factors in policy development and related policy variations.
Introduction
Personalized medicine, here referring to the use of genetics or genomics to guide health care decisions, is changing clinical practice and challenging policy decision making.1,2 Personalized medicine is particularly relevant in oncology, where a number of these technologies have been pioneered. A key challenge to decision makers is that the clinical evidence is limited for many personalized medicine technologies,3,4 in part because of the inherent characteristics of the US diagnostic regulatory system, and because these technologies are less likely to be studied in randomized clinical trials.5 Yet there are now several examples of personalized medicine that have been adopted in care and covered by health insurance.6,7
This study examines the overarching issue of what strategies private payers use to develop policy for personalized medicine.8 Private payers insure more than two thirds of the US population. Their policy decisions are critical factors in access to new technologies and their use in practice.9,10 The topic of how payers make decisions is important to examine, because it identifies the evidence needed for payer decisions and helps clinicians understand payer policies and their impact on clinical practice.11,12 Our objective is to describe the strategies private payers used to develop coverage policy for Oncotype DX (Genomic Health, Redwood City, CA), a novel 21-gene assay.
Methods
Oncotype DX Test
Oncotype DX is a gene expression profiling test that helps determine the probability of breast cancer recurrence and potential benefit from chemotherapy in estrogen receptor–positive node-negative breast cancers. The test categorizes recurrence risk as low, intermediate, or high. Patients with low recurrence scores are less likely to relapse and less likely to benefit from chemotherapy; high recurrence scores indicate higher probability of relapse and higher likelihood of chemotherapy benefit.13,14 Although it had been known that some patients with breast cancer would not benefit from chemotherapy, decision methods were limited before Oncotype DX. Oncotype DX is relatively expensive (approximately $3,500) compared with many diagnostic tests, and for patients with intermediate recurrence scores, Oncotype DX may not change treatment decisions. As a laboratory-developed test, Oncotype DX did not require approval by the US Food and Drug Administration (FDA). Evidence on clinical effectiveness of Oncotype DX is still developing in at least one current large study, TAILORx [Trial Assigning IndividuaLized Options for Treatment (Rx)].15 Oncotype DX has now gained broad use, coverage, and reimbursement. We examined this test rather than those of competitors (eg, MammaPrint; Agendia, Amsterdam, the Netherlands), because it is most commonly used.
Study Data and Methods
We used mixed methods research, including literature review and focused interviews. The literature review was developed to describe selected payer policies for Oncotype DX as well as to identify relevant clinical guidelines and original clinical studies. We examined Oncotype DX coverage policies for date of establishment and policy content. We examined clinical guidelines and clinical studies for publication date. We then used the literature review findings to construct a timeline of evidence development and payer coverage decisions for Oncotype DX and to inform our interviews.
We conducted focused interviews with seven representatives of six private US health plans in December 2008. The interviewed payers were major national insurers, including Aetna (Hartford, CT), Kaiser Permanente (Oakland, CA), Humana (Louisville, KY), UnitedHealth Group (Minneapolis, MN), and WellPoint (Indianapolis, IN); one insurer declined to be identified. Together they represent more than 113 million enrollees.16 Interviewees were senior executives actively engaged in coverage policy decision making for their organizations, including decisions on Oncotype DX. Interviewees were asked about:
Factors considered in coverage decision making on Oncotype DX.
Perception of the strength of evidence for Oncotype DX at the time of coverage decision and how it affected policy.
Features of resulting policies for Oncotype DX.
Results
Timing of Coverage Decisions, Clinical Studies, and Guidelines
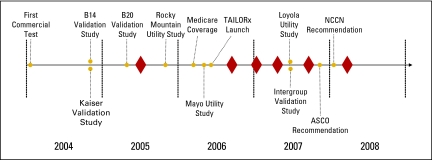
Oncotype DX became commercially available in January 2004. Public payer coverage began with a local Medicare decision in California in January 2006.17 By the time of our interviews, all interviewed payers covered Oncotype DX, but the timing of coverage decisions spanned from 2005 to 2008. We identified seven clinical studies of Oncotype DX in the adjuvant setting published between January 2004 and May 2008. The timing of clinical study publication and when coverage decisions were made varied: one payer granted coverage when three published studies were available, three payers made coverage decisions after five clinical studies were available, and two payers made coverage decisions after at least seven clinical studies were available. One interviewed payer made a coverage decision after ASCO and the National Comprehensive Cancer Network issued recommendations on Oncotype DX (in November 200718 and January 2008,19 respectively). Only one interviewed payer covered Oncotype DX before the California Medicare coverage decision. Figure 1 and Table 1 illustrate timing of coverage decisions for the interviewed payers relative to evidence development and other events.
Figure 1.
Timeline of clinical evidence (gold circles) and payer coverage decisions (red diamonds) for Oncotype DX. Exact dates of payer coverage decisions are not provided to protect payer anonymity. TAILORx, Trial Assigning IndividuaLized Options for Treatment (Rx); NCCN, National Comprehensive Cancer Network.
Table 1.
| Study or Guideline | Description | Date of Announcement or Publication |
|---|---|---|
| Kaiser validation study20 | Validation study among 4,964 Kaiser patients | December 2004, 27th San Antonio Breast Cancer Symposium |
| B14 validation study21 | Validation study of 668 patients in NSABP B-14 trial | December 2004, New England Journal of Medicine |
| B20 validation study22 | Validation study of 651 patients in NSABP B-20 trial | May 2005, 41st ASCO Annual Meeting |
| Local Medicare coverage decision17 | Contractor in California grants coverage of Oncotype DX | January 2006 |
| Rocky Mountain utility study23 | Retrospective analysis of clinical utility of Oncotype DX affecting chemotherapy decisions for 68 patients | December 2005, 28th San Antonio Breast Cancer Symposium |
| Intergroup E2197 validation study24 | Validation study of 776 patients in Intergroup E2197 trial | June 2007, 43rd ASCO Annual Meeting |
| Mayo utility study25 | Analysis of clinical utility of Oncotype DX affecting chemotherapy decisions for 31 Mayo patients | June 2007, 43rd ASCO Annual Meeting |
| TAILORx study15 | Large prospective study of clinical validity, utility, and health outcomes of Oncotype DX | Launched May 2006 |
| Loyola study26,27 of clinical utility | Prospective study of clinical utility of Oncotype Dx in 89 patients | June 2007, 43rd ASCO Annual Meeting |
| Inclusion in ASCO recommendations18 | ASCO update recommends Oncotype Dx | November 2007 |
| Inclusion in NCCN guidelines19 | NCCN clinical guidelines include Oncotype DX | January 2008 |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; TAILORx, Trial Assigning IndividuaLized Options for Treatment (Rx); NCCN, National Comprehensive Cancer Network.
Factors Used to Develop Policy
All payers reported clinical effectiveness as the most important factor in their coverage decisions, and our review of Oncotype DX policies suggested that the clinical literature was well cited. Payers noted their preference for health outcomes evidence (ie, the impact on patient disease and survival), but they acknowledged that for Oncotype DX, this evidence would evolve over 10 to 15 years as the TAILORx trial progressed. For Oncotype DX, four payers expressed willingness to base their decisions on the intermediate end point of clinical utility, which they defined as evidence that the test affects clinical decisions. One example of an intermediate end point was demonstrated in a study conducted at the Rocky Mountain Cancer Centers (Greenwood Village, CO), which showed that Oncotype DX recurrence scores changed physician recommendations for adjuvant chemotherapy.28
Payers also reported using factors arising from the health care system, in conjunction with clinical evidence, to make coverage policy decisions. Payers stated that these factors helped them overcome the uncertainties caused by a lack of clinical effectiveness evidence. For Oncotype DX, payers reported that the following health care system factors informed their coverage decisions: patient and physician adoption, coverage by a local Medicare provider in California, endorsement of medical societies, and the fact that the test did not undergo the FDA approval process (in contrast, MammaPrint received FDA approval). Table 2 provides examples of the health care factors payers considered.
Table 2.
Health Care System Factors Considered by Payers to Inform Coverage Policy
| Factor | Effect | Payer Comments |
|---|---|---|
| Patient and provider adoption | Patients and/or providers start to ask for or use test and file claims for test; prompts closer test review for coverage | “There was demand for the test and that did influence us . . . . If there is pressure from the community and providers, we will do a review sooner. The other thing that influenced us was the test was ordered, and the patient didn't have much say in it, and if we don't approve that test, the member is suddenly left with a $3,000 bill, on top of dealing with cancer. That put an influence in terms of not necessarily making a decision to cover, but making a decision: Do we need to review the test?” |
| Coverage by local Medicare contractor in California | Decision creates status quo for other insurers; may tip decision toward coverage | “We reviewed the studies of clinical utility and said, 'There is clinical utility data on it, and Medicare covers it . . . .' So these two things ultimately played a role for us.” |
| Endorsement by medical societies | Inclusion in clinical guidelines suggests standard of care; tips decision toward coverage | “What we found with Oncotype was that it wasn't the new information that came out but rather a broadening sense of consensus about how it may be used in terms of patient preferences . . . . Our committee reviewed the data and reviewed the NCCN recommendations and largely reflected the use based on the NCCN recommendations with some minor changes.” |
| Regulation | Test is not FDA approved; potentially tips decision away from coverage; some insurers believe evidence would be better with FDA regulation | “It certainly gives a test some credibility that the evidence was re-looked at [by the FDA], although the FDA looks at safety and efficacy and not clinical utility.” |
Abbreviations: NCCN, National Comprehensive Cancer Network; FDA, US Food and Drug Administration.
Payer Perceptions of Evidence at Time of Policy Decisions
Although all payers used clinical evidence as a primary decision factor, they reported different interpretations of its strength. Payers described the clinical evidence on Oncotype Dx at the time of their decisions as reasonably persuasive (n = 2), evolving (n = 2), or insufficient (n = 2). Of interest, payers who issued decisions approximately at the same time varied in this assessment. One of the two payers granting coverage in early 2007 noted the evidence available then as sufficient, whereas another payer reported it as insufficient but still covered Oncotype DX based on other factors.
Similarly, payers perceived health care system factors differently. Three payers reported patient and provider adoption as an important factor, whereas the other three noted them as unimportant. Where adoption was a factor, payers reported that signs of broader adoption, such as increased number of claims, served as triggers for a closer policy review. One payer took into account medical society (ASCO and the National Comprehensive Cancer Network) recommendations, whereas the other five granted coverage before these recommendations. Only one payer reported the local Medicare coverage decision in California as a key factor. Payers were unconcerned that Oncotype DX did not go through the FDA approval process. However, some suggested that FDA review may have improved the evidence base for Oncotype DX. All payers stated explicitly that cost-effectiveness analyses do not influence coverage decisions and did not affect decisions for Oncotype DX.
Oncotype DX Policy Features
Our review found salient differences among Oncotype DX coverage policy features for interviewed payers. They varied in whether prior authorization was required, attestation that there were no predetermined factors for chemotherapy, attestation that a discussion about use of test results was held with patient, requirement that surgery and pathology were completed before test order, requirement that the test be ordered by the physician administering chemotherapy, and retrospective review by the payer of chemotherapy use based on Oncotype DX results.
The payers stated that their coverage policy features for Oncotype DX reflected the clinical evidence. Payers varied in their specific concerns about the appropriate use of the test. Two payers felt that the available evidence was sufficient and did not have concerns about improper test use. These payers did not have prior authorization policies in place. The other payers were concerned about inappropriate test ordering and not using test results in decisions; these payers developed policies to mitigate these concerns.
Discussion
Decisions Varied in Timing and Structure Despite Widespread Coverage
Personalized medicine is changing clinical practice, and payers are being challenged to develop strategies to manage technologies emerging in this field. This study suggests that major US private payers are able to develop policies for new technologies like Oncotype Dx. However, the timing and structure of policies differ among payers. Some private payers developed Oncotype Dx policies earlier than their counterparts, and some payers have stricter utilization policies. Whether policy variation is warranted is not elucidated by this study. Elsewhere, studies have examined the variability among health plan coverage policies. Steiner et al29 found variability in health plan coverage for laser therapies, indicating that variation in policy led to variation in patient access to these therapies. Klabunde et al30 examined variation in colorectal screening policies, suggesting that they may be a factor in colorectal cancer screening rates. We suggest that additional studies on variability among coverage policies for cutting-edge technologies such as personalized medicine will help explain and potentially mitigate the impact of policy variation on physicians and patients.
Different Perceptions of Clinical Evidence and Application of Health Care System Factors May Produce Coverage Variation
Our research suggests that coverage decisions are informed by both clinical evidence and health care system factors, and when clinical evidence is less certain, other factors may play more important roles. For example, one payer believed that the clinical evidence for Oncotype DX was weak but granted coverage based on its adoption by oncologists, which indicated to the payer that Oncotype DX was becoming a standard of care. We identify only the health care system factor categories that influenced decisions, but we did not examine how to measure their contribution. Research has similarly suggested that in addition to clinical evidence, other factors inform coverage decisions. Steiner et al29 found that for laser therapy coverage decisions, payers considered competition factors (ie, coverage provided by other payers), legal factors (eg, whether denial of coverage could be legally challenged), and economic and other factors (eg, severity of condition).22 Meckley et al31 suggested that clinical society recommendations strongly influence reimbursement of personalized medicine technologies and—as found in our study—that cost effectiveness and type of regulatory oversight do not. Future research might continue to examine how health care system factors are used in decisions. We suggest that improving both the clinical evidence and our understanding of how health care system factors are applied by payers is important.
Policy Development and Clinical Practice
Our findings suggest that in the case of novel technologies, not only policy features but also timing of coverage by various payers may have implications for clinical practice. For oncology practices that accept multiple insurance plans, variation may be particularly challenging. In the case of Oncotype Dx, private payers in this study implemented coverage differently over a 4-year span, which potentially created challenges in use of Oncotype Dx in practice. However, payers also described that clinical practice similarly affects policy development for new technologies via the level of adoption by clinicians and patients, which can trigger a technology review or policy development. Additional research of the mutual impacts of policy development and clinical practice may be important as more technologies enter the oncology market.
This report describes how payers are developing strategies for coverage policy decisions for personalized medicine, a field often characterized by promising interventions with uncertain clinical evidence and high cost. In making coverage policy decisions, a key approach for payers seems to be the integration of health care system factors and clinical evidence. Our study found that coverage policies vary by payer organization, suggesting that variation may be a result of both type of evidence used and perceptions of that evidence. Future studies should elucidate more specifically what factors contribute to policy decisions when clinical evidence is uncertain and should examine the implications of policy variation for clinical use of novel technologies.
Acknowledgment
We thank our interviewees, including Joanne Armstrong (Aetna), Lee Newcomer (UnitedHealth Group), Louis Hochheiser (Humana), Alan Rosenberg (WellPoint), Jed Weissberg (Kaiser Permanente), and others. Supported by Grants No. R01CA101849 and P01CA130818 (K.A.P.) from the National Cancer Institute, Bethesda, MD, and an unrestricted grant from the Blue Shield of California Foundation, San Francisco, CA. The funding organizations and sponsors had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or approval of the manuscript. The views presented are solely the opinions of the authors.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Julia R. Trosman, Genomic Health Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Julia R. Trosman, Stephanie L. Van Bebber, Kathryn A. Phillips
Financial support: Julia R. Trosman, Kathryn A. Phillips
Administrative support: Kathryn A. Phillips
Collection and assembly of data: Julia R. Trosman, Stephanie L. Van Bebber
Data analysis and interpretation: Julia R. Trosman, Stephanie L. Van Bebber
Manuscript writing: Julia R. Trosman, Stephanie L. Van Bebber, Kathryn A. Phillips
Final approval of manuscript: Julia R. Trosman, Stephanie L. Van Bebber, Kathryn A. Phillips
References
- 1.President's Council of Advisors on Science and Technology. Priorities for Personalized Medicine. 2008. http://www.whitehouse.gov/files/documents/ostp/PCAST/pcast_report_v2.pdf.
- 2.Aspinall MG, Hamermesh RG. Realizing the promise of personalized medicine. Harv Bus Rev. 2007;85:108–117. [PubMed] [Google Scholar]
- 3.Phillips KA. Closing the evidence gap in the use of emerging testing technologies in clinical practice. JAMA. 2008;300:2542–2544. doi: 10.1001/jama.2008.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury MJ, Berg A, Coates R, et al. The evidence dilemma in genomic medicine. Health Aff (Millwood) 2008;27:1600–1611. doi: 10.1377/hlthaff.27.6.1600. [DOI] [PubMed] [Google Scholar]
- 5.Frueh FW. Real-world clinical effectiveness, regulatory transparency and payer coverage: Three ingredients for translating pharmacogenomics into clinical practice. Pharmacogenomics. 2010;11:657–660. doi: 10.2217/pgs.10.46. [DOI] [PubMed] [Google Scholar]
- 6.Newcomer LN. Insurers and ‘targeted biologics’ for cancer: A conversation with Lee N. Newcomer—Interview by Barbara J. Culliton. Health Aff (Millwood) 2008;27:w41–w51. doi: 10.1377/hlthaff.27.1.w41. [DOI] [PubMed] [Google Scholar]
- 7.Ginsburg GS, Willard HF. Genomic and personalized medicine: Foundations and applications. Transl Res. 2009;154:277–287. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Mossavar-Rhamani Center for Business and Government, John F. Kennedy School of Government, Harvard University. Health Care Delivery Covered Lives: Summary of Findings. 2007. http://www.hks.harvard.edu/m-rcbg/hcdp/numbers/Covered%20Lives%20Summary.pdf.
- 9.Department of Health and Human Services. Coverage and Reimbursement of Genetic Tests and Services: Report of the Secretary's Advisory Committee on Genetics, Health, and Society. 2006. http://oba.od.nih.gov/oba/sacghs/reports/CR_report.pdf.
- 10.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 11.Chalkidou K, Lord J, Fischer A, et al. Evidence-based decision making: When should we wait for more information? Health Aff (Millwood) 2008;27:1642–1653. doi: 10.1377/hlthaff.27.6.1642. [DOI] [PubMed] [Google Scholar]
- 12.Deverka PA. Pharmacogenomics, evidence, and the role of payers. Public Health Genomics. 2009;12:149–157. doi: 10.1159/000189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchionni L, Wilson RF, Wolff AC, et al. Systematic review: Gene expression profiling assays in early-stage breast cancer. Ann Intern Med. 2008;148:358–369. doi: 10.7326/0003-4819-148-5-200803040-00208. [DOI] [PubMed] [Google Scholar]
- 14.Recommendations from the EGAPP Working Group: Can tumor gene expression profiling improve outcomes in patients with breast cancer? Genet Med. 2009;11:66–73. doi: 10.1097/GIM.0b013e3181928f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute. The TAILORx breast cancer trial: TAILORx—Testing personalized treatment for breast cancer. http://www.cancer.gov/clinicaltrials/digestpage/TAILORx.
- 16.Atlantic Information Services. Health plan enrollment: Market share data—Top 25 US health plans, ranked by total medical enrollment. http://www.aishealth.com/MarketData/MCEnrollment/MCEnrol_mc01.html.
- 17.Womack C. Medicare contractor to reimburse Genomic Health's Oncotype Dx: Step toward broader PGx coverage? Pharmacogenomics Reporter. 2006. http://www.genomeweb.com/dxpgx/medicare-contractor-reimburse-genomic-health-s-oncotype-dx-step-toward-broader-p.
- 18.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 19.Genomic Health. Genomic Health reports recommendation of Oncotype DX in National Comprehensive Cancer Network Guidelines. 2008. http://investor.genomichealth.com/ReleaseDetail.cfm?ReleaseID=284249.
- 20.Genomic Health: Kaiser Permanente press release. Kaiser Permanente research—Study of early stage breast cancer patients identifies women at low risk of breast cancer mortality. 2004. http://investor.genomichealth.com/ReleaseDetail.cfm?ReleaseID=172514.
- 21.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 22.Genomic Health. Multiple studies presented at the American Society of Clinical Oncology meeting show consistent positive results for the Oncotype DX breast cancer assay. 2005. http://investor.genomichealth.com/ReleaseDetail.cfm?ReleaseID=172510.
- 23.Genomic Health. Genomic Health announces study results demonstrating Oncotype DX changed adjuvant treatment approach for 25 percent of early-stage breast cancer patients. 2005. http://investor.genomichealth.com/ReleaseDetail.cfm?=181485.
- 24.Genomic Health. Genomic Health announces positive results from first study using Oncotype DX in node-positive breast cancer patients. 2007. http://investor.genomichealth.com/ReleaseDetail.cfm?ReleaseID=246852.
- 25.Kamal AH, Loprinzi CL, Reynolds C, et al. How well do standard prognostic criteria predict oncotype DX (ODX) scores? J Clin Oncol. 2007;25(suppl):21s. abstr 576. [Google Scholar]
- 26.Genomic Health. Genomic Health announces Oncotype DX recurrence score influences treatment decisions for physicians and patients in prospective study. 2007. http://investor.genomichealth.com/ReleaseDetail.cfm?ReleaseID=246832.
- 27.Lo SS, Norton J, Mumby PB, et al. Prospective multicenter study of the impact of the 21-gene recurrence score (RS) assay on medical oncologist (MO) and patient (pt) adjuvant breast cancer (BC) treatment selection. J Clin Oncol. 2007;25(suppl):22s. doi: 10.1200/JCO.2008.20.2119. abstr 577. [DOI] [PubMed] [Google Scholar]
- 28.Oratz R, Paul D, Cohn AL, et al. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract. 2007;3:182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner CA, Powe NR, Anderson GF, et al. Technology coverage decisions by health care plans and considerations by medical directors. Med Care. 1997;35:472–489. doi: 10.1097/00005650-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, Riley GF, Mandelson MT, et al. Health plan policies and programs for colorectal cancer screening: A national profile. Am J Manag Care. 2004;10:273–279. [PubMed] [Google Scholar]
- 31.Meckley LM, Neumann PJ. Personalized medicine: Factors influencing reimbursement. Health Policy. 2010;94:91–100. doi: 10.1016/j.healthpol.2009.09.006. [DOI] [PubMed] [Google Scholar]