Abstract
Purpose
Women with BRCA1 and BRCA2 mutations have an elevated risk of breast cancer and ovarian cancer, but also of developing second primary breast cancer. BRCA1/2 mutation carriers with breast cancer must choose between breast conservation (BCT) and mastectomy (M) yet data on outcomes are limited. The purpose of this study is to compare BCT to M in BRCA1/2 carriers.
Methods
655 women with BRCA1/2 mutations diagnosed with breast cancer and treated with BCT (n=302) or M (n=353) were identified and underwent follow up to assess local, regional and systemic recurrence.
Results
Local failure as first failure was significantly more likely in those treated with BCT compared to M, with a cumulative estimated risk of 23.5% vs. 5.5%, respectively, at 15 years (p<0.0001); 15-year estimates in carriers treated with BCT and chemotherapy was 11.9% (p=0.08 when compared to M). Most events appeared to be second primary cancers rather than failure to control the primary tumor. The risk of contralateral breast cancer was high in all groups, exceeding 40%, but was not statistically significantly different by use of adjuvant radiotherapy (RT) or not, suggesting no added risk from scatter RT at 10 and 15 years. There were no differences seen in regional or systemic recurrences between the BCT and M groups, and no difference in overall survival.
Conclusions
BRCA1/2 mutation carriers with breast cancer have similar survivals whether treated with M or BCT. However, women undergoing BCT have an elevated risk of a second in-breast event that is significantly reduced in the presence of chemotherapy. Contralateral breast cancer events are very common.
Keywords: hereditary breast cancer, BRCA1/2, breast conservation, mastectomy, radiotherapy
Introduction
Women with inherited germline BRCA1 or BRCA2 (BRCA1/2) mutations have an approximate 55%–85% cumulative risk of developing breast cancer by age 70 [1,2] Thus, knowledge of expected outcomes for BRCA1/2 mutation carriers following various breast cancer treatments is needed. Breast-conserving therapy (BCT), defined as surgical excision and radiotherapy (RT), has been shown in multiple trials to result in comparable rates of tumor control and survival as mastectomy (M) in women with sporadic early stage disease [3,4]. Only limited data exist comparing outcomes following BCT versus M exclusively in women with known BRCA1/2 mutations. Estimates of local recurrence and contralateral new breast cancers following BCT compared with M could aid in surgical decision-making in a BRCA1/2 mutation carrier newly diagnosed with breast cancer. Here we compare the clinical outcomes in women with BRCA1/2 mutations treated with BCT to outcomes in similarly staged BRCA1/2 mutation carriers treated with M.
Methods
Study Design
Participants were women with a BRCA1 or BRCA2 mutation and stages I–III breast cancer treated with either BCT (breast conserving surgery and breast RT) or M and who had consented to institutional review board (IRB) approved longitudinal studies. They included women evaluated and followed in high-risk and radiation oncology clinics of 9 institutions located in the United States, Spain and Israel, and Australian and New Zealand women enrolled in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) [5]. All BRCA1/2 mutations were considered deleterious based on generally recognized criteria. Women with sequence variants of uncertain significance in BRCA1 or BRCA2 were excluded from the data set. Patients were included if there was detailed pathology and treatment records, with at least 6 months of clinical follow-up after breast cancer diagnosis. All patients were ascertained from kConFab or clinic databases at the time of this study regardless of recurrence or survival status. When the institutional databases were queried, 655 cases met eligibility criteria: 302 treated with BCT and 353 treated with M (241 without post-mastectomy RT, 103 with post-mastectomy RT, and 9 for which RT information was unknown). Clinical data were abstracted from medical records or (for kConFab participants) self-reported using a validated questionnaire [6]. Data from the contributing institutions was entered into a centralized database at the University of Michigan. Unique identifiers were assigned to maximize confidentiality per IRB compliance guidelines.
Statistical Methods
Patient, clinical and treatment characteristics were compared by local therapy. Statistical comparisons were made using the chi-square test statistic for categorical characteristics and Student's t-test for continuous characteristics.
Time-to-event endpoints included first failure of treatment, diagnosis of contralateral breast cancer (CBC), and overall and breast cancer-specific survival. A local failure as first failure included all breast/chest wall cancer recurrences whether locally isolated or concurrent with regional or distant failure. Regional failures consisted of a recurrence in the axillary, supraclavicular, infraclavicular, or internal mammary regions.
Overall survival estimates were calculated using the product-limit method of Kaplan and Meier [7]. Rates of first failure, CBC development, and breast cancer-specific survival were estimated using the cumulative incidence method, in order to appropriately account for competing events [8]. Asymptotic point-wise standard error estimates for the cumulative incidence estimator were calculated by the method of Pepe [9].
Cox regression models were constructed to detect significant associations between the patient, clinical, and treatment characteristics with local and distant first failure, overall and breast cancer-specific survival, and development of CBC. The time-to-event endpoints were censored at the time of competing events, if present. The following characteristics were included in analyses for local and distant first failure, and overall and breast cancer specific survival: age at diagnosis, menopausal status at diagnosis, BRCA mutation (1 or 2), breast cancer histology, T-stage, estrogen and progesterone receptor status, number of axillary lymph nodes removed, number of positive axillary nodes, use of chemotherapy, use of hormone therapy, and whether or not RT was a component of adjuvant therapy for M patients. Several clinical/treatment characteristics were modeled in a time-dependent fashion, and their inclusion depended upon the time-to-event outcome modeled. Those characteristics were development of ovarian cancer and whether patients underwent a prophylactic bilateral oophorectomy and/or a prophylactic contralateral M. For local first failure, time to prophylactic bilateral oophorectomy was modeled in a time-dependent fashion. For survival endpoints, time to bilateral oophorectomy, time to CBC, and time to ovarian cancer were modeled. For analysis of contralateral events, synchronous contralateral cases (within 180 days of the primary cases) were excluded (12 cases). Thus, the analyzable sample for CBC development was 643 cases.
P-values are reported for univariate associations and for each covariate when adjusted for all other covariates in full multivariable models. Hazard ratios are reported with 95% confidence intervals for significantly associated covariates.
Results
Study Populations
Comparisons between BCT (n=302) and M (n=353) cohorts by patient, tumor and treatment characteristics are shown in Table 1. A similar comparison was performed for the 353 M patients treated with (103) and without (250) post-mastectomy RT. Factors that differed significantly are shown in Table 2. Additional analyses by mutation status demonstrated that when compared with BRCA2 mutation carriers, BRCA1 mutation carriers were more likely to be pre-menopausal (85.3% vs. 68.6%, p=0.002); have estrogen receptor (ER) negative cancers (79.9% vs. 29.5%, p<0.0001); and were less likely to receive hormone therapy (no hormone therapy 77.2% vs. 47.6%, p<0.0001). BRCA2 cancers more commonly had positive axillary nodes (BRCA2: 36.1% vs. BRCA1: 22.4%, p=0.06).
Table 1.
Patient, tumor and treatment characteristics by surgery type
| Characteristic | Lumpectomy | Mastectomy |
|---|---|---|
| Frequency, n | 302 | 353 |
| Patient age at biopsy, years (P = 0.13) | ||
| Mean (Std. Dev.) | 41.9 (9.4) | 43.0 (9.8) |
| Median | 40.5 | 41.9 |
| Minimum – Maximum | 20.1 – 85.0 | 23.6 – 81.5 |
| Patient age at biopsy, n (%) (P = 0.35) | ||
| ≤ 35 | 74 (24.5) | 71 (20.1) |
| 36 – 50 | 179 (59.3) | 216 (61.2) |
| ≥ 50 | 49 (16.2) | 66 (18.7) |
| Race (P = 0.14) | ||
| White | 278 (92.1) | 312 (88.4) |
| Black | 10 (3.3) | 9 (2.6) |
| Hispanic | 9 (3.0) | 17 (4.8) |
| Other/Unknown | 5 (1.7) | 15 (4.3) |
| Menopausal status at primary (P = 0.003) | ||
| Pre- | 240 (79.5) | 240 (68.0) |
| Post- | 52 (17.2) | 89 (25.2) |
| Peri- | 10 (3.3) | 24 (6.8) |
| BRCA gene mutation (P = 0.01) | ||
| 1 | 197 (65.2) | 197 (55.8) |
| 2 | 105 (34.8) | 156 (44.2) |
| Histology (P = 0.07) | ||
| Infiltrating ductal | 258 (85.4) | 292 (82.7) |
| Lobular or Infiltrating ductal & lobular | 10 (3.3) | 26 (7.4) |
| Medullary or other | 34 (11.2) | 35 (9.9) |
| Clinical stage (P = 0.0007)a | ||
| 1 | 158 (52.3) | 145 (41.1) |
| 2 | 130 (43.1) | 170 (48.2) |
| 3 | 12 (4.0) | 37 (10.5) |
| Unknown | 2 (0.7) | 1 (0.3) |
| Pathologic T-stage (P = 0.001) | ||
| T0/T1 | 214 (70.8) | 203 (57.5) |
| T2 | 81 (26.8) | 125 (35.4) |
| T3 | 4 (1.3) | 16 (4.5) |
| Unknown | 3 (1.0) | 9 (2.6) |
| Estrogen receptor (P = 0.006)a | ||
| Positive | 90 (29.8) | 126 (35.7) |
| Negative | 154 (51.0) | 131 (37.1) |
| Borderline | 3 (1.0) | 3 (0.9) |
| Unknown | 54 (17.9) | 93 (26.4) |
| Progesterone receptor (P = 0.06)a | ||
| Positive | 73 (24.2) | 98 (27.8) |
| Negative | 154 (51.0) | 143 (40.5) |
| Borderline | 3 (1.0) | 0 |
| Unknown | 72 (23.8) | 112 (31.7) |
| Final microscopic surgical margins (P = 0.003)a | ||
| Positive | 16 (5.3) | 4 (1.1) |
| Negative | 248 (82.1) | 272 (77.1) |
| Close | 0 | 4 (1.1) |
| Unknown | 38 (12.6) | 73 (20.7) |
| Nodal surgery | ||
| Yes | 292 (96.7) | 350 (99.2) |
| Total lymph nodes removed (P = 0.95) | ||
| Mean (Std. Dev.) | 14.8 (8.5) | 14.7 (8.5) |
| Median | 14 | 14 |
| Minimum – Maximum | 1 – 48 | 1 – 51 |
| Positive lymph nodes removed (P = 0.004) | ||
| 0 | 210 (71.9) | 223 (63.4) |
| 1 – 3 | 62 (21.2) | 76 (21.6) |
| 4+ | 20 (6.9) | 53 (15.1) |
| No | 10 (3.3) | 1 (0.3) |
| Unknown | 0 | 2 (0.6) |
| Radiotherapy | ||
| No | 0 | 241 (68.3) |
| Yes | 302 (100) | 103 (29.2) |
| Unknown | 0 | 9 (2.6) |
| Chemotherapy (P = 0.20)a | ||
| No | 82 (27.2) | 108 (30.6) |
| Yes | 219 (72.5) | 231 (65.4) |
| Unknown | 1 (0.3) | 14 (4.0) |
| Hormone therapy (P = 0.09)a | ||
| No | 202 (66.9) | 210 (59.5) |
| Yes | 90 (29.8) | 125 (35.4) |
| Tamoxifen | 81 (90.0) | 106 (84.8) |
| Other | 9 (10.0) | 19 (15.2) |
| Unknown | 10 (3.3) | 18 (5.1) |
| Adjuvant therapy (P = 0.35) | ||
| Yes | 254 (84.1) | 287 (81.3) |
| No | 48 (15.9) | 66 (18.7) |
| Bilateral oophorectomy (P = 0.28)a | ||
| No | 141 (46.7) | 150 (42.5) |
| Yes | 161 (53.3) | 203 (57.5) |
| Prior to primary diagnosis | 9 (3.0) | 30 (8.5) |
| After diagnosis, prior to failure | 16 (5.3) | 10 (2.8) |
| After diagnosis, after failure | 12 (4.0) | 9 (2.6) |
| After diagnosis, without failure | 114 (38.0) | 147 (41.6) |
| Timing unknown | 10 (3.3) | 7 (2.0) |
| Prophylactic contralateral mastectomy (P < 0.0001)a | ||
| No | 256 (84.8) | 214 (60.6) |
| Yes | 44 (14.6) | 134 (38.0) |
| Unknown | 2 (0.6) | 5 (1.4) |
Comparison between surgical groups made omitting the unknown/missing category.
Table 2.
Patient, tumor and treatment characteristics for mastectomy patients with and without radiotherapy
| Characteristic | (+) Radiotherapy | (−) Radiotherapy |
|---|---|---|
| Frequency, n | 103 | 250 |
| BRCA gene mutation (P = 0.025) | ||
| 1 | 48 (46.6) | 149 (59.6) |
| 2 | 66 (53.4) | 101 (40.4) |
| Clinical stage (P < 0.0001)a | ||
| 1 | 16 (15.5) | 129 (51.6) |
| 2 | 55 (53.4) | 115 (46.0) |
| 3 | 31 (30.1) | 6 (2.4) |
| Unknown | 1 (1.0) | 0 |
| T-stage (P = 0.0001) | ||
| T0/T1 | 43 (41.8) | 160 (64.0) |
| T2 | 42 (40.8) | 83 (33.2) |
| T3 | 15 (14.6) | 1 (0.4) |
| T unknown | 3 (2.9) | 6 (2.4) |
| Nodal surgery | ||
| Total lymph nodes removed (P = 0.004) | ||
| Mean (Std. Dev.) | 16.9 (8.9) | 13.8 (8.1) |
| Median | 15 | 14 |
| Minimum-Maximum | 5–51 | 1–40 |
| Positive lymph nodes removed (P < 0.0001) | ||
| 0 | 33 (32.0) | 190 (76.3) |
| 1–3 | 30 (29.1) | 46 (18.5) |
| 4+ | 40 (38.8) | 13 (5.2) |
| Chemotherapy (P = 0.02)a | ||
| No | 23 (22.3) | 85 (34.0) |
| Yes | 79 (76.7) | 152 (60.8) |
| Unknown | 1 (1.0) | 13 (5.2) |
| Prophylactic contralateral mastectomy (P = 0.01)a | ||
| No | 71 (68.9) | 143 (57.2) |
| Yes | 28 (27.2) | 106 (42.4) |
| Unknown | 4 (3.9) | 1 (0.4) |
Comparison between surgical groups made omitting the unknown/missing category
Patterns of Recurrence
Local and regional failures
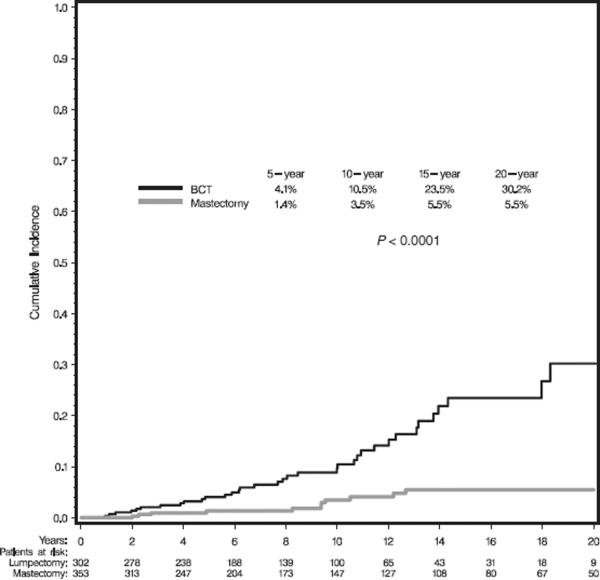
With a median follow-up of 8.2 and 8.9 years for BCT and M patients, respectively, the cumulative incidence estimates of local failure as first failure were significantly greater following BCT than M (p<0.0001), Figure 1. Despite higher stage breast cancers among M patients who received post-M RT compared to those who did not (as shown in Table 2), local recurrence rates were similar due to RT treatment effect (data not shown). Median time to local failure was 7.8 years for BCT patients and 9.4 years for M.
Figure 1.
Cumulative incidence estimates for local failure as first failure by type of local treatment.
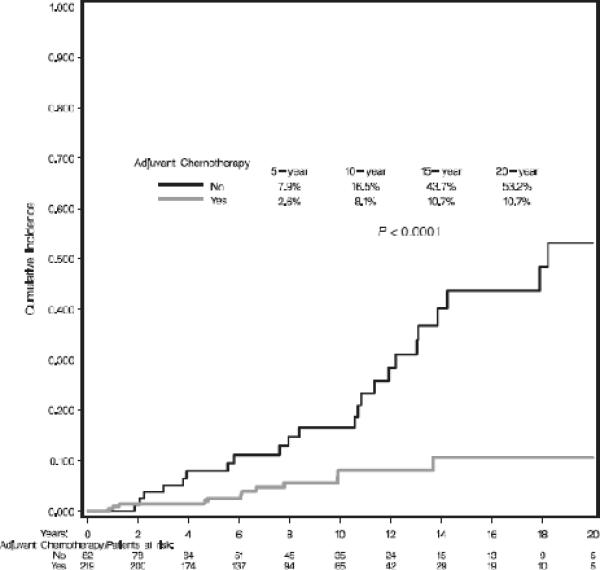
Univariate and multivariate analyses for each patient, tumor and treatment characteristic were performed for the total sample and within BCT and M groups. The results of the multivariate analyses are shown in Table 3. Type of local therapy was the only factor significantly predicting local recurrence within the entire cohort. Multivariate analyses within treatment cohort revealed type of gene mutation and not receiving adjuvant chemotherapy (Figure 2) to be independent predictors of recurrence among patients treated with BCT. Rates of local failure were compared between the 219 patients treated with BCT who received chemotherapy and the entire cohort of 353 women treated with mastectomy. Although more local failures were observed in women treated with BCT and chemotherapy compared to mastectomy, the results did not significantly differ (ie, 8.1% vs. 3.5% at 10 years; 10.7% vs. 5.5% at 15 years, respectively; p=0.08). Analyses within the BRCA1 and BRCA2 BCT subgroups suggested a reduction in recurrence with use of hormonal therapy particularly in BRCA2 carriers (p=0.08 for BRCA2 and p=0.13 for BRCA1). Oophorectomy did not significantly impact rates of local failure among BCT patients (total BCT cohort: HR=0.88, p=0.75; BRCA1 subset: HR=1.63, p=0.27; BRCA2 subset: HR=0.20; p=0.125). Among patients treated with M, the presence of invasive lobular cancer was the only factor significantly associated with local failure.
Table 3.
Significant multivariate hazard ratios for local failure as first failure
| Sample/Characteristic | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Total sample (N = 655) | ||
| Treatment decision | ||
| BCT | 4.5 (2.3–8.9) | <0.0001 |
| Mastectomy | 1.0 | |
| BCT sample(N = 302) | ||
| Gene mutation | ||
| BRCA1 | 1.00 | |
| BRCA2 | 2.9 (1.2 – 7.1) | 0.019 |
| Adjuvant chemotherapy | ||
| Yes | 1.0 | |
| No | 5.4 (2.3 – 13.3) | 0.0001 |
| Mastectomy sample (N = 353) | ||
| Histology | ||
| Infiltrating ductal carcinoma (IDC) | 1.0 | |
| IDC + lobular/Lobular carcinoma | 9.9 (2.1 – 47.1) | 0.003 |
| Medullary/Other | 2.7 (0.4 – 17.3) | 0.289 |
Figure 2.
Cumulative incidence estimates for local failure as first failure for patients choosing breast conservation by use of adjuvant chemotherapy.
For the 35 patients treated with BCT who experienced an in-breast tumor recurrence (IBTR), information regarding location of the lesion in the breast and histology of the initial cancer and subsequent failure was available in 23 cases. Seven of 23 recurrences (30%) were in the same quadrant and of the same histology as the initial cancer, consistent with a true recurrence. The remaining 16 (70%) cases developed recurrences either in a different quadrant from the primary, were of a different histology, or both. For the 11 patients treated with M who had a local isolated first failure, 2 of 11 (18%) failures were a different histology when compared to the initial tumor histology; the remaining 9 (82%) patients had similar histologies at diagnosis and recurrence.
The incidence estimates of regional failures as component of first failure did not vary significantly by local treatment group (data not shown).
Distant failures
The cumulative incidence estimates of distant failure as first failure did not differ significantly by type of local therapy, with 10- and 15-year rates of failure in BCT patients of 7.1% and 11.1%, and in M patients 7.4% and 9.1% (p=0.80). Factors significantly impacting distant failure included a BRCA2 mutation (BRCA 2 vs. BRCA 1: HR 1.9, p=0.02); histology (invasive lobular vs. ductal cancer: HR 3.2, p=0.009) and positive axillary nodes (4 or more positive vs. 0: HR 2.6, p=0.006). Significant factors on multivariate analysis were the presence of an invasive lobular component (HR 3.1; p=0.01) and a BRCA2 mutation (HR1.9; p=0.05).
Contralateral Breast Cancers
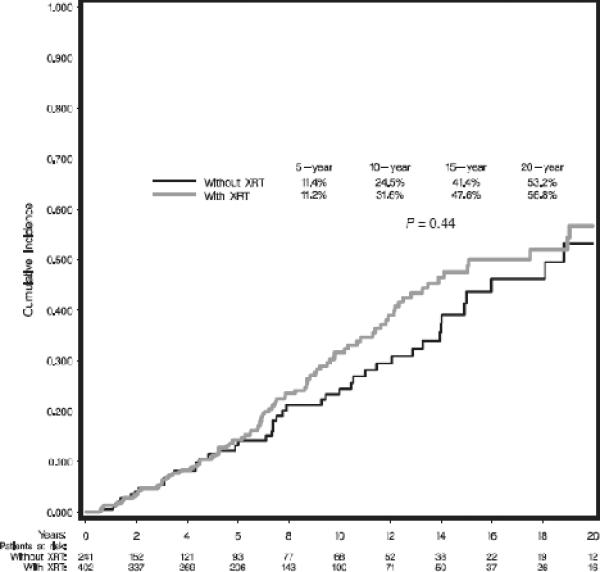
Patients who presented with synchronous bilateral breast cancers were excluded from this analysis for CBC, reducing the analytical sample size to 643 of which 148 women (23.0%) developed a CBC. The cumulative incidence estimates for developing CBC were analyzed by the use of adjuvant RT; results did not demonstrate a significant difference, as shown in Figure 3 (p=0.44), suggesting no increase in CBC from scatter RT. Analysis by local treatment (BCT, M without RT, M with RT) also did not demonstrate significant differences (data not shown).
Figure 3.
Cumulative incidence estimates of contralateral breast cancer by use of adjuvant radiotherapy.
The presence of a BRCA1 compared to BRCA2 mutation was associated with a 1.8-fold increase in CBC (p=0.003). Young age at diagnosis was also associated with increased risk, with patients ≤35 years having a 1.8-fold increased risk relative to women 36–50 years of age (p=0.04); women over 50 years did not significantly differ in rates of CBC compared to women 36–50 years old (HR=1.2, p=0.46). Upon multivariate analysis, no factors emerged as independent predictors for developing a CBC.
Breast Cancer-Specific Survival and Overall Survival
No significant difference in breast-cancer specific or overall survival was observed by local treatment type. Breast cancer-specific survivals with BCT were 93.6% and 91.7% at 10 and 15 years vs. 93.5% and 92.8% with M (p=0.85). Overall survivals with BCT group were 92.1% and 87.3% at 10 and 15 years and 91.8% and 89.8% with M (p=0.73). Factors significantly associated with breast cancer-specific mortality were presence of an infiltrating lobular cancer (HR 4.3; p=0.01) and the development of a CBC (HR 2.5, p=0.02). For overall survival analyses, since mortality is known to be related to patient age, models were adjusted for patient age at time of diagnosis. The only factor significantly related to increased rates of death on multivariate analysis was the development of ovarian cancer (HR 5.0, p=0.0001).
Discussion
The overall results demonstrated a significantly increased risk of local failure in BRCA1/2 mutation carriers treated with BCT compared to carriers treated with M; however, breast cancer-specific and overall survival were comparable. The significant differences in rates of local failure by local therapy sharply contrast with the similar rates reported in multiple randomized trials comparing BCT with M [3,4,10,11]. Given the low prevalence of BRCA1/2-associated breast cancer and the older median age of women in the randomized trials, these studies included predominantly women with sporadic rather than hereditary breast cancer.
As shown by others, the different location and histology in the majority of IBTR's compared to the original cancers and the increased median time to recurrence relative to sporadic breast cancers observed in the present study, is suggestive of the development of new breast cancers rather than true recurrences [12]. Of note, in patients treated with M, our results demonstrated a prolonged median time of 9.4 years to local failure considerably longer than the average of 2–3 years reported in most surgical series [13], also suggestive of the development of new cancers. Future studies using genetic profiling of the primary and secondary lesion are needed to definitively categorize these events.
Predictors of local failure varied between BCT and M. Chemotherapy was the only independent predictor in those treated with BCT, and the reduction was consistent with that observed in many BCT studies of sporadic breast cancer [14,15]. In fact, rates of local control at 10 and 15 years in carriers treated with BCT and chemotherapy were not significantly different from rates following mastectomy although a trend in second events was observed. These results may suggest an increased chemosensitivity in BRCA1/2-associated breast cancers as recently suggested by Fourquet and colleagues [16] and should be followed over time. Given the increasing usage of chemotherapy for invasive breast cancer, this reduction in breast events is potentially highly relevant for current practice. Our results also suggested a benefit in local control in BRCA2 carriers treated with hormonal therapy, consistent with other studies [17, 18].
Oophorectomy was not found to significantly impact rates of local failure in analyses among all patients treated with BCT or in subset analyses of those with positive estrogen or progesterone receptors or by mutation type. While the exact reason for this is uncertain, it is important to note that 73% of these women received chemotherapy, 30% received hormonal therapy and approximately 80% were premenopausal at diagnosis. Only 16% patients received no adjuvant therapy. Chemotherapy induces menopause in the majority of women over age 40 [19]. Thus, the lack of a discernable effect of oophorectomy in this study may reflect the fact that many of the patients were likely rendered post-menopausal with adjuvant therapy.
Earlier reports have shown variable rates of IBTR in BRCA1/2 mutation carriers compared to age-matched women with sporadic breast cancer treated with BCT [20–26]. In a previous paper by Pierce et al of 160 early stage BRCA1/2 carriers matched to 445 women with sporadic disease, rates of IBTR in the carriers at 10 and 15 years were 12% and 24% compared to 9% and 17% for controls (p=.19) [20]. The outcomes that differ between the previously published report and the current analysis are primarily the comparison groups and the rate of recurrence in these groups; the rates of IBTR are comparable between the two studies. There is some overlap of carriers between the previously published report and the current analysis; however, patient data has been updated and the overall BCT data set has expanded. Despite this, the rates of IBTR in the two papers are remarkably similar, with 10- and 15-year estimates in the current series of 10.5% and 23.5%.
Despite increased rates of local failure following BCT compared with M in the present report, no differences were observed in rates of distant failure or disease-specific or overall survival between the two cohorts. This is contradictory to recent prospective studies which strongly support a causal relationship between increased rates of local failure and subsequent distant spread [10]. While our findings may be a result of the limited sample size of the two cohorts, they may also be the result biologic differences of BRCA1/2 associated breast cancer. Local failure in women with sporadic breast cancer may generally represent a true local recurrence and a biologically more aggressive phenotype rather than the second primary cancers suggested in the current report. As mentioned above, BRCA1/2 related breast tumor may have enhanced sensitivity to chemotherapy 16. Furthermore, close follow-up of BRCA1/2 mutation carriers may have led to early detection and treatment of new cancers thus minimizing the opportunity for distant spread. Our results will need to be followed over time and validated in prospective studies, but at present, our data at 10 and 15 years do not suggest an adverse effect of a local recurrence/new primary upon systemic failure.
We acknowledge the limitations of the current study. Although the study consists of patients followed prospectively in high risk clinics, it is a predominantly prevalent rather than incident cohort which results in survivorship and ascertainment biases. Patients attended high risk clinics or were enrolled in kConFab sometimes many years after their first breast cancer diagnosis, so patients who died early from their breast cancer are less likely to be included in this study. Therefore, our estimates for overall and breast-cancer specific survival may be higher than for a population-based incident cohort of mutation carriers. Similarly, those with more than one breast cancer diagnosis may have been more likely to have been ascertained by kConFab and the high risk clinics. Thus, the frequency of second breast cancer events and CBC seen in this study may also overestimate the true rate that would be seen in a population-based incident cohort. Likewise, biases may be hidden within treatment choice (BCT versus M) that cannot be fully adjusted for by the available patient and tumor characteristics. However, it should be noted that patient data in both cohorts was acquired in the same manner, and that all data submitted from the collaborating institutions of patients who met the study criteria were included in the analysis. Ideally, these patient groups should be randomized to treatment (BCT versus M). However, given that the surgical decision-making process is highly personal and based upon complicated therapeutic concerns, it is doubtful whether such a study could be ethically performed.
In conclusion, our results demonstrate a significantly greater risk of local recurrence but no difference in distant failure or disease-specific or overall survival for patients with a deleterious BRCA1/2 mutation treated with BCT when compared to those treated with M. Within the BCT-treated group, use of adjuvant chemotherapy was significantly associated with reduced rates of IBTR. Among women who received adjuvant chemotherapy and BCT, local control did not significantly vary compared to control rates in women treated with mastectomy. These findings should be considered when patients with BRCA1/2-associated breast cancer are considering their local treatment options and for patients considering BCT, discussion of the role of chemotherapy not only for systemic control but also local control would be relevant. CBC was common in all patient subgroups whether treated with BCT or M. Thus, preventive strategies for the contralateral breast require strong consideration upon diagnosis of a BRCA1/2-associated breast cancer.
Acknowledgments
This research was supported by the Breast Cancer Research Foundation (LJP, SDM); the Colebatch Clinical Research Fellowship of the Cancer Council Victoria (KAP), the National Health and Medical Research Council (NHMRC) of Australia (#145684, 288704, 454508) and grants from the National Breast Cancer Foundation, the NHMRC and by the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia (kConFab); Susan G. Komen Breast Cancer Foundation (BGH); Dana Farber/Harvard Cancer Center SPORE in Breast Cancer (JEG); and Cancer Genetics Network (HHSN21620074400C) (SMD).
We thank follow-up study investigators, research nurses and staff at kConFab and all study sites, the heads and staff of the Australian and New Zealand Family Cancer Clinics, and the families who contribute to kConFab and to this international collaboration.
Footnotes
Presented, in part, at the 2010 European Breast Cancer Conference, Barcelona, Spain, March 2010
Conflict of Interest No authors have a conflict of interest with the content of this manuscript.
REFERENCES
- 1.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 2.Begg CB, Haile R, Borg A, et al. Variation of breast cancer risk amond BRCA1/2 carriers. JAMA. 2008;299:194–201. doi: 10.1001/jama.2007.55-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 5.Mann GJ, Thorne H, Balleine RM, et al. Analysis of cancer risk and BRCA1 and BRCA2 mutation prevalence in the kConFab familial breast cancer resource. Breast Cancer Research. 2006;8:R12. doi: 10.1186/bcr1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips KA, Milne RL, Buys S, et al. Agreement between self-reported breast cancer treatment and medical records in a population-based breast cancer family registry. J Clin Oncol. 2005;23:4679–86. doi: 10.1200/JCO.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JASA. 1958;53:457–481. [Google Scholar]
- 8.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representation of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Pepe M. Inference for events with dependent risks in multiple endpoints studies. JASA. 1991;86:770–778. [Google Scholar]
- 10.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GF, Veronesi U, Clough KB, et al. Cancer; consensus conference on breast conservation; Milan, Italy. April 28 to May 2, 2005; 2006. pp. 242–250. [DOI] [PubMed] [Google Scholar]
- 12.Turner BC, Harrold E, Matloff E, et al. BRCA1/BRCA2 germline mutations in locally recurrent breast cancer patients after lumpectomy and radiation therapy: Implications for breast-conserving management in patients with BRCA1/BRCA2 mutations. J Clin Oncol. 1999;17:3017–3024. doi: 10.1200/JCO.1999.17.10.3017. [DOI] [PubMed] [Google Scholar]
- 13.Donegan WL, Perez-Mesa CM, Watson FR. A biostatistical study of locally recurrent breast carcinoma. Surg Gynecol Obstet. 1966;122:529–540. [PubMed] [Google Scholar]
- 14.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–73. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–37. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 16.Fourquet A, Stoppa-Lyonnet D, Kirova YM, et al. Familial breast cancer: Clinical response to induction chemotherapy or radiotherapy related to BRCA1/2 mutations status. Am J Clin Oncol. 2009;32:127–131. doi: 10.1097/COC.0b013e31817f9e1c. [DOI] [PubMed] [Google Scholar]
- 17.Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter prospective study. J Clin Oncol. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–56. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 19.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–29. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 20.Pierce LJ, Levin AM, Rebbeck TR, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006;24:2437–2443. doi: 10.1200/JCO.2005.02.7888. [DOI] [PubMed] [Google Scholar]
- 21.Verhoog LC, Brekelmans CTM, Seynaeve C, et al. Survival and tumour characteristics of breast cancer patients with germline mutations of BRCA1. Lancet. 1998;351:316–321. doi: 10.1016/s0140-6736(97)07065-7. [DOI] [PubMed] [Google Scholar]
- 22.Eccles D, Simmonds P, Goddard J, et al. Familial breast cancer: An investigation into the outcome of treatment for early stage disease. Familial Cancer. 2001;1:65–72. doi: 10.1023/a:1013867917101. [DOI] [PubMed] [Google Scholar]
- 23.Robson M, Levin D, Federici M, et al. Breast conservation therapy for invasive breast cancer in Ashkenazi women with BRCA gene founder mutations. J Natl Cancer Inst. 1999;91:2112–2117. doi: 10.1093/jnci/91.24.2112. [DOI] [PubMed] [Google Scholar]
- 24.Haffty B, Harold E, Khan A, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002;359:1471–1477. doi: 10.1016/S0140-6736(02)08434-9. [DOI] [PubMed] [Google Scholar]
- 25.Kirova YM, Stoppa-Lyonnet D, Savignoni A, et al. Risk of breast cancer recurrence and contralateral breast cancer in relation to BRCA1 and BRCA2 mutation status following breast-conserving surgery and radiotherapy. Eur J Cancer. 2005;41:2304–2311. doi: 10.1016/j.ejca.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Etienne C, Barile M, Gentilini O, et al. Breast-conserving surgery in BRCA1/2 mutation carriers: Are we approaching an answer? Ann Surg Oncol. doi: 10.1245/s10434-009-0638-7. DOI 10.1245/s10434-009-0638-7. [DOI] [PubMed] [Google Scholar]
- 27.Oh JL, Bonnen M, Outlaw ED, et al. The impact of young age on locoregional recurrence after doxorubicin-based breast conservation therapy in patients 40 years old or younger: How young is “young”? Int J Radiat Oncol Biol Phys. 2006;65:1345–1352. doi: 10.1016/j.ijrobp.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 28.de Bock GH, van der Hage JA, Putter H, et al. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: Long-term results of European Organization for Research and Treatment of Cancer studies. Eur J Cancer. 2006;42:351–356. doi: 10.1016/j.ejca.2005.10.006. [DOI] [PubMed] [Google Scholar]