Abstract
We carried out a definition of the species group to which Conus praecellens A. Adams 1854 belongs using a combination of comparative morphological data, molecular phylogeny based on standard genetic markers and toxinological markers. Prior to this work, Conus praecellens was generally postulated to belong to a clade of similarly high-spired, smaller Conusspecies such as Conus pagodus Kiener, 1845, Conus memiae (Habe & Kosuge, 1970) and Conus arcuatus Broderip & Sowerby, 1829. The molecular phylogeny and toxinological data demonstrate that these prior hypotheses are incorrect, and that instead, Conus praecellens is in a branch of Conus that includes Conus stupa (Kuroda, 1956), Conus stupella (Kuroda, 1956), Conus acutangulus Lamark, 1810 and surprisingly, some species that are morphologically strikingly different, Conus mitratus Sowerby, 1870 and Conus cylindraceus Broderip & Sowerby, 1830. A more careful analysis of the morphologically diverse forms assigned to Conus praecellens suggests that from the Philippine material alone, there are at least three additional undescribed species, Conus andremenezi, Conus miniexcelsus and Conus rizali. A reevaluation of protoconch/early teleoconch morphology also strongly suggests that Conus excelsus Sowerby III, 1908 is related to these species. Together, the different data suggest a clade including the 10 species above that we designate, the Turriconus (Shikama and Habe, 1968) (clade; there are additional distinctive forms within the clade that may be separable at the species level. The phylogenetic definition using the multidisciplinary approach described herein provides a framework for comprehensively investigating biodiverse lineages of animals, such as the cone snails.
Introduction
The evolutionary histories of biodiverse Conus lineages are a challenge to elucidate. In part this is because the genus is so speciose (about 700 species) but also because most prior data in the literature is morphological. The usual approach is to characterize each species in the lineage based on its shell morphology and to evaluate phylogenetic relationships using additional anatomical data, when available.
Prior attempts to divide Conus into subgeneric groups have been based largely on shell morphology. In this work, we focus on one particular branch of Conusthat includes the species known as Conus praecellens. Several previous attempts to determine which species are most closely related to Conus praecellenshave grouped C.praecellenswith other high-spired forms (Fig. 1) that are collected in deep offshore locations. Some of the specific prior hypotheses that have been proposed are summarized in Table 1.
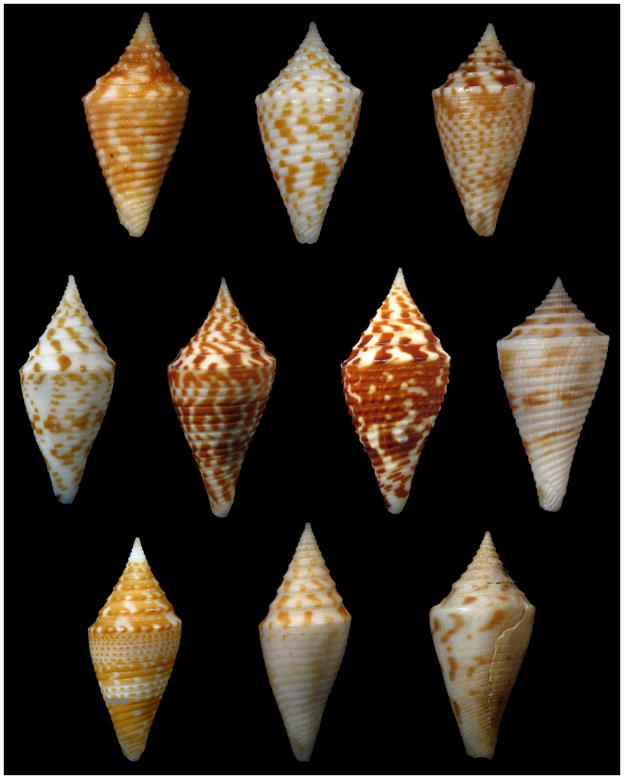
Figure 1.
High-spired Conusspecies previously postulated to be related to Conus praecellens : Top from left: Conus acutangulus , “typical form”, Conus acutangulus , “deep-water form”, Conus nereisMiddle from left: Conus praecellens , “Aliguay form”, Conus praecellens , “sowerbii form”, Conus andremenezi , new species, Conus pagodus , Bottom from left: Conus miniexcelsus , new species, Conus rizali , new species, Conus arcuatus. All of the specimens shown are from the Philippines, except for Conus arcuatus.
Table 1.
Previous Taxonomic Assignments for Conus praecellensand Conus acutangulus
| Marsh (1964): | DaMotta (1991): | Okutani (2000): |
|---|---|---|
| Conasprella | Conasprella | Conasprella |
| praecellens | praecellens | praecellens |
| pagodus | pagodus | pagodu |
| arcuatus | arcuatus | gracatapi |
| acutangulus | acutangulus | |
| wakayamaensis | ||
| Kermasprella | Endemnoconus | |
| acutangulsu | memiae | |
| memiae | wakayamaensis | |
| wakayamaensis | ione | |
| nereis | ||
| jaspideus | ||
In the most comprehensive modern treatise on IndoPacific Conusspecies (Röckel el al., 1995), Conus praecellensis regarded as most closely related to Conus acutangulus. In most of the schemes shown in Table 1 (Marsh, 1964; Okutani, 2000), Conus praecellensis grouped together with Conus acutangulusin the subgenus ConasprellaThiele, 1929. The designated type of Conasprellais “C. cancellatus ” (=C. pagodus ). Another species generally thought to belong to this subgenus is the Eastern Pacific Conus arcuatus. In one of the proposals (da Motta, 1991), Conus praecellensand Conus acutangulusare in two different subgeneric groups: Conus praecellensin Conasprellaand Conus acutangulusin Kermasprella Powell, 1958 (which this author regards as a subgenus of the genus Profundiconus Kuroda, 1956). Among the species included with Conus acutangulusin Kermasprellaare forms such as Conus memiae and Conus nereis(Petuch, 1979), the latter regarded by Röckel et al., 1995 as a form of Conus wakayamaensis(Kuroda, 1956). Thus, the high-spired Conusspecies including Conus praecellenswere either all grouped together in Conasprella, or were divided into a praecellens/pagodusgroup (Conasprella) and an acutangulus/memiae group (Kermasprella).
These suggestions based on shell morphology can be independently evaluated using molecular data. If morphology is used exclusively the traits may be subject to selection forces which do not reflect common descent. Distinguishing similarity by descent (reflecting the phylogeny) from similarity directed by selection (convergence or parallelism) is problematic without independent data corroborating the morphological evidence. Hence a widespread attempt to define biodiversity using molecular markers, notably a segment of the COI gene, has led to the “barcode initiative”. Although this initiative has been widely implemented, workers that need to identify field specimens require a more seamless integration of the molecular with the morphological data.
Our first goal is to define a phylogenetic tree with clades that reflect the branching pattern and in turn the evolutionary history of the species. Molecular data provides independent evidence for such a phylogeny and provides a useful organizing framework for an in-depth study of species-rich groups. The morphological traits mapped onto such a tree distinguish the respective roles of common descent and selection in the evolutionary process
In this work, we focus on the definition of the putative clade that includes Conus praecellens. Using 12SrRNA sequences, we specifically evaluate which Conus species are most closely related to Conus praecellens. In addition to using a molecular phylogeny to assess morphology-based taxonomy, we have gathered molecular data in the form of the genes that encode toxins expressed in the venom ducts of cone snails. As will be shown, these highly specialized “exogenes” (Olivera, 2006) are useful in defining discrete branches of a large biodiverse lineage such as the cone snails. This three-pronged approach defines a more complete picture of the evolutionary history of these biodiverse cone snails with the result that previous morphologically-informed hypotheses may be more objectively assessed.
Material and Methods
Specimen Collection
Most forms in the Conus praecellens complex are collected offshore from 30–250 meters in depth. The bulk of Philippine specimens in collections assigned to this species were collected (together with such species as Tibia fusus(Linnaeus, 1758) and Xenophora solaris(Kosuge & Nomoto, 1972) around 1960, primarily from a few classical fish trawler localities (Maqueda Bay in Samar Is, Tayabas Bay in Luzon); at the time, these were mostly identified as Conus sowerbii (see Reeve, 1849; Springsteen and Loebrera, 1986). Because other forms in the praecellens complex are mostly from even deeper water, these were less well represented in collections; most specimens available in museums are poorly preserved and/or dead-collected. However, the combination of gill net and hookah collections in the Cebu/Bohol area of the Central Philippines and intensive small trawl collections around the Island of Aliguay has increased accessibility to several forms in the Conus praecellens complex. Some of these are smaller specimens that were sparsely represented in earlier collections. A range of live-collected specimens with preserved protoconchs has become available, which has facilitated the reevaluation of the Conus praecellens species complex.
Phylogenetic Analysis
We aligned sequences using Clustal X (Larkin et al., 2007) and refined by eye using MacClade (Maddison and Maddison, 2005).
The tree was inferred using MrBayes (Huelsenbeck et al., 2001; Ronquist and Huelsenbeck, 2003). The run comprised 1,000,000 generations with the first 25% of the sampled generations discarded as burn-in trees. Two MCMCMC runs (metropolis-coupled Monte-Carlo markov-chain), using four chains each, were used to thoroughly explore tree space. Convergence of the likelihoods was determined by comparing the average standard error of the difference (ASED) in split frequencies between the two runs and by comparing plots of the log-likelihood after the burnin to the end of the runs. Optimality was also judged adequate when the PSRF (Potential scale reduction factor) for the total tree length and for each model parameter reached 1.00.
Identification and Sequencing of genomic clones encoding O-Superfamily peptides
Genomic DNA was prepared from 50 mg each of Conus acutangulus, Conus mitratus, Conus pracellens and Conus stupa tissues using the Gentra PUREGENE DNA Isolation Kit Kit (Gentra Systems, Minneapolis, MN) according to the manufacturer’s standard protocol. These genomic DNAs were used as templates for polymerase chain reaction (PCR) with oligonucleotides corresponding to conserved 5’ intron and 3’ UTR sequences of omega and delta prepropeptides. The resulting PCR products were purified using the High Pure PCR Product Purification Kit (Roche Diagnostics, Indianapolis, IN) following the manufacturer’s suggested protocol.
The eluted DNA fragments were annealed to pNEB206A vector and the resulting products transformed into competent DH5a cells, using the USER Friendly Cloning Kit (New England BioLabs, Beverly, MA) following manufacturer’s suggested protocol. The nucleic acid sequences of the resulting omega and delta toxin-encoding clones were determined according to the standard protocol for Automated sequencing.
Morphometric Analysis
Using dial calipers, we measured maximum diameter (mm) and total length (mm; including spire height) of species within the praecellens complex. Relative diameter was calculated as the ratio of maximum diameter to total length. All species were represented by multiple samples. In view of the low between-sample variation, we calculated a single mean relative diameter for each species.
RESULTS
Systematic Descriptions of Three New Species of Conus(by Baldomero M. Olivera and Jason Biggs).
Superfamily CONOIDEA Fleming, 1822
Family CONIDAE Fleming, 1822
Subfamily CONINAE Rafinesque, 1815
Genus ConusLinne, 1758
Conus andremenezi, Olivera and Biggs, new species. (see Fig. 2)
Figure 2.
Two morphospecies with non-“ praecellens-like” protoconchs from Aliguay. All of the specimens shown are from Aliguay Island, Philippines, except the lower left specimen which was collected from southern Japan. Top row: Conus andremenezi ; Bottom row: Conus miniexcelsus. Note the generally finer pattern and narrower shell shape of Conus miniexcelsuscompared to Conus andremenezi. In addition the spiral ribbons of the body whorl of Conus miniexcelsusare smooth, but are distinctly crenulated in Conus andremenezi.
Types
The holotype is deposited in the Marine Science Institute at the University of the Philippines; paratypes are deposited at the Academy of Natural Sciences of Philadelphia, Philadelphia, Pennsylvania (ANSP 421619); the Museum National d’Histoire Naturelle, Paris France (MNHN 21131); the Field Museum of Chicago, Chicago, Ill. (FMHN 312461); the Harvard Museum of Comparative Zoology, Cambridge Mass (MCZ 361611); Zoological Museum of Moscow State University, Moscow, Russia (Lc-37964) and the Bailey–Matthews Museum, Sanible Is., Florida (BMSM 38672).
Type locality
The type locality for Conus andremenezi is Aliguay Island, Philippines, where most specimens in the type series have been collected by commercial fishermen using small trawls at depths of ca .150m. Another established locality is off Panglao, Bohol from Balicasag Island to Momo Beach where the species has been collected by tangle nets in deeper water (~200–300m).
Range
From the Central to Northern Philippines, probably to Viet Nam and possibly much further West (see discussion below). In the recent book of Thach (2005), the specimen figured as Conus praecellens(Plate 61, Fig. 34) is likely to be a specimen of Conus andremenezi.
Description
Biconical in shape, mature specimens from 25–53mm. Moderately solid, and with a relatively high spire, and generally broader than most related forms (D/L≈0.47). Last whorl is broadly conical, with raised spiral ribs that are not smooth but always undulating (and in some specimens, the ribs seem to have arch-like protuberances, instead of a continuous smooth rib). These raised ribs on the body whorl are well separated from each other, with interstices that have axial scales between them.
The body whorl has an off-white ground color with characteristic purplish-brown maculations that occur in zones; in the two darker zones, the maculations generally cover more spiral ribs and extend into the interspaces (although there is considerable variation). The larval shell is broken off in most specimens, but when preserved it is a rounded triangular shape, translucent, very light yellowish brown or off white; the protoconch is followed by 2 white early teleoconch whorls that are lightly nodulose and angled at the periphery. The maculations begin to appear on the periphery of the 3rd or 4th teleoconch whorl, and typically these are more closely spaced to each other than are the larger maculations in the later spire whorls.
The sculpture on the spire whorls is a very diagnostic feature: the spiral ribbons on the larger spire whorls are raised, relatively narrow to very narrow, and always far apart. The wide spacing on the spire whorls between narrow raised spiral ribs is a diagnostic trait of this species; in most similar forms, the spiral ribbons or ridges are much closer together and are more like flattened ribbons, broad and shallow. The broad shape, purplish-brown maculations, undulating spiral ribs and widely spaced ribs on the spire are characteristic features that separate the species from similar forms.
Etymology
This species honors the memory of Andre Menez, one of the giants of the field of toxinology.
Discussion
When the protoconch is broken off, this species is difficult to separate from some closely related forms, that are potentially variants of Conus praecellens. Most specimens can generally be differentiated by the distinctive purple-brown color, the broader shell, the widely-spaced spiral ribbons on the spire whorls, and when preserved, the triangular protoconch. Most specimens in the type series come from Aliguay.
There is a group of Philippine specimens, not from the type locality, which we tentatively assign to this species. These were collected by the Musorstrom expeditions of the MNHN, Paris to Lubang Island/Mindoro. Several large mature specimens of Conus andremenezi, all dead collected, were examined. All of these were collected at depths between 160–198 meters; at more shallow collection stations, this form was absent and a narrower Conus praecellensvariety was present. This provides a more accurate estimate of the depth at which this species occurs.
Finally, there is a small specimen figured by Röckel et al. (Plate 54, Fig. 14) that appears to be a juvenile of Conus andremenezi; if the identity of this specimen can be verified, it extends the range of this species across the entire Indian Ocean since the specimen is reported to be from Somalia. Thus, although almost all of specimens examined were from the Central Philippines, there is strong evidence for the occurrence of the species in the Northern Philippines, and the possibility that it may have a geographic distribution that is much wider is raised by the Somali specimen in the Raybaudi-Massila collection.
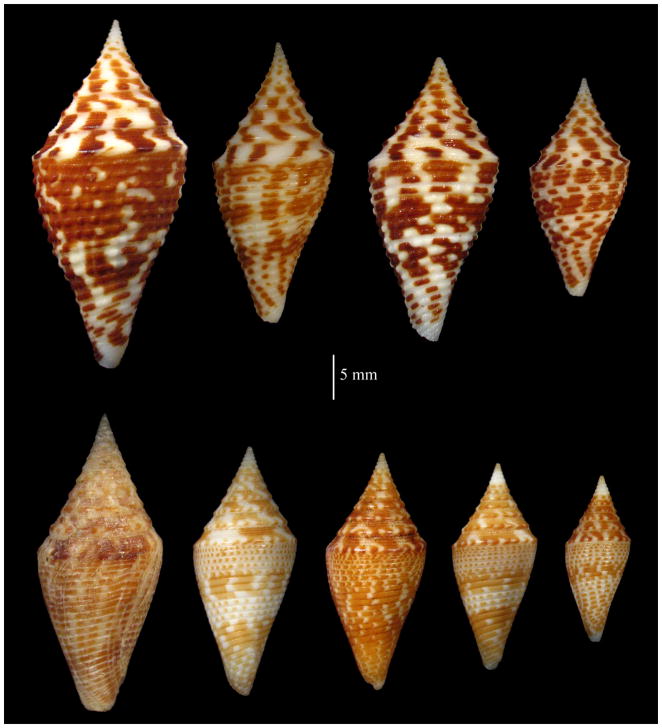
Conus miniexcelsus , Olivera and Biggs, new species (see Figs 3, 4)
Figure 3.
Three distinctive forms with “ praecellens-like” protoconchs. Top row is a series of Conus praecellens , “sowerbii forms”. Bottom is Conus praecellens , “Aliguay form”. In the middle row are two specimens of Conus rizali , new species. The diagnostic morphological differences between the three forms are discussed in the text.
Figure 4.
An illustration of the “miniexcelsus -like complex”. Shown are five specimens (left five) and close ups of their respective protoconchs (right five). Left, top and bottom: Conus miniexcelsus , middle: Conus excelsus , and right top and bottom: Conus acutangulus. Although the shells are different in pattern and size at maturity, note the similar purplish brown translucent protoconchs, followed by the ivory white early teleoconch whorls before the regular shell pattern is initiated. Typical Conus acutangulus(top right) and the “deep-water form” (bottom right) are both distinctly more nodulose while there is a striking similarity between Conus excelsusand Conus miniexcelsusin their protoconchs and early teleoconch whorls.
2008 Conus praecellens f. subaequalisRobin, Encyclopedia of Marine Gastropods,
p 424, fig 14 (non C. subaequalis , Sowerby III)
Types
The holotype is deposited at the Marine Science Institute at the University of the Philippines, paratypes are deposited at the Field Museum, Chicago, Illinois (FMNH 312462); the Museum National d’Histoire Naturelle, Paris France (MNHN 21132); the Harvard Museum of Comparative Zoology, Cambridge, Massachusetts (MCZ 361609); the Academy of Natural Sciences of Philadelphia, Philadelphia Pennsylvania (ANSP 421620); Zoological Museum of Moscow State University, Moscow, Russia (Lc-37965) and the Bailey-Matthews Museum, Sanibel Is., Florida (BMSM 38673).
Type locality
Aliguay Island, Philippines. Most specimens in the type series were collected by the commercial small-trawl operations off Aliguay Island, at depths of 30–150m.
Range
Presently known from the Central Philippines to Wakayama, Japan (Paratype #23).
Description
A moderately small shell; adult size range, 25–37mm. High-spired, with both spire and body whorl having a straight outline, making the shell narrowly biconical. The larval shell has 3–3.5 whorls, translucent brownish or purplish. There are 9–11 teleoconch whorls, the first three being ivory white without any maculations, providing a notable contrast to the translucent colored protoconch. At around the fourth teleoconch whorl, broad brownish maculations appear, centered around the periphery. The ground color is white, with chestnut brown maculations. On the body whorl there are a series of flat spiral ribbons. The shell pattern on the body whorl can be divided into 3–5 zones. The most posterior, next to the suture, are a series ~ 6 spiral ribbons with extremely fine chestnut brown maculations. These are followed by a zone with 3 noticeably broader spiral ribbons that have deeper brown and larger maculations. In most specimens, this is followed by three spiral ribbons that have a finer maculated pattern (but not as fine as in the spiral ribbons in the first zone, closest to the suture). The remainder of the shell towards the tip is covered by spiral ribbons that are darker in color and more heavily maculated; typically the first 3 to 4 are darker than those towards the anterior end of the shell, although there is considerable variation in this regard. In some specimens, the light zone continues to the anterior of the shell. A distinguishing characteristic of this species are the maculations on the whorls closest to the suture, which are generally extremely fine in pattern and greater in number than for any other similar species, followed by the thicker, darker brown spiral ribbons at the center of the body whorl. These features are clearly illustrated in the specimens shown in Figure 3 of the main text.
Etymology
The name proposed emphasizes some striking and unexpected similarities to Conus excelsusdespite the considerable disparity in size, (see Text).
Discussion
Conus miniexcelsusis a distinctive form, most easily confused with Conus praecellens. However, as discussed above and shown in Fig. 3, the differences in protoconch and early teleoconch morphology between the two species are consistent distinguishing characters. This feature puts Conus miniexcelsusin the same group as Conus acutangulus , Conus andremenezi and Conus excelsus(except that the spire of Conus acutangulushas strong tubercules at the sutures). Conus andremenezi is generally larger, with coarse maculations that are purplish brown in color instead of chestnut. Conus miniexcelsusis probably most similar to Conus excelsus , although there is a striking difference in size at maturity. The two Japanese specimens examined are more solid and chunky than the Aliguay material.
Conus rizali, new species, Olivera and Biggs. (see Fig. 3)
Types
The holotype will be deposited at the Marine Science Institute at the University of the Philippines; paratypes are deposited at the Acadamy of Natural Sciences of Philadelphia, Philadelphia, Pennsylvania (ANSP 421621); the Harvard Museum of Comparative Zoology, Cambridge, Massachusetts (MCZ 361610) and in the Museum National d’Histoire Naturelle, Paris France (MNHN 21133).
Type locality
All type specimens were obtained from commercial dealers in the Philippines, and the exact collection locality of the types could not be verified. Springsteen and Leobrara show a figure of Conus rizali (labeled Conus subaequalis ) indicating Punta Engaño, Cebu, suggesting that these were probably collected by fishermen using tangle nets at depths of 100–200m.
Range
Philippines.
Description
The species is medium sized; specimens examined are 26–39mm in length. The shell is biconic, with an unusually tall, straight and sharply pointed spire and a straight sided body whorl, sharply angled at the shoulder. The larval shell has 2 whorls, and is praecellens-like but somewhat proportionally broader than for most specimens of Conus praecellens ; this is followed by 3 teleoconch whorls that have a characteristic white matte surface, somewhat crinkly; starting with the fourth teleoconch whorl, there are 8–9 maculated spire whorls.
The body whorl is characterized by shallow spiral ribbons with only a narrow interstitial space between them; these are broadly maculated in light yellow brown. Characteristically, immediately below the periphery, the first spiral ribbon lacks maculations, leaving a white zone. Although there is some variation, the maculations are much lighter in color than in related forms (paratype 2 almost completely lacks maculations in the body whorl). Of all of the similar forms, this species has the most narrow outline (D/L=0.397±.011); specimens of Conus praecellensfrom Aliguay, which are generally narrower than the sowerbii form have a D/L=0.44, and for Conus miniexcelsus ; (D/L=0.416) these are both narrower than Conus andremenezi.
Although Röckel et al., put this species in synonymy with Conus praecellens , we believe that it is a distinctive form that can readily be separated from specimens assigned to Conus praecellens. The narrower outline, the shallow ribbons on the body whorl, and differences in protoconch/early teleoconch morphology separate Conus rizali from other related forms.
Etymology
This species is named in honor of Jose Rizal, the National Hero of the Philippines. Dr. Rizal, who was executed by the Spanish Colonial Administration in 1898, collected shells as a hobby.
Discussion
Conus rizali refers to the form previously figured as Conus subaequalis Sowerby III, 1870 by authors. This name was used by Springsteen and Leobrara, and by Lim and Wee; however, the specimen recently figured by Robin (2008) as Conus subaequalisis not Conus rizali but Conus minexcelsus. A specimen was also figured by Röckel et al (Plate 54, Figure 6) but they refer this to Conus praecellens. Conus rizali is sufficiently distinct so that it can immediately be picked out from other related forms discussed in the text of the paper: the narrow outline of the shell sets it immediately apart, and in fact the form that is most similar in outline is Conus gratacapi from Japan, which is an unrelated species. This species has only been intermittently collected over the last four decades, and never in large numbers. As has been discussed in detail by Röckel et al, and is shown in the original figure of Sowerby (which Röckel et al., reproduced), the specimens that we assign to Conus rizali are clearly not conspecific with the figure of Conus subaequalis , which likely refers to a different form in the Conus praecellens complex.
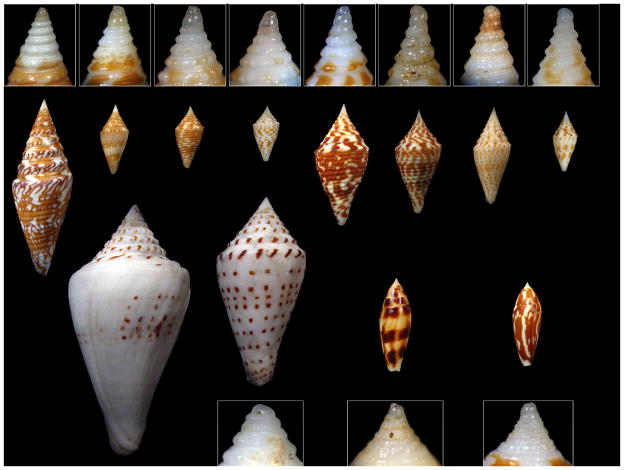
Figure 6.
Distinctive forms proposed to belong to the Turriconusclade. Top row (showing the shell and in the inset, a close-up of the corresponding protoconch), from left to right: Conus excelsus; miniexcelsus; acutangulus , “typical form”; acutangulus , “deep-water form”; andremenezi; praecellens , “sowerbii form”; rizali; praecellens , “Aliguay form”. Lower row, from left to right: Conus stupa; stupella; mitratus; cylindraceus. The protoconch of Conus stupais not shown; it is extremely eroded in the figured specimen.
Morphological definition of species in the Conus praecellens complex: Two “ miniexcelsus -like” forms
In this section, we describe and define two distinctive forms in the Conus praecellens complex from the Philippines. As will be defined in the Discussion, the “Conus praecellens complex” can be divided into two broad groups on the basis of protoconch morphology, the “ praecellens-like” forms and the “ miniexcelsus -like” forms.
The two miniexcelsus -like forms from Aliguay Island (i.e., those with non-praecellens-like protoconchs), which we proposed to designate as new species, are discussed first. The Aliguay specimens of these two forms are easily distinguishable from each other (see Fig. 4). Since both of these miniexcelsus -like forms were apparently unnamed, these are formally described in the section above. The appendix summarizes the individual type specimens on which the new taxa are based.
Conus andremenezi, new species (See Figure 2)
This form may be similar or identical to Conus bicolor Sowerby (I 1833), which is a preoccupied name. Sowerby then provided a new name in 1841, Conus sinensis. Röckel et al., (1995) felt that “taxonomic status of Conus bicolor/Conus sinensis(Sowerby ii, 1841) remains disputable because the type specimen is lost and the type figure (Plate 54, Fig. 3) does not match C. praecellensin a satisfying way: the pictured shell has a comparatively low spire…is somewhat bulbous below the shoulder and its color pattern consists of brown axial flames…we favor synonomy with Conus praecellens.” The figure shown by Röckel et al. (originally drawn from “Conus bicolor”) is similar to the form we describe above as C. andremenezi; we have not adopted the name Conus sowerbii for this form because the syntype in the British Museum does not appear to be conspecific with C. andremenezi.
We believe that this form is clearly distinguishable from typical Philippine specimens of C. praecellens ; first, the protoconch is not “ praecellens-like”; second, this form is generally broader and has a characteristic purplish-brown coloration. Furthermore, on the body whorl, there are raised but not flattened spiral ridges that undulate, with a wide space between ribbons with axial scales between the spiral ridges. More consistently, the sculpture on the spiral whorls has narrow, raised ridges, widely spaced from each other. This suite of characteristics consistently distinguishes this species from C. praecellensof similar size (see Fig. 1) and from Conus miniexcelsus(see next species); Conus rizali is even more distinctive from C. andremenezi. There are a group of small Conus praecellensthat are most easily confused with Conus andremenezi ; these are discussed under Conus praecellensbelow.
Conus miniexcelsus , new species (See Figure 2)
This very distinctive species is characterized by its relatively narrow shell outline (D/L 0.42 vs. 0.47 for C. andremenezi ), the multispiral protoconch of 2 – 3 whorls, which is translucent and distinctly brownish or purplish and contrasts in its color with the first 2 – 2 teleoconch whorls that are ivory white. In most specimens, these white whorls are smooth or have, at most, nearly obsolete tubercles. This is followed by 6–10 maculated teleoconch whorls that are grooved and have strong axial structure so that the upper part of each spire whorl has a distinctly tiled appearance. The body whorl has shallow spiral ribbons with regular, brown maculations that have a characteristic pattern described in the Appendix in detail. The colored, translucent protoconch contrasting in color with the first two shiny white teleoconch whorls, combined with the slender shape and the very fine maculated pattern are diagnostic of this distinctive species. There is considerable variation in how dark the maculations are; a range of variations is shown in Figure 4. A full description of this new species was provided in the previous section; detailed measurements of all the types are provided in the appendix. Almost all specimens have been collected by small trawls in Aliguay from 30–70 fathoms, but occasional specimens have also been collected using gill nets off Balicasag Island. One specimen assigned to this species from Southern Japan is included in the type series (see Fig 4).
Overview and description of the “ praecellens-like forms”
Conus praecellensremains a confusing taxon, and the scheme proposed below is not entirely satisfactory; multiple forms have been assigned to this species by various workers. Even after the two “miniexcelsus -like forms” are separated as new species, what remains still comprises a confusing set of specimens, most of which we are provisionally retaining in C. praecellens. We believe that the retention of diverse forms within C. praecellenswill prove to be only an interim solution, and new morphologically similar species will be identified once a more extensive molecular and morphological analysis has been carried out over a wider suite of specimens from a greater geographic range.
There is a widely illustrated specimen designated as “a possible syntype” of Conus praecellensfrom the British Museum. This is atypical of specimens assigned to C. praecellensfrom the Philippines. This possible syntype from the China Sea is lighter in color and finer in sculpture on the body whorl than either of the two major Philippine varieties that we include in C. praecellens. The first group, “the Aliguay form,” which is small and light colored, has been extensively collected both by the small dredge operations in Aliguay Island, and is the form illustrated in Fig. 3. A second more variable group that we refer to as the “sowerbii forms,” include larger specimens that vary considerably in shell pattern, shape and size. These comprise most specimens collected by fish trawlers in the period from 1955–1965, particularly from two localities, Tayabas Bay and Maqueda Bay. A third group is that referred to by previous authors as Conus subaequalisand is described above as Conus subaequalis. The three forms are shown in Fig. 5.
Figure 5.
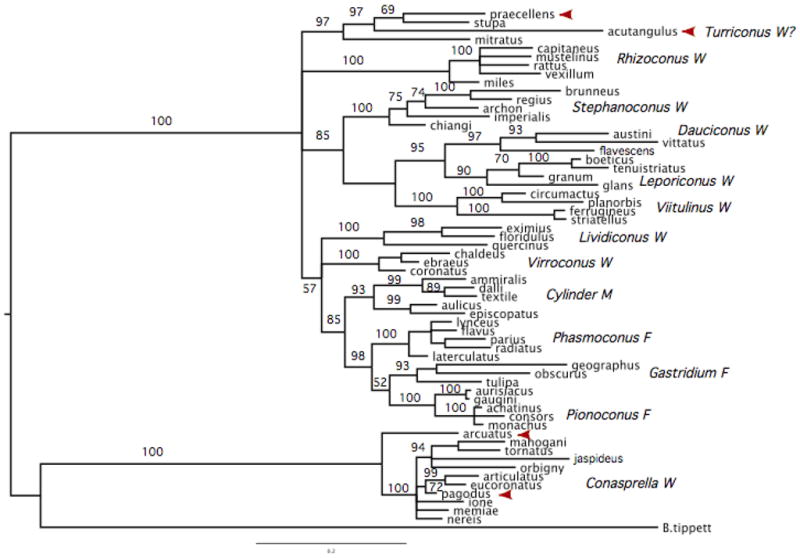
Phylogenetic tree of some Conus species based on 12SrRNA sequences. Species shown in Figure 1 are indicated with arrows. Branches are labeled with Bayesian confidence values (posterior probabilities expressed as percentages). These data clearly separate Conus acutangulusand Conus praecellensfrom the Conasprellaspecies with 100% confidence and join them with C. stupaand C. mitratuswith 97% confidence.
Conus praecellens(Aliguay form) (See Figure 3)
The series of specimens that we assign to C. praecellens , “Aliguay form”, appear to be the closest to the type in the British museum in shell pattern; these have mostly been collected in 30–80 fathoms off Aliguay Island, between Mindanao and Bohol in the Philippines. The Philippine specimens are smaller than the Bristish museum “syntype” (average size 24mm); key features that distinguish this form are a blunt, paucispiral protoconch of two whorls, the relatively smaller size, and the chestnut color of the maculations. Details of the spire sculpture and body whorl that are also diagnostic are delineated below.
The shells of this form (see Fig. 5) typically have 2–2½ protoconch whorls, the first being quite spherical and inflated, and the second narrower and more elongate. The first two teleoconch whorls are typically white and flattened compared to the protoconch whorls, and they are knobbed on the periphery, while the two protoconch whorls are smooth. There are 8 to 9 whorls maculated with a chestnut brown color, with the first one or two maculated whorl(s) also knobbed. The early teleoconch whorls are characterized by a deep spiral groove on the upper section of the whorl; these spiral grooves gradually increase in number as the whorls get larger; these are narrow furrows that can be bisected by axial sculpture that varies considerably in strength; in specimens where the axial sculpture is strong, the area immediately adjacent to the suture looks as if it were tiled, since the combination of the spiral grooves and the axial sculpture divide the area between grooves into square sections. The broader part of the spiral whorl is smooth to the periphery; the lower suture is below the sharply angled periphery.
Conus praecellens , “sowerbii forms” (See Fig. 3)
There are forms in the C. praecellens complex most commonly found in collections; most specimens were collected by trawlers around 1960 in great numbers in the Maqueda and Carigara Bays of Samar Island, and in Tayabas Bay of Southwestern Luzon, from Jolo Island in the Sulu Sea. The “sowerbii forms” are larger and more densely maculated than the specimens of the “Aliguay form” described above. There is considerable variation in shell morphology: some specimens are slender and narrow with fine sculpture; others appear to be much broader at the shoulders with an overall coarser sculpture. However, when preserved, the protoconchs of all of these have the typical highly inflated first whorl, with only two, pearly white protoconch whorls. A range of specimens collected from various Philippine localities, all with typical “ praecellens-like” protoconchs that are well preserved are shown in figure 5 (the contrast between these and the “Aliguay form” is also illustrated in the figure).
Given the distinctive (and similar) protoconchs of both the Aliguay and the sowerbii forms, we have provisionally assigned these in C. praecellens. However, further characterization of both the radular and gut morphology, as well as a molecular characterization, may prove that these are distinct from each other, and that there are additional separable species embedded in the “sowerbii forms”, a possibility that clearly needs to be further evaluated.
Conus praecellens , other distinctive varieties.
A smaller form of Conus praecellenswas recently collected by the Muséum National d’Histoire Naturelle Paris Museum, off Aurora, Eastern Luzon, Philippines. These specimens were notable because they were very similar to Conus andremenezi , but much smaller. They are easily separable from Conus andremenezi because they have the typical “ praecellens-type” protoconch. These look very different from the two Conus praecellensforms described above. We note that in general, Conus andremenezi is a larger species; however, there have been juvenile Conus andremenezi specimens collected using lumum-lumum nets in the Camotes Sea, along with specimens of the Conus praecellens , Aliguay form, described above. It seems likely that only juvenile specimens of Conus andremenezi are collected at this locality because lumun-lumun nets were used, and it presumably takes longer than the 3 months lumun-lumun nets are laid out for Conus andremenezi to reach full maturity. These small specimens of Conus andremenezi do have the characteristic protoconchs of that species, though they are somewhat lighter in color than the Aliguay series. In contrast, the variety of Conus praecellenscollected off Aurora (trawled at a station 83 meters in depth) have the praecellens-type protoconch. At the same site, the MNHN expedition collected two dead, somewhat eroded specimens, that were larger in size, in a trawl 189–307 meters in depth that are likely to be true Conus andremenezi.
In addition, a species was recently described as Conus beatrix, Poppe and Tagaro, 2006. We have not had an opportunity to examine the holotype of this species; it may be that these represent a series of unusually pale specimens, possibly continuous with the Conus praecellens , Aliguay form, described above. If this were the case, and further molecular evidence shows that these are separable from Conus praecellens , then these authors will have provided a potentially valid species name for Conus praecellensAliguay form.
Conus rizali , new species (See Figure 3)
This distinctive form was previously recognized by Springsteen and Leobrera and by Lim and Wee as morphologically separable from any of the other forms assigned to C. praecellensor C. acutangulus. In their treatment of this complex, Springsteen and Leobrera (1986) provided a figure of this form, to which the name Conus subaequalisSowerby III, 1870 was assigned, with the locality Punta Engaño, Cebu, Philippines. This locality suggests that the specimens they examined were collected by gill nets in deep water. A similar specimen was illustrated by Röckel et al. (Plate 54, #6) and labeled “Conus praecellens ” from Davao, Philippines (likely collected by tangle nets off Balut Island, Davao). In our description of Conus rizali (see section above), the name we propose for this form, we discuss a number of distinguishing morphological characteristics. The name assigned by both Springsteen and Leobrera and Lim and Wee, Conus subaequalisdoes not appear to refer to this form (Röckel et al., reproduce the original figure of Sowerby which refers to a smaller shell, broader in outline with seemingly more deeply colored maculations than Conus rizali ).
The slender, high-spired and biconical shape of C. rizali is similar to the Japanese Conus gratacapai , a poorly-understood, rare, deep-water species. Several museum specimens of C. gratacapai were examined, including some paratypes of the latter. Several striking morphological differences indicate that the two forms are not conspecific. Most notably, C. gratacapai does not have the sutural structure of C. rizali ; there is a smooth transition between whorls in the former, but a peripheral overhang between spire whorls in the latter. Another obvious difference are the light brown maculations of C. rizali , which are absent in C. gratacapai Pilsbry, 1904. The spire sculpture of the two forms differs significantly as well.
Other “miniexcelsus-like” forms: Conus excelsusand Conus actangulus
The presence of a translucent multispiral brownish or purplish protoconch, followed by several ivory white teleconch whorls is a striking morphological feature of Conus minexcelsus. In this respect, two well-known species are “ miniexcelsus -like”: Conus excelsusand Conus acutangulus. Both have the same characteristic translucent protoconch and ivory white early teleconch whorls (see Fig 6). All of these forms have the body whorl covered by spiral ribbons. The major difference is in the highly nodulose whorls of Conus acutangulus , versus the smoother whorl of Conus minexcelsus. Conus excelsusis somewhat intermediate in this respect. There are also the striking and obvious differences in size, C. excelsusbeing by far the largest.
Conus excelsusSowerby III, 1908 (See Figure 4)
This is one of the largest species in the group (to over 100 mm). The protoconch consists of about 3.25 whorls, with a maximum diameter of about 1 mm. The protoconch whorls are grayish, and early teleoconch whorls are bright white that then begin to have brown radial blotches of varying size in the later whorls. Early teleoconch whorls have one deep spiral groove, increasing to up to 4 spiral grooves in the later whorls. Although body whorl can be almost smooth, some specimens have variably spaced, axially striate spiral groups separated by granulose ribbons. Most specimens in the Philippines are from Balut Island, Davao, collected by gill nets at depths of approximately 100–150 fathoms. Although C. excelsusis strikingly different in size, the body whorl sculpture, the protoconch and early teleoconch whorls show such strong similarities to C. miniexcelsusthat a close genetic relationship between the two species seems highly likely.
Conus acutangulusLamarck, 1810 (See Figure 4, 6)
Among Philippine specimens, there appear to be two varieties of Conus acutangulus , the typical form (Fig. 4, top row, right), which conforms to the neotype designated by Kohn (Kohn, 1981) and a variety that we will refer to as the “deep-water form” (Fig. 4, bottom row, right). This is a well-known species, and the only issue is whether the two distinct forms described below are conspecific, or whether they represent two different species.
“Typical form (description after Röckel et al., 1995)”. This is a small to medium sized shell; the larval shell is multispiral with 3 to 4 whorls. The teleoconch whorls are strongly tuberculate for at least the first 8 post-nuclear whorls, a distinctive characteristic. The body whorl has strong spiral ribbons or ribs that are separated by grooves with strong axial threads. The shell is largely brown, except for small, scattered white blotches at the shoulder and center. The aperture is white. This form is collected in relatively shallow water, typically between 3 to 20 meters. Divers in Batangas Bay, Luzon, collected most Philippine specimens; more recently divers in Nucnucan, Bohol, have collected the typical form. Fully mature specimens are 25–38 mm in length, D/L 0.50–0.53. Typical fully mature non-Philippine specimens are also illustrated by Röckel et al.1995 (Plate 54, Figs. 19 and 21).
“Deep-water forms”. The deep-water varieties typically occur between 30 to 150 meters, and are collected either by dredging or gill nets. These forms do not have the brown to dark brown color of the typical variety, but are mostly white with sparse light orange-brown or grayish flecks. Generally smaller than the typical form, most specimens are 16–22 mm in length. This form has been collected in Aliguay and Pamilacan Islands. A comparison of available deeper-water specimens assigned to C. acutangulusreveals considerable variation that needs to be more carefully investigated and defined. These forms clearly occur outside the Philippines; Röckel et al., illustrate a specimen from the Solomon Islands (Plate 54, Fig. 17).
Molecular Phylogeny: relationship of Conus praecellensto other high-spired Conusspecies
Most of the forms investigated in this article were collected in the Central Philippines, primarily by gill nets or trawlers offshore. A number of smaller Conusspecies of similar shape, including several diverse forms assigned to Conus praecellens , are collected in this way (Fig 1). As described above, in most taxonomic work, Conus praecellensis either explicitly discussed or implicitly grouped with similarly shaped, high-spired, small, deep-water Conusspp. even when authors do not endorse a specific phylogenetic scheme (for example see Robin, 2008; Röckel et al., 1995; Walls, 1979).
Table 1 summarizes previously proposed taxonomy based on shell morphology, which can be assessed using molecular data. For comparison, a phylogenetic tree based on 12S mitochondrial DNA includes C. praecellensand C. acutangulus , along other vermivorous, molluscivorous, and piscivorous Conusspecies (see Fig 5).
This molecular phylogeny assigns C. praecellensand C. acutangulusto the same clade. The surprising, yet the clear cut result is that most other species proposed to be included in Conasprellawith C. praecellensby previous workers based on shell morphology actually cluster in a branch extremely divergent from most Conus. The type species of Conasprella, C. pagodus , is on this very distant branch, which we refer to as the “Conasprellaclade”. Additional molecular data support these findings (Bandyopadhyay et al., 2008). These data, discussed below suggest that the degree of divergence makes it untenable to keep these species (C. pagodus , etc.) within the same genus as other Conusspp.
Definition of a clade based on morphological and molecular data:
The combination of the morphological analysis of the various species and distinctive forms above combined with the available molecular data (see Fig. 5) provides a framework for defining the group of Conusspp. most related to C. praecellensand C. acutangulus. As discussed above, some of the superficially similar high spired Conusspecies are not at all closely related based on the molecular data. In the Philippines, there are 10 species and 12 distinctive forms which we assign to this group that we designate the Turriconusclade (Conus excelsus= Conus nakayasui , type species). A comprehensive taxonomic revision of the genus Conusis currently being carried out by A. Kohn; we suggest that Turriconusis a distinct branch within the major group of species that together, comprise the genus Conus.
The molecular work definitively excludes a number of Conusspp. from Turriconus ; clearly, species such as Conus pagodus, Conus memiae, Conus nereis , and other deep-water species with high spires such as Conus boholensis(Petuch, 1979), Conus eugrammatus(Bartsch & Rheder, 1943), as well as non-Philippine species such as Conus jaspideus(Gmelin, 1791) and Conus arcuatus , though morphologically similar to C. praecellens , do not belong in the Turriconusclade. The 10 species and 12 distinctive forms that comprise the Turriconusclade in the Philippines are shown in Figure 6, and summarized in Table 2; as outlined in the table, these fall into 4 groups that will be discussed in turn.
Table 2.
Morphologically Distinct Forms in the Turriconusclade (Conus excelsus , type species).
| Group I | Conus excelsus |
| miniexcelsus | |
| andremenezi | |
| acutangulustypical form* | |
| acutangulusdeep water form | |
| Group II | Conus praecellens“sowerbii forms”* praecellens“Aliguay form rizali |
| Group III | Conus mitratus * Cylindraceus |
| Group IV | Conus stupa stupella* |
Peptide toxins belonging to the O-superfamily have been determined for these forms.
The first group is the excelsus/acutangulusgroup, with 4 species and 5 distinctive forms. This group includes the type of Turriconus , Conus excelsus. The second is the praecellensgroup with 2 species and 3 distinctive forms. The third is the mitratusgroup of at least 2 species and fourth, the stupagroup.
The first group of species, the excelsus/acutangulusgroup, is characterized by a generally triangular protoconch, without the spherical, inflated first protoconch whorl. The protoconch is translucent, brownish purplish or light yellowish in color; and followed by two or more much whiter teleoconch whorls. The 4 species in this group are Conus excelsus, Conus miniexcelsus, Conus andremenezi and the two forms of Conus acutangulusdescribed above.
In the second group, the praecellensgroup, the characteristic feature is a white protoconch of two whorls, with the first protoconch whorl being inflated and spherical (i.e., “ praecellens-like”). The characteristic shape of this protoconch is diagnostic of this group (as shown in Fig. 6); there may be more species than are recognized here, since this is a rather variable assemblage of forms as is discussed above. C. praecellensand C. rizali are the two species in this group, with at least two distinctive forms assigned to C. praecellens.
The third group in Turriconus , based on molecular data and expanded using morphological similarities, is the Conus mitratusgroup. These species have much more elongated body whorls than are found in the two groups above. They seem to share the characteristic white early teleoconch whorls before the mature coloration is expressed with the first group. On morphological grounds, we assign two species to this group: Conus mitratusand Conus cylindraceus(Broderip & Sowerby, 1830). The molecular evidence in this paper is only provided for Conus mitratus ; however, corroborative molecular evidence for Conus cylindraceushas been obtained by others (C. Meyers, personal communication).
The fourth group in the Turriconusclade is a subgenus (Kurodaconus ) recognized as distinctive by some workers; the molecular evidence suggests these species should be included in Turriconus. There are two species in this group, Conus stupaand Conus stupella; it is the opinion of several workers on Conusthat these may not be separable species, since they always appear to occur together. This suggestion needs to be further evaluated. These forms differ from Groups I and II by the smooth body whorl, while the species in these groups have the spiral ribbons or ribs.
The overall hypothesis, based on the combined molecular and morphological data, is presented in Table 2. We used this working hypothesis as a guide to analyze toxins in the putative species of the Turriconusclade, from which DNA samples were available (marked by asterisks in Table 2).
Using Toxinological markers to evaluate the Turriconus clade:
The hypothesis presented in Table 2 was experimentally tested using a toxinological analysis. Since cone snails are venomous animals, they use toxins in their venom to capture prey, defend against predators and for competitive interactions. Since each species presumably has a different spectrum of prey, predators and competitors, the genes encoding venom components are “exogenes,” which diverge very rapidly as new species evolve. The peptide toxins that are present in Conusvenoms are encoded by only a few gene superfamilies; these are predicted to undergo accelerated evolution.
Conuspeptide genes are examples of exogenes (Olivera, 2006); their gene products act exogenously, targeting other animals (instead of acting endogenously within the cone snail itself). A considerable amount of prior work has demonstrated that each Conusspecies has its own distinct complement of venom peptides, with the same peptides not found in venoms of even closely-related species. What would be predicted when a group of closely-related species is analyzed is that the gene products encoded by a particular conopeptide superfamily will be highly related to each other, but not identical in sequence. This toxinological prediction was used to test if the species proposed to be in the Turriconusclade do indeed have closely related (but not identical) toxins, as would be expected for exogene products.
All species for which DNA was available were analyzed; since no cDNA samples were available, the analysis had to be carried out on genomic DNA. The gene superfamily used for the analysis was the O-superfamily; it is possible to determine the sequence of the mature toxins because there is a conserved intronic sequence that borders the mature toxin region (see Materials and Methods) thus, PCR primers can be used to determine peptide toxin sequences from each species. The O-superfamily has diverged into two branches (Olivera et al., 1999; Terlau and Olivera, 2004) one hydrophilic, which includes the ω- and κ-conotoxins from fish hunting cone snail venoms (the “ω-branch”) and the second highly hydrophobic; in fish hunting cone snails this includes the δ and μO-conotoxins (the “δ-branch”). PCR primers used to amplify genes in each branch are different and therefore toxin sequences can be separately obtained. This analysis was carried out and the results are shown in Fig. 7.
Figure 7.
Toxinological analysis. Predicted mature toxin sequences from two distinct branches of the O-superfamily of conopeptides for four members of Turriconus: Conus acutangulus, Conus mitratus, Conus praecellensand Conus stupa. An independent comparison of the toxin sequences from the two branches, the hydrophilic ω and the hydrophobic δ, each demonstrate the close relationship between the different species of the Turriconus clade analyzed.
It is clear from the figure that representatives of all four species groups, separated using our aforementioned morphological/molecular phylogenetic analyses, yielded homologous O-superfamily peptide sequences that fall into both the ω- and the δ-branches; all of which share a high degree of sequence identity. Moreover, as predicted by the exogenomic hypothesis (Olivera, 2006), these peptide sequences have diverged from each other. Therefore, the postulated accelerated evolution of these exogenes, which, in turn, is an indicator of species divergence, is indeed observed.
Discussion
This study has used three types of data: comparative morphology, molecular phylogeny based on standard gene markers and toxinological markers (i.e., peptide toxin sequences). This three-pronged effort was aimed at branch definition in a biodiverse lineage, the venomous cone snails, which led to a specific phylogenetic hypothesis. As is typical, none of the individual data sets were as comprehensive or complete as might be desired; nevertheless, the combination made the phylogenetic framework proposed compelling.
Morphological evidence: importance of the protoconch/early teleoconch
In earlier work of Springsteen and Leobrera, two forms in the C. praecellens complex from the Philippines were separated, the commonly trawled form (designated Conus sowerbii ), and a second much more slender form, with lighter yellowish brown maculations, assigned to Conus subaequalis(see Plate 71, Figures 1 and 2 of Springsteen and Leobrera). This treatment was subsequently adopted by Lim and Wee (1992). Thus, while Walls (1979) and Röckel et al., only recognized C. praecellensand C. acutangulus , these workers recognized three distinctive forms from the Philippines/Southeast Asia (“C. sowerbii ” (= C. praecellens ), “C.subaequalis ” and C. acutangulus ).
One reason that the definition of forms has been challenging is because most available specimens did not have good protoconch (or early teleoconch) whorl preservation. There is a tendency for Philippine specimens in this group from many localities to have a black tarry layer covering the spire of the shell, which is routinely removed by commercial shell dealers using acid, a treatment that destroys key features critical for morphological differentiation. In the analysis below, we used these “compromised” specimens in our morphometric analyses but focused primarily on the few specimens with well-preserved protoconch and early teleoconch whorls for discriminating between forms. Once consistent differences in protoconch and early teleoconch morphologies were established, additional morphological characters were used to help separate distinctive forms. This general approach was used for the morphological definition of forms described above.
The basic approach is illustrated in Figure 8, which show two forms collected off Aliguay Island; these have proven to be particularly illuminating. Both would have been assigned by Röckel et al. (1995) to Conus praecellens. In Fig. 8, the specimen on the right is a specimen of what we refer to as the Aliguay form of C. praecellens ; the other specimen (on the left) is what named Conus miniexcelsus , new species (above). The top panel of Fig. 8 shows the shells of the two specimens, which are approximately the same size. Although their shell patterns differ, the considerable variation observed in this complex led to a rather confused situation in the past. The major morphological observation that changed this situation is illustrated in the lower panel of Fig. 8, which shows a magnified view of the well-preserved protoconch and early teleoconch whorls of the two specimens; we believe that the differences depicted are sufficiently diagnostic to definitively assign the specimens illustrated into two distinct groups (i.e., Conus praecellensand Conus miniexcelsus ). The multispiral brownish protoconch of C. miniexcelsuscontrasts with the protoconch of the specimen assigned to C. praecellens ; the early teleoconch whorls are also distinctively different. Using this as the major criterion for separating forms makes it simpler to identify other shell morphological characteristics that consistently differ, even though each individual character might have a considerable range of variation. This approach has provided a much more consistent suite of characters to allow a definition of different morphospecies.
Figure 8.
Comparison of two morphospecies collected in Aliguay Island, Philippines. The specimen at the left is Conus miniexcelsus , and on the right is Conus praecellens , “Aliguay form”. Top: The whole shell; Bottom: A close-up of the protoconch and first few teleoconch whorls. Note the different shape and color of the protoconchs and the characteristic switch in Conus miniexcelsusfrom a triangular translucent purplish brown protoconch, to the ivory white first teleoconch whorls, and finally to the normal maculated pattern. There are characteristic sculptural differences in the early teleoconch whorls between the two specimens as well.
Using this criterion, the Philippine forms previously assigned to C. praecellensfall into two separable groups: a group that has a characteristic protoconch of 2-2 whorls, with a “paucispiral” first whorl (i.e., rounded and somewhat inflated); the protoconchs of these forms are pearly white, and we have referred to these as the “ praecellens-like forms.” The specimens that do not have this very characteristic type of protoconch, herein collectively referred to as the “miniexcelsus -like forms”, have the first protoconch whorl not rounded, nor inflated. The entire protoconch of the latter generally has a more triangular outline when compared to the praecellens -like forms; these protoconchs are typically translucent-brown, translucent-yellowish or off white. Although this color can be subtle, the contrast to the pure white early teleoconch whorls is usually diagnostic.
The molecular evidence
A major conclusion from the molecular analysis is that C. praecellensand C. acutangulusdo not belong in the Conasprellaclade. Instead, they form a distinct branch among the major group of species in Conus. Another unexpected result from the molecular phylogenetic analysis is the other species, Conus stupaand Conus mitratus , branch within the same well-supported clade as C. acutangulusand C. praecellens. In particular, Conus mitratushas strikingly different shell morphology from C. praecellensand C. acutangulus. In all previous taxonomic work, Conus mitratusand Conus stupahave been assigned to different subgenera from Conus praecellensand Conus acutangulus.
The contribution of toxinological markers
Our starting point for defining the clade of cone snails that includes Conus praecellenswere the previous proposals in the literature for subgenera (or genera, when Conuswas split into multiple genera) that included Conus praecellens. In most proposals, Conus praecellenswas, in effect, proposed to be related to other high-spired, deep-water species such as Conus pagodus , Conus memiae and Conus arcuatus , which are usually assigned to Conasprella. In some of the prior hypotheses, this group of species was split in two; e.g., Conasprellaand Kermasprella(da Motta, 1991), or Conasprellaand Endemnoconus(Okutani, 2000). Although these various proposals differ in detail, they all group C. paraecellensand C. acutanguluswith species such C. pagodus, C. memiae and C. arcuatus. However, both the molecular phylogenetic results using standard molecular markers and the exogenomic data using toxinological markers are inconsistent with all of these hypotheses; only Conus praecellensand Conus acutangulusappear to be closely related to each other by the latter two criteria. All of the other species previously grouped with Conus praecellensin prior phylogenetic proposals based on shell morphology are now assigned to a distant and different branch of cone snails from Conus praecellens /Conus acutangulus(see phylogenetic tree in Fig. 2).
Instead, the combined data led to a new and strikingly different phylogenetic framework for the Conusspecies comprising the branch that includes Conus praecellens ; this proposal is summarized in Fig. 7 and Table 2. An entirely unexpected set of species appears to be more closely related to Conus praecellens(in Groups 3 and 4 in Table 2). These Conusspecies (C. stupa, C. stupella, C. mitratus , and C. cylindraceus ) were never previously proposed to be in the same clade/subgenus as C. praecellensand C. acutangulus.
The use of toxinological markers has buttressed the molecular phylogenetic analysis. The presence of highly similar peptide toxins that belong to the O-superfamily of conopeptides indicates that the various groups that branch together with Conus praecellensare indeed related, using an independent toxinological data set.
The Turriconus clade: overview
A more comprehensive morphological analysis reveals that various distinctive forms previously assigned to Conus praecellensare likely not conspecific; three new species were described and additional distinct forms defined. Thus, Conus praecellensand the newly described species, Conus miniexcelsus, Conus rizali , and Conus andremenezi are now proposed to be species in the same clade.
Finally, the morphological analysis focused on the importance of protoconch/early teleoconch whorl morphology. These morphological characters strongly suggest that Group 1 in Table 2 should include Conus excelsusgiven its strikingly similar protoconch/early teleoconch morphology to C. acutangulusand C. miniexcelsus. Clearly, there is a difference in size: Conus excelsusis much larger at maturity. We would predict that the molecular phylogeny and toxinology of C. excelsuswill reveal a particularly close affinity to Conus miniexcelsus(which is, in part, the basis for the proposed name of the latter). Unfortunately, Conus excelsusis rare (and also a highly-prized shell collector’s treasure — good specimens can sell for over $1000) and we have been unable to obtain a live specimen to date. However, we propose to call the entire group the Turriconusclade, with C. excelsusas the designated type. In terms of species diversity, it would appear that Groups 1 and 2 in Table 2 are the dominant species groups of this clade — except for the differences in protoconch morphology detailed above, they are all high-spired species with the body whorls characterized by spiral ribs or ribbons. Conus excelsusis the type species of the proposed subgenus Turriconus.
All three types of data used for this investigation need to be extended. A more extensive molecular phylogeny needs to be carried out on all of the forms indicated in Table 2, including, in particular, the designated “distinctive forms” so that an evaluation of whether these are separable species can be carried out. The new species we have proposed need to be rigorously evaluated both by the standard molecular markers as well as by their toxin genes. More refined molecular phylogeny should also allow a better resolution of how the various forms in the proposed Turriconusclade are related to each other and to other species of Conus. In addition, the exogenomic analysis, while in agreement with the molecular phylogeny, also needs to be extended to all of the species in Turriconus , as well as to other gene superfamilies expressed in venom ducts (in addition to the O-gene superfamily that was shown in Fig. 8). The determination of venom peptide sequences by this type of analysis is, in itself, of considerable intrinsic value, since it would allow the predicted gene products from each species to be chemically synthesized and directly tested for functional activity. Finally, the morphological analysis to date is based only on gross shell morphology. Clearly, other morphological features, particularly the internal anatomy, need to be evaluated; these will serve as an independent test of the phylogenetic hypothesis presented. Potentially, common (distinguishing?) morphological features of species in this clade of Conusmay be discovered.
The interaction between the three prongs that are the basis of the approach used in this manuscript has a potential synergy that goes beyond clade definition. The morphological analysis of distinctive forms within the Conus praecellens complex identifies candidates that may or may not be different species, separable from the ones already recognized. Using standard molecular phylogeny will help to define this; however, using exogene analysis should be even more definitive: if these were indeed separable species, then none of the toxin gene products should exactly overlap in their sequence, because of the hypermutation in exogenes that accompanies speciation events.
If a distinctive form was truly a separable species, different toxin sequences would be predicted — if it were merely a variant of the same species, then identity in most venom peptide sequences should be found (except for allelic differences). In some of the well-known species (e.g., Conus textile , Linnaeus, 1758 and Conus striatusLinnaeus, 1758) that are distributed all over the Indo-Pacific, it has been shown that major venom peptides have the same sequence, even from variants whose shells may be distinguishable from each other because of the long period it may have taken for a species to spread across the entire Indo-Pacific, from the Red Sea to Hawaii.
The inclusion of exogenes in the investigation of biodiversity has a significance that goes beyond differentiating between morphologically closely related species. The divergence of exogenes from one species to the next is indicative of different biology, shaped by different selection pressures. This is essentially a molecular readout of the deeper biological/historical/ecological differences between species that might look morphologically similar. Thus, the characterization of toxin genes in the case of Conusnot only serves as a tool for branch definition, but is a potentially important entry point toward a more profound understanding of the biological differences between species, a molecular readout that could provide insights into the complex changes that accompany the speciation events that give rise to a biodiverse lineage of animals such as the cone snails.
Acknowledgments
This work was supported by Program project grant GM48677 from the National Institutes of General Medical Sciences. We are grateful to Adam Baldinger of the Harvard Museum of Comparative Zoology, Philippe Maestrati and Philippe Bouchet MNHN, Paris and Paul Callomon Gary Rosenberg, ANSP, Philadelphia for the loan of specimens used for this study. We are grateful to Chris Meyers for making his sequences for C. cylindraceusavailable to us.
Appendix New Species of Conus in the TurriconusClade
Table I.
Conus andremenezi, summary of type specimens
(Figures of Types are Cross-referenced)
| Length | Width | Locality (PI) | Depository (Cat#) | |
|---|---|---|---|---|
| Holotype (Figs 1, 2, 6) | 36.7 | 16.9 | Aliguay | MSI |
| Paratype 1 | 52.9 | 21.7 | Aliguay | * |
| Paratype 2 | 40.1 | 29.1 | Aliguay | * |
| Paratype 3 | 40.6 | 20.1 | Aliguay | * |
| Paratype 4 | 43.1 | 22.0 | Aliguay | * |
| Paratype 5 | 42.7 | 20.4 | Aliguay | MCZ 361611 |
| Paratype 6 | 26.8 | 12.4 | Aliguay | * |
| Paratype 7 (Fig 2) | 29.4 | 14.0 | Balicasag Is. | Lc-37964 |
| Paratype 8 | 44.0 | 20.9 | (Philippines) | * |
| Paratype 9 | 46.9 | 20.9 | (Philippines) | * |
| Paratype 10 | 28.7 | 13.6 | (Philippines) | * |
| Paratype 11 (Fig 2) | 23.0 | 10.7 | (Philippines) | MSI |
| Paratype 12 | 29.6 | 13.0 | (Philippines) | FMHN 312461 |
| Paratype 13 | 33.2 | 15.4 | (Philippines) | BMSM 38672 |
| Paratype 14 | 26.0 | 12.6 | (Philippines) | * |
| Paratype 15 | 43.3 | 21.4 | (Philippines) | * |
| Paratype 16 | 26.6 | 13.1 | (Philippines) | * |
| Paratype 17 | 31.5 | 14.6 | (Philippines) | * |
| Paratype 18 | 39.5 | 18.5 | Aliguay | * |
| Paratype 19 | 35.8 | 17.1 | Aliguay | ANSP 421619 |
| Paratype 20 | 27.7 | 13.1 | Aliguay | * |
| Paratype 21 | 30.5 | 15.4 | Aliguay | * |
| Paratype 22 | 29.2 | 13.8 | Panglao Is. | MNHN 21131 |
| Paratype 23 | 46.9 | 21.8 | Olango Is. | * |
| Paratype 24 | 37.4 | 16.7 | Aliguay | * |
| Paratype 25 | 36.4 | 17.6 | Aliguay | * |
| Paratype 26 | 34.6 | 15.4 | Aliguay | * |
| Paratype 27 | 44.6 | 21.9 | Aliguay | * |
| Paratype 28 | 48.7 | 22.3 | Aliguay | * |
| Paratype 29 | 38.6 | 18.7 | Aliguay | * |
| Paratype 30 | 33.9 | 15.3 | Aliguay | * |
| Paratype 31 | 35.1 | 15.4 | Aliguay | * |
| Paratype 32 | 42.5 | 20.1 | Aliguay | * |
| Paratype 33 | 35.2 | 17.0 | Aliguay | * |
| Paratype 34 | 37.3 | 17.6 | Aliguay | * |
| Paratype 35 | 36.8 | 17.7 | Aliguay | * |
| Paratype 36 | 21.6 | 9.3 | Camotes Sea | * |
| Paratype 37 | 23.8 | 11.2 | Camotes Sea | * |
Table II.
Conus miniexcelsus , summary of type specimens
| Length | Width | Locality (PI) | Depository (Cat#) | |
|---|---|---|---|---|
| Holotype (Figs 1, 2, 6, 8) | 22.0 | 19.6 | Aliguay | MSI |
| Paratype 1 | 27.7 | 11.8 | Aliguay | * |
| Paratype 2 (Fig 2) | 27.3 | 11.6 | Aliguay | MSI |
| Paratype 3 | 29.0 | 12.5 | Aliguay | * |
| Paratype 4 | 27.4 | 10.8 | Aliguay | * |
| Paratype 5 | 28.3 | 11.4 | Aliguay | * |
| Paratype 6 | 25.5 | 10.5 | (Philippines) | * |
| Paratype 7 | 31.1 | 11.6 | (Philippines) | Lc-37965 |
| Paratype 8 | 31.5 | 13.1 | (Philippines) | FMNH 312462 |
| Paratype 9 | 28.9 | 12.3 | (Philippines) | * |
| Paratype 10 | 35.1 | 13.7 | (Philippines) | * |
| Paratype 11 | 27.6 | 11.6 | (Philippines) | MCZ 361609 |
| Paratype 12 | 28.3 | 12.5 | (Philippines) | ANSP 421620 |
| Paratype 13 | 36.6 | 15.0 | (Philippines) | * |
| Paratype 14 | 35.8 | 14.7 | Panglao Is. | BMSM 38673 |
| Paratype 15 | 35.5 | 13.2 | (Philippines) | * |
| Paratype 16 | 27.2 | 11.5 | Aliguay | MNHN 21132 |
| Paratype 17 | 26.9 | 11.5 | Aliguay | * |
| Paratype 18 (Fig 2) | 18.5 | 7.4 | Aliguay | MSI |
| Paratype 19 | 25.5 | 11.4 | Aliguay | * |
| Paratype 20 | 33.7 | 13.5 | Aliguay | * |
| Paratype 21 | 32.5 | 13.6 | Balicasag Is. | * |
| Paratype 22 | 15.8 | 6.4 | Panglao Is. | * |
| Paratype 23 | 33.3 | 14.2 | Minabe, Wakayama, Japan | * |
| Paratype 24 | 28.8 | 12.4 | Olango Is. | * |
Table III.
Conus rizali , summary of type specimens
| Length | Width | Locality (PI) | Depository (Cat#) | |
|---|---|---|---|---|
| Holotype (Figs 3, 6) | 26.6 | 12.4 | Philippines | MSI |
| Paratype 1 | 36.3 | 14.0 | Philippines | * |
| Paratype 2 (Figs 1, 3) | 26.1 | 10.2 | Philippines | MSI |
| Paratype 3 | 34.5 | 13.9 | Philippines | * |
| Paratype 4 | 38.2 | 15.8 | Philippines | ANSP 421621 |
| Paratype 5 | 37.3 | 14.8 | Philippines | MCZ 361610 |
| Paratype 6 | 39.0 | 14.5 | Philippines | MNHN 21133 |
| Paratype 7 | 37.8 | 14.2 | Philippines | * |
| Paratype 8 | 35.9 | 14.4 | Balut Is. | * |
MSI — Marine Science Institute, University of the Philippines, Quezon City, Philippines
ANSP — Academy of Natural Sciences, Philadelphia, PA, USA
MNHN — Muséum national d’Histoire naturelle, Paris, France
MCZ — Museum of Comparative Zoology, Harvard University, Cambridge, MA, USA
BMSM — Bailey-Matthews Shell Museum, Sanibel FL, USA
FMHN — Field Museum of Chicago, Chicago IL, USA
Lc — Zoological Museum of Moscow State University, Moscow, Russia
These specimens will be donated to public museums, but have not yet been assigned.
Footnotes
Support for this work was provided by grants from the NIHGMS PO1 GM048677 (to BMO) and the NIHGMS Diversity Supplement Fellowship 3 PO1 GM048677-13S1 (to JSB)
Literature Cited
- Bandyopadhyay PK, Stevenson BJ, Ownby JP, Cady MT, Watkins M, Olivera BM. The mitochondrial genome of Conus textile, coxI-coxII intergenic sequences and Conoidean evolution. Molecular Phylogenetic Evolution. 2008;46:215–23. doi: 10.1016/j.ympev.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Motta AJ. A systematic classification of the gastropod family Conidae at the generic level. La Conchiglia; Rome: 1991. [Google Scholar]
- Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–4. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lim CF, Wee VTH. Southeast Asian Conus, a seashells book. Seaconus Private Limited; 1992. [Google Scholar]
- Maddison DR, Maddison WP. MacClade 4.08. 2005 [Google Scholar]
- Marsh JA. Cone Shells of the World. Jacaranda Press; Melbourne: 1964. [Google Scholar]
- Okutani T. Marine Mollusks in Japan. Tokai University Press; Tokyo: 2000. pp. 619–667. [Google Scholar]
- Olivera BM. Conus peptides: biodiversity-based discovery and exogenomics. Journal of Biological Chemistry. 2006;281:31173–7. doi: 10.1074/jbc.R600020200. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Walker C, Cartier GE, Hooper D, Santos AD, Schoenfeld R, Shetty R, Watkins M, Bandyopadhyay P, Hillyard DR. Speciation of cone snails and interspecific hyperdivergence of their venom peptides. Potential evolutionary significance of introns. Annals of New York Academy of Science. 1999;870:223–237. doi: 10.1111/j.1749-6632.1999.tb08883.x. [DOI] [PubMed] [Google Scholar]
- Reeve LA. Conus. L. Reeve & Co; London: 1849. Monograph of the genus. [Google Scholar]
- Robin A. Encyclopedia of Marine Gastropods. XENPHORA and ConchBooks; Hackenheim, Germany: 2008. [Google Scholar]
- Röckel D, Korn W, Kohn AJ. Manual of the Living Conidae (Vol. I: Indo-Pacific Region) Verlag Christa Hemmen; Wiesbaden, Germany: 1995. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Shikama T, Habe T. A New Japanese Cone, Turriconus nakayasui with Reference to Embrikena stupa group. Venus (the Japanese Journal of Malacology) 1968;26:3–4. [Google Scholar]
- Springsteen FJ, Loebrera FM. Shells of the Philippines. Carfel Seashell Museum; Manila, Philippines: 1986. [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Thach NN. Shells of Vietnam. ConchBooks; Hackenheim, Germany: 2005. [Google Scholar]
- Walls JG. Cone Shells. A synopsis of the living Conidae. TFH Publications Inc. Ltd; Hong Kong: 1979. [Google Scholar]