Abstract
BACKGROUND
Histologic inflammation correlates with the degree of baseline olfactory dysfunction in patients with chronic rhinosinusitis (CRS), however factors associated with improvement in olfactory status after endoscopic sinus surgery (ESS) remain elusive.
OBJECTIVE
Our purpose was to compare histopathologic findings in CRS patients with olfactory loss and evaluate whether inflammatory markers can predict long-term olfactory improvement after ESS.
METHODS
Adult (≥18 years) patients with CRS were prospectively enrolled after electing ESS due to failed medical management. Mucosal tissue specimens were collected at the time of surgery and underwent pathlogic review in a blinded fashion. Subjects completed the 40-item Smell Identification Test (SIT) preoperatively and at least 6 months postoperatively. Multivariate logistic regression was used to identify histologic factors associated with postoperative improvement in SIT score.
RESULTS
The final cohort was comprised of 101 patients with a mean follow-up of 16.7 ± 6.0 months. Mean mucosal eosinophil count was higher in patients with hyposmia and anosmia (p<0.001). Patients with preoperative anosmia were more likely to have greater severity of BM thickening compared to subjects with hyposmia or normosmia (p=0.021). In patients with olfactory dysfunction, 54.7% reported olfactory improvement of at least 4 points on postoperative SIT scores. After controlling for nasal polyposis, histologic variables were not associated with postoperative improvement in olfaction.
CONCLUSIONS
Patients with severe olfactory dysfunction were more likely to have mucosal eosinophilia and basement membrane thickening on ethmoid histopathologic examination compared to normosmics. The presence of specific histologic inflammatory findings did not however predict olfactory improvement after surgery.
Keywords: Chronic rhinosinusitis, olfaction, histology, eosinophils, eosinophilia, pathology, sinusitis, endoscopic sinus surgery, inflammation
INTRODUCTION
Olfactory loss is considered a defining symptom of chronic rhinosinusitis (CRS) according to the latest American Academy of Otolaryngology – Head and Neck Surgery (AAOHNS) clinical practice guideline.1 An estimated 500,000 surgical procedures are performed each year to address symptoms related to CRS, including olfactory dysfunction.2 A common question posed by patients with CRS and olfactory loss is the likelihood that endoscopic sinus surgery (ESS) will improve their sense of smell. Substantial evidence exists which supports the efficacy of ESS to improve most sinusitis-specific symptoms including nasal obstruction, facial pressure, and nasal discharge.3–6 Despite these findings, improvement in olfaction following ESS has been less consistent and more difficult to predict.7–10
Olfactory deficits in the setting of CRS have long been attributed to a conductive mechanism, wherein mucosal edema or polyps restrict airflow to the olfactory cleft.7 More recently, the importance of a sensorineural component has been recognized.11 In this sensorineural mechanism, direct damage to the neuroepithelium from chronic inflammation results in olfactory loss, independent of whether odorant molecules have access to the olfactory mucosa. In a study by Kern, mucosal biopsies were taken both from the sinuses and olfactory cleft in patients undergoing ESS for CRS.11 A similar inflammatory infiltrate of lymphocytes, macrophages, and eosinophils was found in both regions of the sinonasal cavity. The extent of mucosal inflammation appeared to correlate with the degree of baseline olfactory dysfunction, however definitive conclusions could not be made. We have previously published histopathologic findings from this cohort of patients with CRS.12,13 In these studies, the presence of specific inflammatory markers, namely eosinophilia, was found to correlate not only with baseline disease severity but also quality-of-life outcomes after ESS. What remains unknown is whether these same histopathologic findings correlate with baseline olfactory dysfunction and whether they can be used to predict olfactory outcomes after ESS. The purpose of the present study was to compare histopathologic findings in CRS patients with varying levels of olfactory loss and evaluate whether these findings can predict baseline olfactory dysfunction and long-term olfactory improvement after endoscopic sinus surgery.
METHODS
Study Population
Adult (≥18 years) subjects with CRS were prospectively enrolled from an academic, tertiary care rhinology practice after electing to undergo ESS. All subjects were diagnosed with CRS according to guidelines established by the Rhinosinusitis Task Force.14 Inclusion criteria required a failure of medical management, including at least three weeks of broad-spectrum or culture directed antibiotics in addition to a trial of topical nasal steroid therapy. Demographic and comorbidity data were collected by the Principal Investigator (PI) during normal in-take procedures and included: age, gender, nasal polyposis, asthma, acetylsalicyclic (ASA) intolerance, allergic rhinitis (confirmed via skin prick testing or modified radioallergic sorbent testing), septal deviation, history of prior sinus surgery, and current smoking. Baseline objective disease severity was assessed using computed tomography (CT) (Lund-Mackay) and endoscopy (Lund-Kennedy) scoring systems.15,16 The Institutional Review Board at OHSU provided approval of study protocol and the informed consent process
Olfactory assessment
Olfactory function was quantified using the Smell Identification Test (SIT, Sensonics Inc., Haddon Heights, NJ). The SIT is a validated, forced choice, “scratch and sniff” test utilizing microencapsulated odorant strips (score: 0–40).17 Patients with preoperative SIT scores ≤ 5 were excluded from further analysis due to possible malingering. Subjects were asked to complete the SIT preoperatively and again at postoperative time points during the course of standard follow-up. Patients with greater than 6 months follow-up were assessed with the longest follow-up point used for outcome analysis.
Histologic Evaluation
All subjects were prescribed perioperative medical management for ongoing sinus symptoms and to prepare the mucosa for ESS. This included an oral prednisone taper seven days prior to surgery (4 days of 30mg/day followed by 3 days of 20mg/day), oral antibiotics, and continued nasal steroid application or allergy therapy when necessary. Sinus mucosal tissue was removed from the ethmoid cavity at the time of surgery. This included the tissue necessary to complete a standard endoscopic ethmoidectomy. Hematoxylin and eosin stains (H&E) of mucosal tissue were performed as previously reported.12 Histological review was performed in a blinded fashion by a single, board-certified surgical pathologist using a binocular microscope.
Histologic analysis was performed with the goal of identifying and quantifying eleven distinct cellular, epithelial, and stromal inflammatory markers (Table 1). The area of densest cellular infiltrate within each sample was utilized to consistently classify subjects. All cellular markers were recorded as absolute number per high power field (HPF). Epithelial markers were categorized into determinations of inflammatory severity: goblet cells were quantified as a percentage of epithelial cells within 200–250μm mucosal fragments, basement membrane (BM) thickening was measured in curettage fragments with optimal transverse orientation, and squamous metaplasia was measured using a semi-quantitative ordinalization. Likewise, all stromal markers were measured and categorized in a semi-quantitative fashion using a similar grading scheme.
Table 1.
Histologic measures of cellular, epithelial, and stromal markers of inflammation
| Histologic markers | Variable: | Measurement: |
|---|---|---|
| Cellular markers: | ||
| Eosinophils | Continuous | Absolute number/HPF |
| Mast cells | Continuous | Absolute number/HPF |
| Lymphocytes | Continuous | Absolute number/HPF |
| Plasma cells | Continuous | Absolute number/HPF |
| Macrophages | Continuous | Absolute number/HPF |
| Neutrophils | Continuous | Absolute number/HPF |
| Epithelial markers: | ||
| Goblet cells | Continuous | Percentage (%) |
| BM thickening | Ordinal | <5μm, 5–10μm, 10–15μm, 15+μm |
| Squamous metaplasia | Ordinal | None; focal/mild; moderate; marked/severe |
| Stromal markers: | ||
| Subepithelial edema | Ordinal | None; focal/mild; moderate; marked/severe |
| Mucosal fibrosis | Ordinal | None; focal/mild; moderate; marked/severe |
HPF = high powered field (×400 power), BM = basement membrane, μm = micrometer
Statistical analysis
Data was collected on standardized clinical research forms and analyzed using SPSS v.17.0 statistical software (SPSS Inc., Chicago, IL.). Patients were categorized according to SIT scores into normosmic, hyposmic, and anosmic groups based on gender adjusted normative distributions (Table 2).18 Differences in demographic factors, clinical characteristics, diagnostic testing, and baseline histologic inflammatory markers were assessed across preoperative olfactory categories using the Kuskall-Wallis and chi-square tests where appropriate. All ordinal measures of histologic review were collapsed into dichotomous measures due to small and zero cell sizes. Subjects with >5 eosinophils/HPF were considered to have a positive indication of eosinophilia.12,19 Postoperative change in SIT scores across olfactory categories was assessed using the Wilcoxon signed rank test. Multivariate logistic regression modeling was used to adjust for independent risk factors which might confound postoperative improvement in SIT score. Preliminary models included all individual histologic variables as the main exposure variables of interest as well as other cofactors with univariate significance (p ≤ 0.25). The main dependent variable of interest was postoperative improvement in SIT score, defined by an absolute postoperative change of ≥ 4 points (10%).20 Covariates were introduced into all bivariate models with each histologic factor to assess confounding. A covariate resulting in an alteration of the effect estimate for each histologic variable by at least 10% was considered a significant confounder. Final models were chosen with manual backwards selection in a stepwise fashion. Mean scores and standard deviations (SD) are reported for descriptive statistics. Odds ratios and 95% confidence intervals are reported for all regression modeling.
Table 2.
Olfactory diagnostic status using gender adjusted normative determinations of Smell Identification Test (SIT) scores
| SIT Score | Olfactory Diagnosis |
|---|---|
| 0 – 5 | Possible malingering |
| 6 – 18 | Anosmia |
| 19 – 33 | Hyposmia (males) |
| 26 – 34 | Hyposmia (females) |
| 34 – 40 | Normosmia (males) |
| 35 – 40 | Normosmia (females) |
RESULTS
Demographic Data and Baseline Clinical Characteristics
A total of 110 patients had histologic samples and preoperative SIT scores available for analysis. One patient was excluded for possible malingering and eight failed to complete postoperative olfactory evaluations, leaving a final cohort of 101 patients. The final cohort had a mean follow-up of 16.7 ± 6.0 months and included 24 anosmics, 40 hyposmics, and 37 normosmics. Baseline demographic and clinical characteristics are shown in Table 3. Patients with olfactory dysfunction were more likely to be older, of male gender, and have worse CT and endoscopy scores. Nasal polyposis, asthma, aspirin intolerance, and allergic rhinitis were also more common in anosmic patients compared to patients with less severe olfactory impairment.
Table 3.
Differences in demographic measures, clinical characteristics, and diagnostic testing across olfactory status for subjects with chronic rhinosinusitis
| Normosmic (n=37) | Hyposmic (n=40) | Anosmic (n=24) | |||||
|---|---|---|---|---|---|---|---|
| mean ± SD | n (%) | mean ± SD | n (%) | mean ± SD | n (%) | p-value | |
| Demographics: | |||||||
| Age | 42.7 ± 12.4 | 50.1 ± 13.4 | 47.5 ± 13.5 | 0.044 | |||
| Follow-up (months) | 16.0 ± 6.6 | 16.6 ± 6.4 | 17.3 ± 4.4 | 0.796 | |||
| Gender | |||||||
| Male | 11 (29.7) | 23 (57.5) | 17 (70.8) | ||||
| Female | 26 (70.3) | 17 (42.5) | 7 (29.2) | 0.004 | |||
| Clinical characteristics: | |||||||
| Nasal polyposis | 10 (27.0) | 18 (45.0) | 22 (91.7) | <0.001 | |||
| Asthma | 12 (32.4) | 8 (20.0) | 21 (87.5) | <0.001 | |||
| ASA intolerance | 2 (5.4) | 2 (5.0) | 7 (29.2) | 0.010 | |||
| Allergic rhinitis | 15 (40.5) | 12 (30.0) | 2 (8.3) | 0.024 | |||
| Septal deviation | 9 (24.3) | 15 (37.5) | 4 (16.7) | 0.166 | |||
| Prior sinus surgery | 18 (48.6) | 20 (50.0) | 14 (58.3) | 0.739 | |||
| Current smoker | 3 (8.1) | 3 (7.5) | 1 (4.2) | 0.825 | |||
| Diagnostic testing: | |||||||
| Lund-Mackay CT score | |||||||
| Preoperative | 10.3 ± 5.5 | 11.8 ± 5.8 | 19.5 ± 4.6 | <0.001 | |||
| Lund-Kennedy endoscopy score | |||||||
| Preoperative | 6.2 ± 5.1 | 7.1 ± 4.1 | 11.6 ± 3.5 | <0.001 | |||
| Postoperative | 4.6 ± 3.7 | 3.9 ± 3.0 | 7.4 ± 4.0 | 0.006 | |||
SD = standard deviation, ASA = acetylsalicylic acid, CT = computed tomography. Bolded p-values are statistically significant at the 0.05 alpha level.
Baseline Histologic Measures
Baseline histologic findings are shown in Table 4. In regards to cellular markers of inflammation, average mucosal eosinophil count was higher in patients with hyposmia and anosmia (p<0.001), although each olfactory group displayed a wide range of eosinophil counts. A significantly higher percentage of subjects with anosmia also presented with eosinophil counts >5/HPF (p<0.001). No differences were found across olfactory categories in regards to absolute counts of mast cells, plasma cells, or macrophages. Lymphocytic cellular markers were present in all ethmoid mucosal samples, with no significant differences between olfactory categories. Neutrophils were not noted in any biopsy samples during histologic review. Patients with preoperative anosmia were more likely to have greater severity of BM thickening compared to subjects with hyposmia or normosmia (p=0.021). No further difference between olfactory groups was seen for any other epithelial or stromal inflammatory markers. When controlling for the presence of polyps, neither eosinophils nor BM thickening continued to be associated with baseline olfactory dysfunction.
Table 4.
Differences in histologic measures of inflammatory markers within olfactory mucosa across preoperative olfactory status.
| Normosmic (n=37) | Hyposmic (n=40) | Anosmic (n=24) | |||||
|---|---|---|---|---|---|---|---|
| Histologic measures | mean ± SD | n (%) | mean ± SD | n (%) | mean ± SD | n (%) | p-value |
| Cellular markers: | |||||||
| Eosinophils | 47.4 ± 88.2 | 57.3 ± 130.7 | 140.0 ± 167.3 | 0.001 | |||
| >5/HPF | 18 (48.6) | 13 (32.5) | 20 (83.3) | <0.001 | |||
| Mast cells | 0.2 ± 0.8 | 0.4 ± 1.3 | 0.08 ± 0.4 | 0.406 | |||
| Lymphocytes | 248.5 ± 405.0 | 335.6 ± 368.1 | 303.4 ± 360.8 | 0.069 | |||
| Plasma cells | 28.5 ± 45.9 | 30.2 ± 52.6 | 33.3 ± 44.8 | 0.578 | |||
| Macrophages | 0.3 ± 1.8 | 0.1 ± 0.6 | 0.3 ± 1.0 | 0.855 | |||
| Epithelial markers: | |||||||
| Goblet cells (%) | 20.4 ± 23.1 | 21.4 ± 22.8 | 18.3 ± 23.3 | 0.822 | |||
| BM thickening ≥5μm | 17 (45.9) | 19 (47.5) | 19 (79.2) | 0.021 | |||
| Squamous metaplasia | 22 (59.5) | 19 (47.5) | 14 (58.3) | 0.522 | |||
| Stromal markers: | |||||||
| Subepithelial edema | 26 (70.3) | 28 (70.0) | 21 (87.5) | 0.236 | |||
| Mucosal fibrosis | 22 (59.5) | 33 (82.5) | 16 (66.7) | 0.079 | |||
SD = standard deviation, HPF = high powered field (×400 power), BM = basement membrane, μm = micrometer. Bolded p-values are statistically significant at the 0.05 alpha level.
Postoperative Change in Olfaction
Mean preoperative and postoperative SIT scores were calculated for each olfactory subgroup (Figure 1). On average, normosmics reported similar olfactory function both preoperatively (36.1 ± 1.4; SIT range 34–39) and postoperatively (34.0 ± 6.5), however some subjects (n=6) did report worse postoperative olfactory status (SIT range: 7–38). Hyposmics reported similar olfactory SIT scores both preoperatively (28.8 ± 4.2; SIT range: 19–34) and postoperatively (31.2 ± 4.9; SIT range: 15–38). Anosmics displayed the greatest overall improvement in olfactory function from average preoperative SIT scores (9.6 ± 2.6; SIT range: 6–15) to mean postoperative results (21.8 ± 10.0; SIT range: 7–36; p<0.001). In patients with olfactory dysfunction, a total of 35 of 64 patients (54.7%) reported olfactory improvement of 4 points or greater on postoperative SIT scores, including improvement in 18/24 anosmics (75.0%) and 17/40 hyposmics (42.5%). As expected, none of the normosmic subjects reported postoperative improvement.
Figure 1.
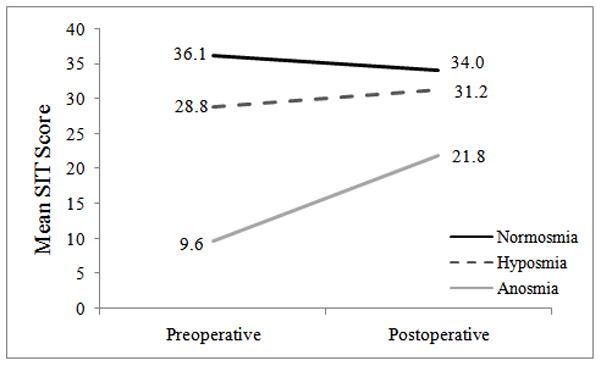
Postoperative trends in olfactory improvement by olfactory status
Univariate Modeling for Olfactory Improvement
Univariate screening for demographic cofactors found a significantly higher percentage of males reporting improvement in olfactory function (68.6%). Clinical cofactors associated with significant improvement in SIT scores included the presence of polyps (p=0.006, OR: 3.6, 95% CI: 1.4, 8.0), asthma (p=0.043, OR: 2.4, 95% CI: 1.0, 5.5), ASA intolerance (p=0.042, OR: 3.9, 95% CI: 1.0, 14.3), and allergic rhinitis (p=0.066, OR: 0.4, 95% CI: 0.1, 1.1). In addition to baseline anosmia (p<0.001, OR: 10.6, 95% CI: 3.6, 30.9) baseline CT scores (p<0.001, OR: 1.1, 95% CI: 1.1, 1.2) and endoscopy scores (p=0.006, OR: 1.1, 95% CI: 1.0, 1.2) were significantly associated with olfactory improvement.
Univariate screening for independent histologic variables found that only subjects with >5 eosinophils/HPF (p=0.166, OR: 1.8, 95% CI: 0.8, 4.1) and subepithelial edema (p=0.155, OR: 2.1, 95% CI: 0.8, 5.9) were marginally associated with improvement in SIT scores. None of the remaining cellular, epithelial, or stromal inflammatory markers was associated with olfactory improvement.
Multivariate Modeling for Olfactory Improvement
In order to control for potential confounding effects, multivariate modeling was used to adjust for demographic and clinical characteristics found to be significant on univariate analysis. On multivariate analysis, the presence of polyps and allergic rhinitis remained highly associated with olfactory improvement, whereas male gender, asthma, and ASA intolerance were no longer significant. After adjusting for the presence of polyps, mucosal eosinophilia was no longer associated with olfactory improvement (p=0.638). Similarly, subepithelial edema was no longer predictive of olfactory improvement after controlling for polyp status (p=0.812).
DISCUSSION
This study sought to determine whether specific histopathologic findings are present in CRS patients with varying degrees of olfactory dysfunction and whether these findings could be used as clinically-relevant predictors of olfactory function after surgery. Mucosal eosinophilia and basement membrane thickening were more commonly seen in patients with severe olfactory dysfunction. This finding was similar to Kern’s study which found more intense inflammation in patients with olfactory dysfunction11. However, when controlling for the presence of polyps, neither eosinophils nor BM thickening were independent predictors of baseline olfactory dysfunction. In fact, 22 (92%) anosmic patients presented with nasal polyposis which increases the likelihood of perfect confounding by polyp status rather than a true association between histopathology and olfactory dysfunction. Given that histologic findings did not correlate with baseline olfactory dysfunction, it was not surprising that histology also failed to predict olfactory improvement after surgery.
The concept of conductive and sensorineural mechanisms of olfactory loss in CRS certainly seems probable based on available evidence.21 However, the relative contribution of each mechanism to olfactory loss is unknown. Furthermore, the mechanism by which available treatments, either surgical or medical, improve olfactory function is not clearly established. For example, patients with nasal polyposis and anosmia will sometimes regain olfactory function after prolonged courses of oral steroids. This outcome may be due to shrinkage of polyps with improved access of odorants to the olfactory cleft, resolution of direct mucosal inflammation in the olfactory cleft, or both.21 Sinus surgery in patients with nasal polyps serves to physically remove polyps and improve airflow throughout the sinonasal cavity. However, surgery also likely decreases the overall state of mucosal inflammation by clearing chronic infection and promoting mucous drainage. Very few studies have sought to directly biopsy the olfactory mucosa in disease states such as CRS. This is not surprising given the difficulty of safely accessing the olfactory cleft and theoretical risks of permanent smell loss. It is seemingly unlikely that elucidation of the precise mechanism of baseline olfactory loss will be possible without further attempts at direct biopsy both before and after available treatments.
In this cohort, 75% of anosmic patients demonstrated improvement in olfactory function after ESS. Despite this improvement, most patients remained in the severely hyposmic range. It remains unknown why these patients were left with significant olfactory dysfunction, given that ESS serves to physically remove obstructing polyps and diminish the overall inflammatory state of the sinonasal mucosa. One possibility is that a certain amount of olfactory loss is permanent. Some have proposed that pathologic insults such as sinusitis may trigger uncompensated increases in olfactory cell death with a net loss of olfactory sensory neurons over time.21–23 Additionally, it is known that olfactory function decreases with age, likely due to the cumulative effects of environmental exposure with replacement of olfactory mucosa by respiratory epithelium.24 If these processes are indeed occurring, then the genetic susceptibility of the host may be as relevant as the specific nature or intensity of the histopathologic insult.
There are several important limitations of this study which increase the chance of a type II error (no association found between histologic inflammation and olfactory improvement when, in fact, one exists). Patients in this limited cohort were on a short course of oral steroids prior to intraoperative ethmoid mucosal sampling. We did not control for steroid use at the time of surgery which has the theoretical potential to decrease the degree of inflammatory markers seen in mucosal specimens. Steroids may effectively reduce the presence of histologic markers and confound (dilute) the relationship between histologic markers and olfactory improvement. Secondly, mucosal specimens were taken from the ethmoid cavity as opposed to the olfactory mucosa. Patients with CRS have sinus mucosal inflammation by definition; however, it is not necessarily true that all patients with CRS also have inflammation of the olfactory mucosa. In the study by Kern et al., a similar inflammatory infiltrate was present in the sinus muocsa and olfactory mucosa; however, the degree of inflammation was generally greater in the sinus mucosa compared to the olfactory mucosa.11 If the severity of olfactory inflammation is truly less than sinus mucosal inflammation, the prevalence of inflammatory markers measured in this study may have been insufficient to show a true association as measured.
CONCLUSION
Olfactory dysfunction was a common finding in patients with CRS. Patients with severe olfactory dysfunction were more likely to have mucosal eosinophilia and basement membrane thickening on ethmoid histopathologic examination compared to those with normal olfaction. Despite these findings, the presence of specific histologic inflammatory findings did not help predict olfactory improvement after surgery. Olfactory loss in the setting of CRS remains a troublesome problem and further study is warranted to understand olfactory outcomes following sinus surgery.
Acknowledgments
This study was made possible by a grant from the National Institute on Deafness and Other Communcation Disorders (R01 DC005805), one of the National Institutes of Health, Bethesda, MD, and support from the Department of Ototlaryngology – Head and Neck Surgery and the Department of Pathology, Oregon Health & Science University, Portland, Oregon.
Grant funded by the National Institute on Deafness and other Communication Disorders, Bethesda, MD. R01 DC005805 (PI: Smith, TL)
Footnotes
The Institutional Review Board at OHSU provided approval and oversight for all research activities.
Public clinical trial registration (http://www.clinicaltrials.gov) ID: NCT00799097
Accepted for oral presentation at the American Rhinologic Society Meeting at 113th Annual Combined Otolaryngological Spring Meetings (COSM) in Las Vegas, NV., April 28-May 2, 2010.
References
- 1.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline. Otolaryngol Head Neck Surg. 2007;137(Suppl):S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 2.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996 National Center for Health Statistics. Vital Health Stat. 1998;13(139):25. [PubMed] [Google Scholar]
- 3.Terris MH, Davidson TM. Review of published results for endoscopic sinus surgery. ENT Journal. 1994;73:574–580. [PubMed] [Google Scholar]
- 4.Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108(2):151–157. doi: 10.1097/00005537-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Gliklich RE, Metson R. Effect of sinus surgery on quality of life. Otolaryngol Head Neck Surg. 1997;117:12–17. doi: 10.1016/S0194-59989770199-2. [DOI] [PubMed] [Google Scholar]
- 6.Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. Am J Rhinol. 2008;22(3):297–301. doi: 10.2500/ajr.2008.22.3172. [DOI] [PubMed] [Google Scholar]
- 7.Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope. 2001;111:409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litvak JR, Fong K, Mace J, et al. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008;118:2225–2230. doi: 10.1097/MLG.0b013e318184e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(2):139–44. doi: 10.2500/ajra.2009.23.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang RS, Su MC, Liang KL, et al. Preoperative prognostic factors for olfactory change after functional endoscopic sinus surgery. Am J Rhinol Allergy. 2009;23(1):64–70. doi: 10.2500/ajra.2009.23.3262. [DOI] [PubMed] [Google Scholar]
- 11.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000;110:1071–1077. doi: 10.1097/00005537-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(4):454–461. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soler ZM, Sauer DA, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality of life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;42(1):64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 15.Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinol. 1993;107:183–184. [PubMed] [Google Scholar]
- 16.Lund VJ, Kennedy DW. Quantification for staging sinusitis. Ann Otol Rhinol Laryngol. 1995;104:17–21. [PubMed] [Google Scholar]
- 17.Doty RL, Newhouse MG, Azzalina JD. Internal consistency and short-term test- retest reliability of the University of Pennsylvania Smell Identification Test. Chem Senses. 1985;10:297–300. [Google Scholar]
- 18.Doty RL. Administration Manual. 3. Haddon Heights, New Jersey: Sensonics; 1995. The Smell Identification TestTM; pp. 1–17. [Google Scholar]
- 19.Kountakis SE, Arrango P, Bradley Dewayne, et al. Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope. 2004;114(11):1895–1905. doi: 10.1097/01.mlg.0000147917.43615.c0. [DOI] [PubMed] [Google Scholar]
- 20.Doty RL, Yousem DM, Pham LT, et al. Olfactory dysfunction in patients with head trauma. Arch Neurol. 1997;54:1131–1140. doi: 10.1001/archneur.1997.00550210061014. [DOI] [PubMed] [Google Scholar]
- 21.Raviv JR, Kern RC. Chronic sinusitis and olfactory dysfunction. Otolaryngol Clin North Am. 2004;37:1143–1157. doi: 10.1016/j.otc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Vent J, Robinson AM, Gentry-Nielson MJ, et al. Pathology of the olfactory epithelium: smoking and ethanol exposure. Laryngoscope. 2004;114:1383–1388. doi: 10.1097/00005537-200408000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Kern RC, Conley DB, Haines GK, Robinson AM. Pathology of the olfactory mucosa: implications for the treatment of olfactory dysfunction. Laryngoscope. 2004;114:279–285. doi: 10.1097/00005537-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Schiffman SS. Taste and smell loses in normal aging and disease. JAMA. 1997;278:1357–1362. [PubMed] [Google Scholar]


