Methylmalonic acidemia is an autosomal recessive metabolic disorder caused by a deficiency in the mitochondrial enzyme, methylmalonyl-CoA mutase (MUT). In this study, Carrillo-Carrasco and colleagues demonstrate that a single intrahepatic injection of AAV8 encoding the Mut gene under control of the liver-specific thyroxine-binding globulin promoter is sufficient to rescue Mut −/− mice from neonatal lethality and provides long-term phenotypic correction.
Abstract
Methylmalonic acidemia is a severe metabolic disorder caused by a deficiency of the ubiquitously expressed mitochondrial enzyme, methylmalonyl-CoA mutase (MUT). Liver transplantation has been used to treat a small number of patients with variable success, and whether liver-directed gene therapy might be employed in such a pleiotropic metabolic disorder is uncertain. In this study, we examined the therapeutic effects of hepatocyte-directed delivery of the Mut gene to mice with a severe form of methylmalonic acidemia. We show that a single intrahepatic injection of recombinant adeno-associated virus serotype 8 expressing the Mut gene under the control of the liver-specific thyroxine-binding globulin (TBG) promoter is sufficient to rescue Mut–/– mice from neonatal lethality and provide long-term phenotypic correction. Treated Mut–/– mice lived beyond 1 year of age, had improved growth, lower plasma methylmalonic acid levels, and an increased capacity to oxidize [1-13C]propionate in vivo. The older treated mice showed increased Mut transcription, presumably mediated by upregulation of the TBG promoter during senescence. The results indicate that the stable transduction of a small number of hepatocytes with the Mut gene can be efficacious in the phenotypic correction of an inborn error of organic acid metabolism and support the rapid translation of liver-directed gene therapy vectors already optimized for human subjects to patients with methylmalonic acidemia.
Introduction
Methylmalonyl-CoA mutase (MUT) (EC 5.4.99.2) is a mitochondrial enzyme that catalyzes the conversion of l-methylmalonyl-CoA into succinyl-CoA in the mitochondrial matrix (Fenton et al., 2001). This reaction is essential for the metabolism of propionyl-CoA, an important intermediate in the degradation of isoleucine, valine and odd-chained fatty acids. Deficiency of MUT causes isolated methylmalonic acidemia (OMIM #251000), a severe disorder of intermediary metabolism associated with multisystemic disease and lethal metabolic instability (Oberholzer et al., 1967; Stokke et al., 1967). Patients with complete absence of enzyme activity are classified as mut0 and have a poor prognosis for long-term survival despite aggressive nutritional and medical management (Matsui et al., 1983; van der Meer et al., 1994; Nicolaides et al., 1998; de Baulny et al., 2005; Dionisi-Vici et al., 2006; Hörster et al., 2007).
Elective liver (Kayler et al., 2002; Nyhan et al., 2002; Hsui et al., 2003; Kaplan et al., 2006b; Kasahara et al., 2006) and/or combined liver–kidney transplantation (van ‘t Hoff et al., 1998; Nagarajan et al., 2005) has been used to treat a subset of mut0 patients. Although solid organ recipients do not experience peripheral metabolic correction after transplantation (van ‘t Hoff et al., 1998; Nagarajan et al., 2005; Kaplan et al., 2006b), they have improved growth and protection from intermittent metabolic crises. These observations suggest that gene and cell therapy directed toward the liver might have therapeutic effects in the treatment of methylmalonic acidemia. However, the variability in the selection of candidates for liver transplantation, timing of the surgery, lack of standard monitoring pre- and postoperatively, and uniqueness in individual biochemical genetic phenotypes of recipient patients have confounded the ability to clearly establish the efficacy of liver transplantation as a treatment for methylmalonic acidemia. Experimental studies will be needed to carefully address this important clinical issue.
We have previously reported the temporal correction of a murine model of mut0 methylmalonic acidemia by adenovirus-mediated gene therapy (Chandler and Venditti, 2008) and described long-term correction in the same model, using a recombinant adeno-associated virus serotype 8 (rAAV8) vector that expressed the Mut gene from the enhanced chicken β-actin (CBA) promoter (Chandler and Venditti, 2010). In wild-type mice, the Mut enzyme is present ubiquitously (Chandler et al., 2007a) but is most highly expressed and active in the liver (Wilkemeyer et al., 1993), an organ that plays a central role in methylmalonyl-CoA metabolism. The rAAV8-CBA-mMut-treated Mut–/– mice showed evidence of transgene expression in numerous organs, including the liver, skeletal muscle, and heart, with significant levels of Mut protein detected in the liver (Chandler and Venditti, 2010). Although the contribution of Mut enzymatic activity from each tissue to the corrected phenotype was not discernable, the sustained improvement in the Mut–/– mice in our earlier rAAV experiments might largely be explained by expression of the Mut transgene in hepatocytes.
In this study, we explore the therapeutic efficacy of liver-directed gene therapy as a potential treatment for methylmalonic acidemia. In addition to targeting a cell type established to be central in the pathology of methylmalonic acidemia (Hayasaka et al., 1982; Krahenbuhl et al., 1991; Chandler et al., 2009; de Keyzer et al., 2009), selective expression of the Mut gene in the liver might help minimize transgene-directed immune responses (Franco et al., 2005) and afford rapid translation to the development of an rAAV8-MUT vector similar to those given to human subjects with hemophilia (Manno et al., 2006) and α1-antitrypsin deficiency (Brantly et al., 2009). An rAAV8 vector that expresses the Mut gene from the liver-specific thyroxine-binding globulin (TBG) promoter (Hayashi et al., 1993; Xiao et al., 1998) was constructed and delivered to neonatal Mut–/– mice. Hepatic expression of the viral Mut transgene completely rescues Mut–/– mice from neonatal lethality, restores growth, and affords long-term survival and yet, exactly as is observed in methylmalonic acidemia patients who have received liver transplants, it does not completely correct biochemical abnormalities. Expression of the Mut gene in a small percentage of stably transduced cells accounts for the clinical effects and is higher in older mice, likely because of senescence upregulation of the transgene from the TBG promoter. The results of our experiments have therapeutic implications for other disorders of intermediary metabolism, particularly inborn errors of organic acid metabolism.
Materials and Methods
Construction and production of rAAVs
The expression construct, p-AAV8-CI-TBG-RGB, was provided by the University of Pennsylvania Vector Core (Philadelphia, PA). The plasmid contains transcriptional control elements that include a cytomegalovirus enhancer, the TBG promoter, and the rabbit globin polyadenylation signal. These elements are flanked by inverted terminal repeat sequences from AAV serotype 2. Murine methylmalonyl-CoA mutase and enhanced green fluorescent protein (GFP) cDNAs were cloned into pAAV2/8.CI.TBG.RBG (see Supplementary Fig. 1 at www.liebertonline.com/hum). The expression vector pAAV2/8.CI.CB7.mMut.RBG has been described and contains the cytomegalovirus enhancer/chicken β-actin promoter driving the expression of the murine methylmalonyl-CoA mutase cDNA (Chandler and Venditti, 2010). The vector genomes were packaged into an AAV8 capsid, purified by cesium chloride gradient purification, and titered by quantitative PCR as previously described (Hayashi et al., 1993; Gao et al., 2002).
Animal studies
Murine experiments were approved and performed according to the regulations and standards of the National Human Genome Research Institute (NHGRI, Bethesda, MD) Animal Care and Use Committee (ACUC). The mice used harbor a deletion of exon 3 in the Mut gene. This mutation abolishes the production of mature RNA, protein, and detectable enzymatic activity (Chandler et al., 2007a). Mice homozygous for this mutation (Mut–/–) display a severe methylmalonic acidemia phenotype that is lethal in the newborn period and is accompanied by progressive elevation of methylmalonic acid (MMA) levels to 2000 μM (normal range, 5–10 μM) at the time of death. Heterozygous animals (Mut+/−) appear normal and have biochemical profiles identical to those of wild-type animals, and were used as controls throughout the study. Immediately before injection, 2 × 1011 or 4 × 1011 vector genome copies (GC) of either the pAAV2/8.CI.TBG.GFP.RGB or pAAV2/8.CI.TBG.mMut.RGB vector were diluted to a total volume of 20 μl with phosphate-buffered saline. Hepatic injections were performed on nonanesthetized neonatal mice, typically within several hours of birth, using a 32-gauge needle and transdermal approach as previously described (Chandler and Venditti, 2010).
Expression analysis
Livers from untreated mice and mice injected in the neonatal period with pAAV2/8.CI.TBG.GFP.RGB were collected at 60 days of life, embedded, frozen immediately on dry ice, and stored at −80°C. Frozen liver sections (thickness, 8 μm) were placed onto glass slides, stained with 4′,6-diamidino-2-phenylindole (DAPI), and mounted with Fluoro-Gel aqueous mounting medium (Electron Microscopy Sciences, Hatfield, PA). Random slides from each liver section were evaluated by confocal microscopy (510 NLO Meta; Carl Zeiss, Oberkochen, Germany) with fluorescein isothiocyanate (FITC) and DAPI filters for the same microscope and camera settings and using the threshold level from untreated control mice as a reference for liver background fluorescence, as previously described (Wang et al., 2010b). The total number of cells was calculated with software and manually validated. GFP-labeled cells were quantified manually by careful examination of 13 randomly selected sections by three independent observers. Each observer evaluated 680 cells and the results were averaged as the number of GFP-positive nuclei per total nuclei visualized. The GFP intensity in each image was also determined.
Quantitative real-time PCR analysis
Mut RNA expression in liver was calculated for untreated Mut–/– mice (n = 3), and for Mut+/− mice (n = 3) and Mut–/– mice treated with 2 × 1011 GC of rAAV8-TBG-mMut, 90 days (n = 3) and 270 days (n = 3) after injection. Total RNA from frozen livers was extracted, using an RNeasy mini kit (Qiagen, Valencia, CA). Residual DNA was digested with DNA-free (Ambion, Austin, TX). Mut and Serpina7 (TBG) transcripts were quantified by real-time PCR, in triplicate, using TaqMan gene expression assays with mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (4352932E), murine methylmalonyl-CoA mutase (Mm00485312_m1), and thyroxine-binding globulin (Mm00626105_m1) probes, all from Applied Biosystems (Foster City, CA), and analyzed with an Applied Biosystems 7500 real-time PCR system. Expression of Mut and Serpina7 was normalized on the basis of liver GAPDH transcript levels.
Vector genome copy number
Genome copy (GC) number was measured by quantitative real-time PCR analysis as described previously. A standard curve was prepared, using serial dilutions of a plasmid carrying the murine Mut cDNA. Genomic DNA was extracted from murine liver samples and 100 ng of DNA was used to determine the genome copy number of rAAV at 90 days (n = 2) and 270 days (n = 3) of life.
Western blotting
Tissue samples were homogenized with a 2-ml Tenbroeck tissue grinder (Wheaton Science International, Millville, NJ) in tissue protein extraction reagent (T-PER; Pierce Protein Research Products/Thermo Scientific, Rockford, IL). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was done with 35 mg of clarified liver extract and transferred to nylon membranes. Western blot analysis was performed with a rabbit polyclonal antibody against murine Mut at a 1:500 dilution (Chandler et al., 2007b) and mouse monoclonal anti-OxPhos (oxidative phosphorylation) complex III core II antibody (A-11143; Invitrogen, Carlsbad, CA) at a 1:4000 dilution; incubation was for 3 hr. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (1858415; Pierce Protein Research Products/Thermo Scientific) at a 1:10,000 dilution or HRP-conjugated goat anti-mouse antibody (1858413; Pierce Protein Research Products/Thermo Scientific) at a 1:30,000 dilution was incubated for 1 hr. Signal was detected with SuperSignal West Pico chemiluminescent substrate (34080; Pierce Protein Research Products/Thermo Scientific).
Metabolic measurements
Blood samples were obtained by retro-orbital sinus plexus sampling, using a sterile glass capillary tube at 24, 60, 90, 120, 180, 270, and 360 days of life, for measurement of plasma methylmalonic acid (MMA) levels. Samples were immediately centrifuged and plasma was collected, diluted in water, and frozen at −80°C until measurements were performed. Plasma MMA levels were quantified by gas chromatography-mass spectrometry with stable isotopic internal calibration as previously described (Marcell et al., 1985; Allen et al., 1993).
In vivo propionate oxidation studies
Mut+/−, untreated Mut–/–, and treated Mut–/– mice were injected intraperitoneally with 200 μg of sodium [1-13C]propionate (Cambridge Isotope Laboratories, Andover, MA). [1-13C]propionate oxidation was measured by collecting expired gas, using an adaptation of a method developed to study propionate oxidation in patients with methylmalonic and propionic acidemia, as previously described (Chandler and Venditti, 2010). Mice were placed into a respiratory chamber that contained a CO2 probe to allow the direct measurement of CO2 generated by each animal. An aliquot of expired air was removed from the chamber at each time point for analysis of 13C enrichment in CO2. The isotope ratio (13C/12C) of the collected breath was measured with a gas isotope ratio mass spectrometer (Metabolic Solutions, Nashua, NH). The percent dose metabolized was calculated as follows: percent dose metabolized = total 13C excreted (mmol)/dose (mmol) × 100.
Statistical analyses
In all instances, p values were considered significant if the value was less than 0.05. Differences in survival between treated groups were analyzed by χ2 test. The weights between treated and untreated mice and differences in metabolite levels were assessed by two-sided, two-tailed unpaired Student t test. The Kruskal–Wallis test was used to determine the statistical significance of differences in measured propionate oxidation rates between groups at 25 min.
Results
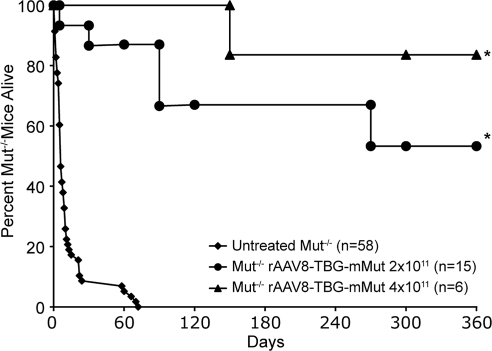
Twenty-one Mut–/– mice received a single intrahepatic injection of either 2 × 1011 GC (n = 15) or 4 × 1011 GC (n = 6) of rAAV8-TBG-mMut at birth. In the untreated mutant group, 94% perished before 21 days of life and only one of the untreated mice was alive by 72 days of life. In contrast, all but one of the Mut–/– mice that received 2 × 1011 GC of AAV8-TBG-mMut (n = 15), and all that received 4 × 1011 GC of AAV8-TBG-mMut (n = 6), were alive at 21 days of life after injection. After weaning on day 24, 93% (14 of 15) of the 2 × 1011 GC-treated mutants were alive (Fig. 1). One year after injection, 53% (8 of 15) of the Mut–/– mice that received 2 × 1011 GC of rAAV8-TBG-mMut and 83% (5 of 6) of the Mut–/– mice that received 4 × 1011 GC were still alive. In the group that received the dose of 2 × 1011 GC (n = 15) one animal died soon after birth, another was found dead at 30 days, one was killed at 90 days to harvest tissue, and another two were reported sick and were killed at 90 days of life. Another two Mut–/– mice from this group were found dead after their cage accidentally flooded at about 270 days of life. Postmortem examination of the treated Mut–/– mice that perished during the study period revealed no evident cause of death or gross abnormalities in any organ on necropsy.
FIG. 1.
Long-term survival of Mut–/– mice after liver-directed gene therapy. Survival (in days) of untreated Mut–/– mice (n = 58) and those treated with either 2 × 1011 GC (n = 15) or 4 × 1011 GC (n = 6) of rAAV8-TBG-mMut in the neonatal period. Mice treated with 4 × 1011 GC of AAV8-TBG-mMut (n = 6) had 84% survival (5 of 6) by 360 days of life compared with 53% (8 of 15) for those treated with 2 × 1011 GC of AAV8-TBG-mMut. Both treated groups had significantly increased survival at 24, 60, and 100 days and beyond (*p < 0.01) compared with the group of untreated Mut–/– mice.
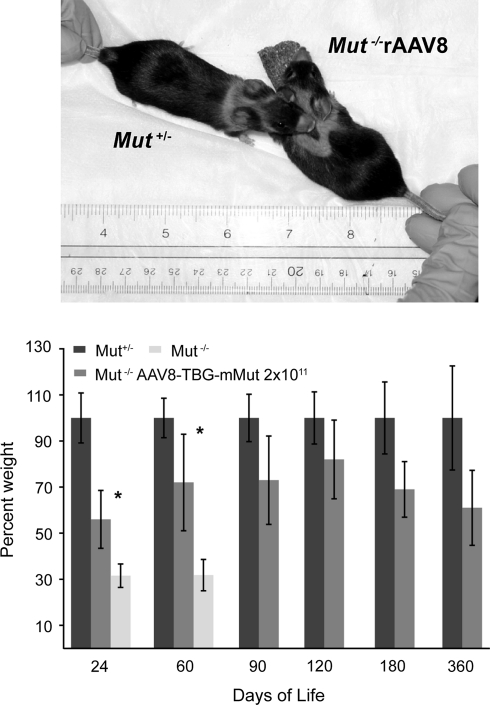
rAAV8-TBG-mMut-treated Mut–/– mice achieved and maintained 60–80% of body weight when compared with their Mut+/− littermates (Fig. 2). Treated Mut–/– mice were significantly greater in size than untreated Mut–/– mice on days 24 and 60 (p < 0.01), and close to the size of sex-matched, heterozygous treated littermates at all time points (Fig. 2, top). There were no differences between the weights of Mut–/– mice treated with 2 × 1011 or 4 × 1011 GC of rAAV8-TBG-mMut (data not presented). Although the older rAAV8-TBG-mMut-treated Mut–/– mice were slightly smaller than heterozygous controls, they were otherwise vigorous and appeared grossly normal. Although formal testing has not been performed, these animals appeared clinically well, with no obvious neurological or behavioral phenotypes (see Supplementary Video 1 at www.liebertonline.com/hum).
FIG. 2.
Improved growth parameters for mice treated with rAAV8-TBG-mMut. Weights were calculated as a percentage of sex-matched Mut+/− littermates. Error bars represent plus and minus 1 standard deviation. Treated Mut–/– mice showed improved growth compared with the group of untreated Mut–/– mice at 24 and 60 days (*p < 0.01). Results for the treated Mut–/– group compared with the treated Mut+/− group were significantly different at all points (p < 0.01). Top: Appearance at 1 year of age of a Mut–/– mouse treated with rAAV8-TBG-mMut (right) and an age- and sex-matched treated Mut+/− littermate (left).
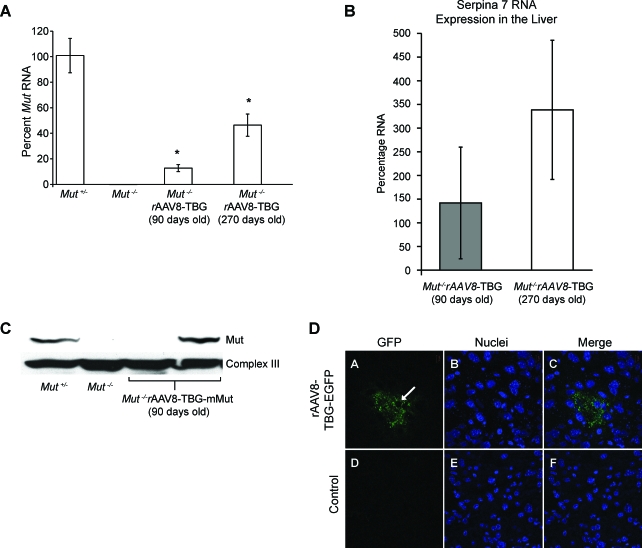
A single intrahepatic injection of rAAV8-TBG-mMut (2 × 1011 GC) at birth produced detectable levels of Mut mRNA in the liver of Mut–/– mice. When compared with the Mut RNA expression of untreated Mut+/− mice (n = 3), Mut–/– mice killed at 90 days had 13% of transcript levels (n = 3) and mice killed at 270 days of life had 46% of transcript levels (n = 3) (Fig. 3A). An immunoblot showed immune-reactive Mut protein in some of these liver samples (Fig. 3C). Transgene copy numbers in genomic DNA extracted from the livers of treated mice averaged 0.05 and 0.06 per diploid genome at 90 days (n = 2) and 270 days (n = 3) of life, respectively. Serpina7 (TBG) mRNA, derived from the endogenous genomic locus, had a 2.4-fold increase in expression at 270 days compared with 90 days of life (Fig. 3B).
FIG. 3.
Increased expression of methylmalonyl-CoA mutase in the liver of mice treated with a single intrahepatic injection of AAV8-TBG-mMut (2 × 1011 GC). (A) Liver Mut mRNA expression was determined by quantitative PCR. Treated Mut–/– mice had significantly higher mRNA expression: 13% at 90 days of life (n = 3; p < 0.01) and 46% at 270 days of life (n = 3; *p < 0.01), compared with untreated Mut+/− mice (n = 3). Untreated Mut–/– mice had less than 0.5% of the Mut mRNA expression of Mut+/− mice. (B) The expression of Serpina7 (TBG) was assayed by quantitative PCR in treated Mut–/– mice. The mice at 270 days (n = 3) had, on average, 2.4-fold increased Serpina7 RNA levels compared with younger animals (n = 3). (C) Immunoblotting showed that one of two treated Mut–/– mice studied at 90 days had hepatic Mut protein levels similar to that of Mut+/− mice. No Mut protein was detected in untreated Mut–/– mice. (D) rAAV-mediated transgene expression in the liver. Shown is GFP expression and localization in liver sections of mice killed 60 days after injection of rAAV8-TBG-GFP at 2 × 1011 GC (A–C) compared with control mice (D–F). GFP expression (A and D) and nuclear DNA (B and E) images were merged (C and F), using LSM Image Browser software (Carl Zeiss). The white arrow indicates an area where the nuclear contour is preserved with green signal present. Color images available online at www.liebertonline.com/hum.
To approximate the amount of hepatic correction achieved in our treated Mut–/– mice, we injected 2 × 1011 GC of rAAV8-TBG-GFP and evaluated the liver of mice 60 days after a neonatal intrahepatic injection. Clusters of cells accounting for approximately 9% of hepatocytes visualized were GFP positive (Fig. 3D). Attempts to perform immunohistochemistry with anti-Mut antibodies were unsuccessful.
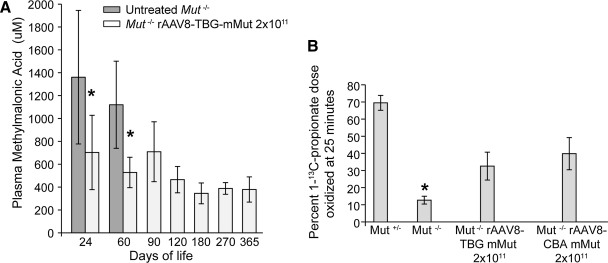
A decrease in plasma MMA concentration was used as an indirect measure of increased Mut enzymatic activity. Plasma MMA concentrations were assayed at 24, 60, 90, 120, 180, 270, and 360 days of life. Mut–/– mice treated with rAAV8-TBG-mMut at 2 × 1011 GC had significantly lower plasma MMA levels compared with untreated Mut–/– mice (Fig. 4A). Treated Mut–/– mice had mean plasma MMA levels of 709 μM (n = 11) and liver Mut mRNA expression of 13% (n = 3) at 90 days of life. At 270 days of life, the treated mice had a mean plasma MMA concentration of 379 μM (n = 8) and liver Mut mRNA expression of 46% (n = 3). There was no difference in MMA concentrations between the 2 × 1011 and 4 × 1011 GC-treated groups (data not presented). Overall, plasma MMA levels in treated Mut–/– mice remained elevated 50 to a 100 times above the levels of Mut+/− mice (5–10 μM).
FIG. 4.
Increase in methylmalonyl-CoA mutase activity in mice treated with 2 × 1011 GC of AAV8-TBG-mMut. (A) Plasma methylmalonic acid (MMA) levels at 24, 60, 90, 120, 180, 270, and 360 days of life in untreated Mut–/– mice and Mut–/– mice treated with 2 × 1011 GC of rAAV8-TBG-mMut. The mean plasma MMA levels of untreated Mut–/– mice were 1,361 μM at 24 days (n = 13) and 1,120 μM at 60 days (n = 6). The mean plasma MMA levels of treated Mut–/– mice were considerably lower at 703 μM in mice 24 days old (n = 12; *p < 0.01) and 528 μM in mice 60 days old (n = 11; *p < 0.01). Plasma MMA levels in older mice stabilized between 345 and 465 μM after 90 days (n = 4–8 at each point). The plasma MMA levels in unaffected Mut+/− mice ranged from 5 to 10 μM. (B) In vivo [1-13C]propionate oxidation in Mut–/– mice 1 year after treatment with 2 × 1011 GC of rAAV8-TBG-mMut or 2 × 1011 GC of rAAV8-CBA-mMut. Mice were injected with 200 mg of sodium [1-13C]propionate and the percentage of dose oxidized was measured at 25 min. rAAV8-TBG-mMut-treated Mut–/– mice (n = 4) oxidized 32.5 ± 8.1% of the injected tracer, compared with 12.6 ± 2.2% by untreated Mut–/– mice (n = 9) (*p < 0.01). rAAV8-CBA-mMut-treated Mut–/– mice (n = 4) oxidized 39.8 ± 9.4% of the injected tracer (p value not significant vs. rAAV8-TBG-mMut-treated mice). Untreated Mut+/− mice (n = 12) oxidized 69.4 ± 4.3% of the injected tracer at the same time point. Error bars surround the 95% confidence intervals.
Whole-animal propionate oxidation capacity, which is dependent on the functional activity of methylmalonyl-CoA mutase, was measured in an in vivo assay. Mut–/– mice at 1 year posttreatment with either 2 × 1011 GC of rAAV8-TBG-mMut (n = 4) or 2 × 1011 GC of rAAV8-CBA-mMut (n = 4) were injected with sodium [1-13C]propionate. The latter group of mice was included as a comparator for long-term, whole-body correction. The in vivo metabolism of this tracer involves a series of enzymatic reactions, including methylmalonyl-CoA mutase, and results in oxidation of [1-13C]propionate to 13CO2 via the Krebs cycle. Assuming normal activity of other enzymatic reactions, an increase in sodium [1-13C]propionate oxidized to 13CO2 by treated mice relative to untreated Mut–/– mice indicates an increase in methylmalonyl-CoA mutase activity. As can be seen in Fig. 4B, Mut+/− mice (n = 12) metabolize approximately 69.4 ± 4.3% of [1-13C]propionate into 13CO2 in 25 min whereas untreated Mut–/– mice (n = 9) oxidize 12.6 ± 2.2% of the dose, with flat enrichment kinetics. At 1 year of age, rAAV8-TBG-mMut-treated Mut–/– mice (n = 4) showed an increased capacity to metabolize [1-13C]propionate and could convert approximately 32.5 ±8.1% of the injected dose into 13CO2 in 25 min, which is not significantly different (p = 0.20) from the 39.8 ± 9.4% range observed in a group of mice treated with 2 × 1011 GC of rAAV8-CBA-mMut.
Discussion
The successful outcomes seen in some patients with methylmalonic acidemia who have received liver transplants led us to perform liver-directed gene delivery experiments in a mouse model that faithfully replicates the severest phenotype of methylmalonic acidemia. We selected the TBG promoter because it has been used in rAAVs that have successfully corrected liver-specific metabolic disorders in mice, such as ornithine transcarbamylase deficiency (Moscioni et al., 2006) and apolipoprotein deficiency (Lebherz et al., 2007), and has been extensively studied in nonhuman primates (Wang et al., 2010a). The use of a hepatotropic rAAV serotype 8 (Gao et al., 2002) further enhanced delivery of the transgene to the liver. Mut RNA levels in rAAV8-TBG-mMut-treated Mut–/– mice were consistent with the GFP expression pattern in the liver seen after treatment with rAAV8-TBG-GFP (Fig. 3), suggesting that the correction of approximately 9% of hepatocytes is responsible for maintaining long-term metabolic stability and phenotypic correction in treated Mut–/– mice.
Gene therapy with either 2 × 1011 GC of rAAV8-TBG-mMut or 2 × 1011 GC of rAAV8-CBA-mMut resulted in comparable phenotypic and metabolic correction of Mut–/– mice. For example, at 60 days the growth effects were similar between the treated mutant groups (72 ± 18% [Fig. 2] vs. 81 ± 15%; p = 0.20), as were the plasma MMA levels (528 ± 133 μM [Fig. 4B] vs. 511 ± 160 μM; p = 0.77). Even 1 year after injection, Mut–/– mice treated with 2 × 1011 GC of rAAV8-TBG-mMut had a propionate oxidative capacity that was equivalent to that observed in mice treated with 2 × 1011 GC of rAAV8-CBA-mMut (Fig. 4B). The hepatic RNA and protein expression seen at later times in Mut–/– mice treated with 2 × 1011 GC of rAAV8-TBG-mMut establish that the TBG promoter can effectively direct long-term expression in a small population of transduced cells, as the GFP reporter experiments show.
Endogenous TBG (Serpina7) transcript levels increased over time, suggesting there was an increase in the promoter-driven expression of Mut from the rAAV-TBGmMut transgene in our treated mice. Such an observation would be consistent with a prior report that established TBG as a senescence-upregulated protein in rodents (Savu et al., 1991). We therefore quantified the expression of TBG RNA at 90 and 270 days of life and found an approximate 2.4-fold increase in the levels of RNA at the later time point (Fig. 3B). Because mice at both the 90- and 270-day time points had similar genome copy levels of the rAAV8-TBG-mMut vector, the most plausible explanation for the apparent increase in Mut transcript levels at 270 days compared with 90 days in the rAAV8-TBG-mMut-treated Mut–/– mice would be transcriptional upregulation from the TBG promoter in the AAV vector, as occurs at the endogenous locus. Further studies will be required to evaluate more thoroughly whether promoter regulation may be used to modulate the effects of gene therapy in methylmalonic acidemia, but these data support the use of an rAAV configured with a liver-specific promoter, especially if such a vector could facilitate a physiological, long-term increase in the expression of a therapeutic viral transgene.
Complete biochemical correction of methylmalonic acidemia, in humans or mice, is unlikely. Patient studies have clearly, and repeatedly, documented failure of metabolites to normalize in liver (Kaplan et al., 2006a) and/or combined liver–kidney transplant recipients (van ‘t Hoff et al., 1998; Nagarajan et al., 2005). In the gene therapy experiments presented here, we evaluated plasma MMA levels to indirectly measure Mut activity, not as a definitive parameter to establish that the disease phenotype had been corrected. Numerous metabolic changes have been documented in methylmalonic acidemia, but the role(s) that each plays in the pathology of this heterogeneous disease remains unclear. AAV8-TBG-mMut-treated Mut–/– mice display substantially increased concentrations of MMA but appear grossly normal, suggesting that a small amount of Mut enzymatic activity can provide long-term phenotypic correction and demonstrating that stably transduced cells persist in the setting of an abnormal metabolic milieu.
The data presented here examine the efficacy of liver-directed gene therapy in a tractable experimental animal model and help clarify some of the confounding issues that have surrounded liver transplantation for methylmalonic acidemia patients. Our results clearly demonstrate the efficacy of this approach and are consistent with the suggestion offered by some authors to perform a liver transplantation as soon as possible as a treatment for this disorder (Morioka et al., 2007). Because the stable transduction of a small number of hepatocytes appears sufficient to obtain therapeutic effects in a mouse model that replicates a severe form of the condition, liver-directed gene therapy would likely benefit patients with methylmalonic acidemia and, possibly, other inborn errors of organic acid metabolism. Last, our vector has been harmonized in design with those currently being used in human clinical trials and is similar to one that has been tested in nonhuman primates (Wang et al., 2010a). This should facilitate the translation of our murine studies to a clinical gene therapy trial for methylmalonic acidemia.
Supplementary Material
Acknowledgments
The authors thank Stephen Wincovitch for confocal microscopy guidance, Denise Larson for performing frozen sections of liver, Cherry Yang and Irene Ginty for mouse care and technical assistance, David M. Bodine IV for providing a critical reading of the manuscript, and the University of Pennsylvania Vector Core for AAV preparations and advice. N.C.C., R.J.C., S.C., and C.P.V. were supported, in part, by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Allen R.H. Stabler S.P. Savage D.G. Lindenbaum J. Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42:978–988. doi: 10.1016/0026-0495(93)90010-l. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Chulay J.D. Wang L. Mueller C. Humphries M. Spencer L.T. Rouhani F. Conlon T.J. Calcedo R. Betts M.R. Spencer C. Byrne B.J. Wilson J.M. Flotte T.R. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J. Venditti C.P. Adenovirus-mediated gene delivery rescues a neonatal lethal murine model of mut0 methylmalonic acidemia. Hum. Gene Ther. 2008;19:53–60. doi: 10.1089/hum.2007.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J. Venditti C.P. Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno associated viral gene therapy. Mol. Ther. 2010;18:11–16. doi: 10.1038/mt.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J. Sloan J. Fu H. Tsai M. Stabler S. Allen R. Kaestner K.H. Kazazian H.H. Venditti C.P. Metabolic phenotype of methylmalonic acidemia in mice and humans: The role of skeletal muscle. BMC Med. Genet. 2007a;8:64. doi: 10.1186/1471-2350-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J. Tsai M.S. Dorko K. Sloan J. Korson M. Freeman R. Strom S. Venditti C.P. Adenoviral-mediated correction of methylmalonyl-CoA mutase deficiency in murine fibroblasts and human hepatocytes. BMC Med. Genet. 2007b;8:24. doi: 10.1186/1471-2350-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.J. Zerfas P.M. Shanske S. Sloan J. Hoffmann V. Dimauro S. Venditti C.P. Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2009;23:1252–1261. doi: 10.1096/fj.08-121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Baulny H.O. Benoist J.F. Rigal O. Touati G. Rabier D. Saudubray J.M. Methylmalonic and propionic acidaemias: Management and outcome. J. Inherit. Metab. Dis. 2005;28:415–423. doi: 10.1007/s10545-005-7056-1. [DOI] [PubMed] [Google Scholar]
- de Keyzer Y. Valayannopoulos V. Benoist J.F. Batteux F. Lacaille F. Hubert L. Chretien D. Chadefeaux-Vekemans B. Niaudet P. Touati G. Munnich A. de Lonlay P. Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr. Res. 2009;66:91–95. doi: 10.1203/PDR.0b013e3181a7c270. [DOI] [PubMed] [Google Scholar]
- Dionisi-Vici C. Deodato F. Roschinger W. Rhead W. Wilcken B. “Classical” organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: Long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J. Inherit. Metab. Dis. 2006;29:383–389. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- Fenton W.A. Gravel R.A. Rosenblatt D.S. The Metabolic & Molecular Bases for Inherited Disease. McGraw-Hill; New York: 2001. Disorders of propionate and methylmalonate metabolism; pp. 2165–2194. [Google Scholar]
- Franco L.M. Sun B. Yang X. Bird A. Zhang H. Schneider A. Brown T. Young S.P. Clay T.M. Amalfitano A. Chen Y.T. Koeberl D.D. Evasion of immune responses to introduced human acid α-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol. Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L. Calcedo R. Johnston J. Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka K. Metoki K. Satoh T. Narisawa K. Tada K. Kawakami T. Matsuo N. Aoki T. Comparison of cytosolic and mitochondrial enzyme alterations in the livers of propionic or methylmalonic acidemia: A reduction of cytochrome oxidase activity. Tohoku J. Exp. Med. 1982;137:329–334. doi: 10.1620/tjem.137.329. [DOI] [PubMed] [Google Scholar]
- Hayashi Y. Mori Y. Janssen O.E. Sunthornthepvarakul T. Weiss R.E. Takeda K. Weinberg M. Seo H. Bell G.I. Refetoff S. Human thyroxine-binding globulin gene: Complete sequence and transcriptional regulation. Mol. Endocrinol. 1993;7:1049–1060. doi: 10.1210/mend.7.8.8232304. [DOI] [PubMed] [Google Scholar]
- Hörster F. Baumgartner M.R. Viardot C. Suormala T. Burgard P. Fowler B. Hoffmann G.F. Garbade S.F. Kölker S. Baumgartner E.R. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut–, cblA, cblB) Pediatr. Res. 2007;62:225–230. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- Hsui J.Y. Chien Y.H. Chu S.Y. Lu F.L. Chen H.L. Ho M.J. Lee P.H. Hwu W.L. Living-related liver transplantation for methylmalonic acidemia: Report of one case. Acta Paediatr. Taiwan. 2003;44:171–173. [PubMed] [Google Scholar]
- Kaplan P. Ficicioglu C. Mazur A.T. Palmieri M.J. Berry G.T. Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol. Genet. Metab. 2006a;88:322–326. doi: 10.1016/j.ymgme.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kaplan P. Ficicioglu C. Mazur A.T. Palmieri M.J. Berry G.T. Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol. Genet. Metab. 2006b;88:322–326. doi: 10.1016/j.ymgme.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kasahara M. Horikawa R. Tagawa M. Uemoto S. Yokoyama S. Shibata Y. Kawano T. Kuroda T. Honna T. Tanaka K. Saeki M. Current role of liver transplantation for methylmalonic acidemia: A review of the literature. Pediatr. Transplant. 2006;10:943–947. doi: 10.1111/j.1399-3046.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- Kayler L.K. Merion R.M. Lee S. Sung R.S. Punch J.D. Rudich S.M. Turcotte J.G. Campbell D.A., Jr. Holmes R. Magee J.C. Long-term survival after liver transplantation in children with metabolic disorders. Pediatr. Transplant. 2002;6:295–300. doi: 10.1034/j.1399-3046.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S. Chang M. Brass E.P. Hoppel C.L. Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J. Biol. Chem. 1991;266:20998–21003. [PubMed] [Google Scholar]
- Lebherz C. Sanmiguel J. Wilson J.M. Rader D.J. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc. Diabetol. 2007;6:15. doi: 10.1186/1475-2840-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R. Glader B. Ragni M. Rasko J.J. Ozelo M.C. Hoots K. Blatt P. Konkle B. Dake M. Kaye R. Razavi M. Zajko A. Zehnder J. Rustagi P.K. Nakai H. Chew A. Leonard D. Wright J.F. Lessard R.R. Sommer J.M. Tigges M. Sabatino D. Luk A. Jiang H. Mingozzi F. Couto L. Ertl H.C. High K.A. Kay M.A. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Marcell P.D. Stabler S.P. Podell E.R. Allen R.H. Quantitation of methylmalonic acid and other dicarboxylic acids in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal. Biochem. 1985;150:58–66. doi: 10.1016/0003-2697(85)90440-3. [DOI] [PubMed] [Google Scholar]
- Matsui S.M. Mahoney M.J. Rosenberg L.E. The natural history of the inherited methylmalonic acidemias. N. Engl. J. Med. 1983;308:857–861. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- Morioka D. Kasahara M. Horikawa R. Yokoyama S. Fukuda A. Nakagawa A. Efficacy of living donor liver transplantation for patients with methylmalonic acidemia. Am. J. Transplant. 2007;7:2782–2787. doi: 10.1111/j.1600-6143.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Moscioni D. Morizono H. McCarter R.J. Stern A. Cabrera-Luque J. Hoang A. Sanmiguel J. Wu D. Bell P. Gao G.P. Raper S.E. Wilson J.M. Batshaw M.L. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol. Ther. 2006;14:25–33. doi: 10.1016/j.ymthe.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nagarajan S. Enns G.M. Millan M.T. Winter S. Sarwal M.M. Management of methylmalonic acidaemia by combined liver–kidney transplantation. J. Inherit. Metab. Dis. 2005;28:517–524. doi: 10.1007/s10545-005-0517-8. [DOI] [PubMed] [Google Scholar]
- Nicolaides P. Leonard J. Surtees R. Neurological outcome of methylmalonic acidaemia. Arch. Dis. Child. 1998;78:508–512. doi: 10.1136/adc.78.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhan W.L. Gargus J.J. Boyle K. Selby R. Koch R. Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur. J. Pediatr. 2002;161:377–379. doi: 10.1007/s00431-002-0970-4. [DOI] [PubMed] [Google Scholar]
- Oberholzer V.G. Levin B. Burgess E.A. Young W.F. Methylmalonic aciduria: An inborn error of metabolism leading to chronic metabolic acidosis. Arch. Dis. Child. 1967;42:492–504. doi: 10.1136/adc.42.225.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savu L. Vranckx R. Rouaze-Romet M. Maya M. Nunez E.A. Treton J. Flink I.L. A senescence up-regulated protein: The rat thyroxine-binding globulin (TBG) Biochim. Biophys. Acta. 1991;1097:19–22. doi: 10.1016/0925-4439(91)90017-4. [DOI] [PubMed] [Google Scholar]
- Stokke O. Eldjarn L. Norum K.R. Steen-Johnsen J. Halovorsen S. Methylmalonic acidemia: A newborn error of metabolism which may cause fatal acidosis in the neonatal period. Scand. J. Clin. Lab. Invest. 1967;20:313–328. [Google Scholar]
- van der Meer S.B. Poggi F. Spada M. Bonnefont J.P. Ogier H. Hubert P. Depondt E. Rapoport D. Rabier D. Charpentier C. Parvy P. Bardet J. Kamoun P. Saudubray J.M. Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J. Pediatr. 1994;125:903–908. doi: 10.1016/s0022-3476(05)82005-0. [DOI] [PubMed] [Google Scholar]
- van ‘t Hoff W.G. Dixon M. Taylor J. Mistry P. Rolles K. Rees L. Leonard J.V. Combined liver–kidney transplantation in methylmalonic acidemia. J. Pediatr. 1998;132:1043–1044. doi: 10.1016/s0022-3476(98)70407-x. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Wang H. Bell P. Grant R. Vandenberghe L.H. Sanmiguel J. Morizono H. Batshaw M.L. Wilson J.M. >The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010a;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Wang H. Bell P. McCarter R.J. He J. Calcedo R. Vandenberghe L.H. Morizono H. Batshaw M.L. Wilson J.M. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol. Ther. 2010b;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkemeyer M.F. Andrews E.R. Ledley F.D. Genomic structure of murine methylmalonyl-CoA mutase: Evidence for genetic and epigenetic mechanisms determining enzyme activity. Biochem. J. 1993;296:663–670. doi: 10.1042/bj2960663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W. Berta S.C. Lu M.M. Moscioni A.D. Tazelaar J. Wilson J.M. Adeno-associated virus as a vector for liver-directed gene therapy. J. Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.