Abstract
Typical neuroleptic drugs elicit their antipsychotic effects mainly by acting as antagonists at dopamine D2 receptors. Much of this activity is thought to occur in the cerebral cortex, where D2 receptors are found largely in inhibitory GABAergic neurons. Here we confirm this localization at the electron microscopic level, but additionally show that a subset of cortical interneurons with low or undetectable expression of D2 receptor isoforms are surrounded by astrocytic processes that strongly express D2 receptors. Ligand binding of isolated astrocyte preparations indicate that cortical astroglia account for approximately one-third of the total D2 receptor binding sites in the cortex, a proportion that we found conserved among rodent, monkey, and human tissues. Further, we show that the D2 receptor-specific agonist, quinpirole, can induce Ca2+ elevation in isolated cortical astrocytes in a pharmacologically reversible manner, thus implicating this receptor in the signaling mechanisms by which astrocytes communicate with each other as well as with neurons. The discovery of D2 receptors in astrocytes with a selective anatomical relationship to interneurons represents a neuron/glia substrate for cortical dopamine action in the adult cerebral cortex and a previously unrecognized site of action for antipsychotic drugs with affinities at the D2 receptor.
The circuit basis of all cortical functions involve a balance between excitation and inhibition, and recent studies in this laboratory have begun to reveal the mechanisms by which interneurons exert control on cognitive processes mediated by prefrontal circuits (1, 2). D2 family receptors are among the neurotransmitter receptors through which inhibitory actions are signaled in the cortex (3–6). Previous light microscopic investigations have revealed that prefrontal cortical neurons express the D2S and D2L isoforms (4). Here, we report the ultrastructural localization of these D2 receptor isoforms. Knowledge of the precise cellular disposition of these receptors could have relevance to the differential effects of typical and atypical neuroleptic drugs as well as the modulation of cognitive processes carried out by prefrontal circuits.
Materials and Methods
Immunocytochemistry.
Affinity-purified anti-D2 polyclonal antibodies were used for anatomical studies at both light and electron microscopic levels, as described in detail in ref. 4. The specificity of these antibodies to both the short and long isoforms of the dopamine D2 receptor has been demonstrated (4). In brief, sections from four perfused monkey brains were incubated with anti-D2 antibodies and further processed by incubation with either biotinylated goat anti-rabbit antibodies (Jackson Immuno-Research) or goat anti-rabbit antibodies coupled to 1.4-nm gold particles (Nanoprobes, Stony Brook, NY). The bound anti-D2 antibodies were visualized by the immunoperoxidase method using the ABC Elite kit (Vector Laboratories) or by silver enhancement of gold particles (Nanoprobes). Double-label immunofluorescence studies were performed by incubating sections with anti-D2 antibodies and mouse monoclonal antibodies to glial fibrillary acidic protein (GFAP; Chemicon) or parvalbumin (Chemicon) followed by incubation with goat anti-rabbit IgG-rhodamine and goat anti-mouse IgG-FITC (both from Jackson ImmunoResearch). For double labeling electron microscopic analysis, sections were incubated in a mixture containing the anti-D2 and anti-GFAP antibodies. The D2 receptor isoforms were visualized by using the immunogold reaction and GFAP by the immunoperoxidase technique.
Isolation of Astrocytes.
Astrocytes were isolated from the cortical gray matter by using a density gradient as described by Farooq and Norton (7). Freshly obtained tissues were sliced and incubated with 0.1% trypsin at 37°C for 45 min. Disaggregated cell suspensions were used for the differential centrifugation. Isolated astrocytes were kept in a CO2 incubator with DMEM (GIBCO) at 37°C for 2 h before they were used for further studies. D2 receptor-deficient mice were generated by gene disruption (8).
Radioligand Binding Assay.
Aliquots of frozen astrocyte samples containing 50 μg of protein were used for binding studies with radiolabeled ligands, as described in refs. 4 and 5. Astrocyte samples were incubated with 2 nM 3H-radioligand for 1 h at 24°C in a total volume of 1 ml. The reaction was terminated by rapid filtration through glass filters and the retained radioactivity was counted. Nonspecific binding was determined by performing the assay in the presence of 2 μM (+)-butaclamol-HCl or raclopride (Research Biochemicals), and background activity was subtracted from total activity.
Calcium Imaging.
Optical recordings of intracellular calcium concentrations were performed essentially as described in ref. 9. Freshly isolated astrocytes were plated on poly-l-lysine-coated coverslips and incubated with 5 μM cell-permeant fluo-3 (fluo-3 acetoxymethyl ester from Molecular Probes). The fluo-3 fluorescence present in the cells was measured with a Bio-Rad MRC-1024 system. While exciting the dye containing cells with light of 488 nm wavelength, variations in intracellular calcium were measured at 522 nm. Fluorescence images were acquired every 3 s. Changes in the Ca2+ concentrations were calculated by dividing the measured fluorescence intensity after drug application (F) by the measured average fluorescence intensity at baseline (F/F0).
Results
D2 Receptors in Interneurons and in Astrocytic Processes.
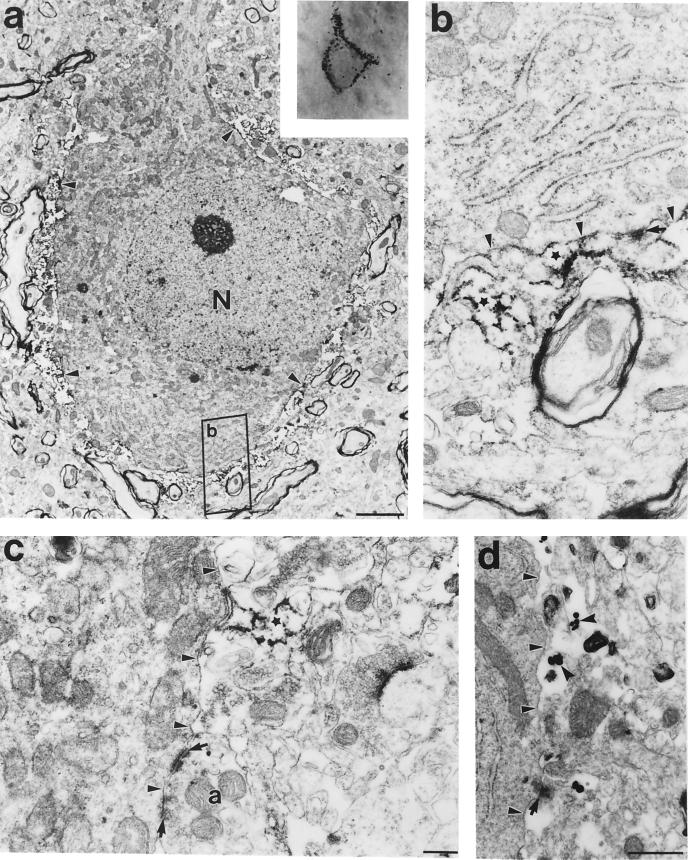
Immunoreactivity of the D2S and D2L isoforms of the dopamine D2 receptor subtype was strongly associated with nonpyramidal neurons (Fig. 1 a–c), as evidenced by coexpression with parvalbumin (Fig. 1d) or glutamic acid decarboxylase (GAD; data not shown). The dark punctate immunolabeling was most often associated with the cell plasma membrane (Figs. 1b and 2 Inset), strongly suggesting a distinct postsynaptic localization of D2 receptors. Analysis of 80 interneurons at the electron microscope revealed two subsets of D2-labeled cells. The larger proportion (60%) of neurons expressed D2 receptor within the cytoplasm (Fig. 3 a and b) and in dendritic shafts (4). However, the other group of 32 neurons exhibited no evidence of cytoplasmic labeling. Instead, immunoreactivity was present in astrocytic processes that enwrapped the soma (Fig. 2 a and b). In both diaminobenzidine (DAB) and gold particle immunoreaction experiments, the reactions were observed outside the cell, adjacent to the plasma membrane (arrowheads), and were not associated with neuronal elements, such as synapses (arrows) (Fig. 2 b–d).
Figure 1.
The short and long isoforms of the dopamine D2 receptor were expressed at similar levels in interneurons of the prefrontal cortex (arrows in a and c). These receptors were concentrated in proximity to the plasma membrane (arrowheads in b); particularly evident in d, where the D2 (red) receptors enwrap the parvalbumin-labeled (green) neuron. The D2 receptors (red) coexpress with GFAP (in green), a marker for astrocytic processes (e and f). The D2 receptors also colocalize with GFAP in isolated astrocytes (g–j).
Figure 2.
Electron micrograph of a D2-immunolabeled cortical neuron (Inset), showing that the D2 receptors are localized exterior to the plasma membrane of the neuron, on surrounding processes (small arrowheads), and not within the neuron (a). At higher magnification in b and c, the peroxidase reactivity was clearly associated with astrocytic processes (stars) and was not present at synaptic sites (arrows). (d) Similarly, immunogold labeling of D2 receptors (notched arrowheads) also confirmed the presence of this receptor in glial astrocytic processes. Small arrowheads point to the plasma membrane of the cell body. (Scale bars: a, 2 μm; b and c, 200 nm; d, 500 nm.)
Figure 3.
A large proportion of the D2-labeled interneurons showed reactivity within the cell body (arrowheads in a and b). An electron micrograph represents the parvalbumin-labeled [diaminobenzidine (DAB)-peroxidase reaction] neuron expressing D2 receptors (immunogold particles). (Scale bars: a, 2 μm; b, 200 nm.)
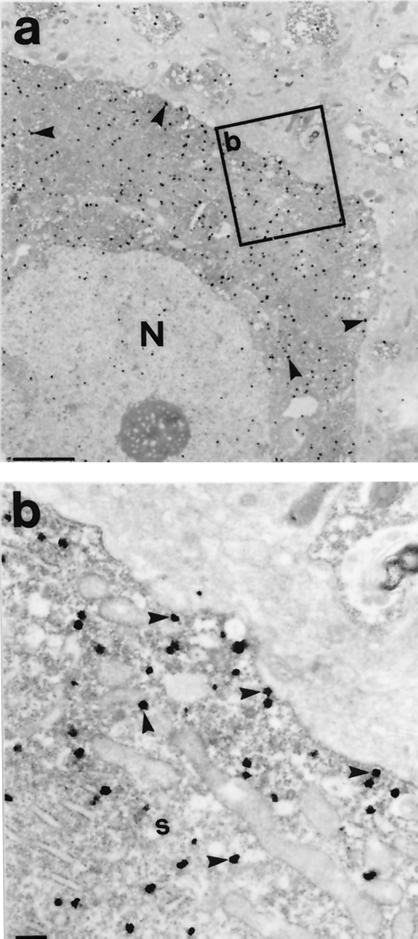
To confirm the localization of D2 receptors in the astrocytes surrounding neuronal soma, we performed double-labeling with antibodies both to the D2 receptor and GFAP, a marker for glial astrocytic processes. Coexpression of the D2 receptors and GFAP was observed at both the light (Fig. 1 e and f) and electron microscopic levels (Fig. 4 a–c). Ultrastructural evidence of colocalization of the D2 receptors with S-100, another astrocytic protein, was also observed (data not shown). GFAP-immunoreactive structures containing D2 receptors were commonly juxtaposed to unlabeled axons and dendrites (Fig. 4 b and c). Close appositions of this kind have previously been described in the cerebral cortex (10, 11), and may serve as release sites for neurotransmitters (10), such as dopamine.
Figure 4.
(a and c) Electron micrographic evidence of double immunolabeling of D2 receptors with GFAP in the prefrontal cortex, where the receptor is labeled with immunogold particles (notched arrowheads) and GFAP by the peroxidase reaction (stars). a shows the colocalization of the D2 receptor with GFAP, whereas b and c show the astrocytic structures making close contacts (arrows) with unidentified axons (a) and dendrites (d). Small arrowheads identify the plasma membrane of the cell body. (Scale bar = 200 nm.)
D2 Receptor-Specific Ligand Binding Sites in Isolated Astrocytes.
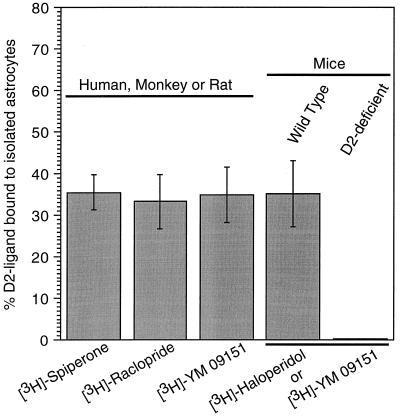
We next isolated cortical astrocytes from tissues obtained from mice, rats, monkey, and human and examined their ability to bind ligands that have specificity for dopamine D2 receptors (e.g., spiperone, raclopride, haloperidol, and YM-09151). Double labeling with antibodies to the D2 receptor and GFAP further confirmed the localization of D2 receptors in isolated astrocytes (Fig. 1 g–j). We found that approximately 32–38% of the total ligand binding activity was associated with astrocytes isolated from these species (Fig. 5). As a control, astrocytes isolated from D2 receptor-deficient mice (8) failed to show D2-ligand binding, suggesting that the binding activity observed in normal animals is specific for D2 receptors (Fig. 5). Other reports have also observed dopamine receptors on glial membranes as well as in glial cell lines by radioligand binding (12, 13), mRNA expression (14), autoradiography (15), and electrophysiology (16). An interaction between the D2 receptor and its antagonists in astrocytes can also be inferred from the recent observation that chronic treatment with D2-antagonist drugs tend to alter the density of glia in prefrontal cortex in rhesus monkey (17).
Figure 5.
Specific binding activity in isolated astrocytes from the cerebral cortex to spiperone, raclopride, haloperidol, and YM-09151, compounds with high affinities at D2 receptor. About 35% of the total binding activity in cortex was observed in the astrocytes isolated, respectively, from human, monkey, rat, and mouse preparations. D2 receptor-deficient mice lacked binding activity, whereas it was present in wild-type mice.
Ca2+ Elevation in Astrocytes on Activation with D2 Agonist.
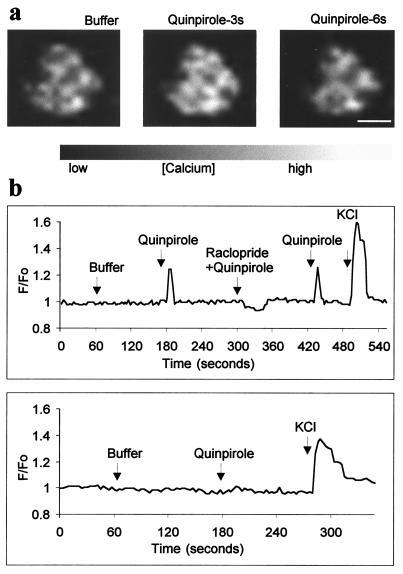
To determine whether the D2 receptor in astrocytes is functional, we used calcium imaging. Application of 150 μM quinpirole, a D2 receptor agonist, produced a temporary increase in intracellular Ca2+ whose F/F0 peaked at 1.25 (Fig. 6a and Table 1) and 1.31 (Fig. 6b and Table 1) in rat and mice, respectively. Typically a subset of astrocytes (25% of 643 cells evaluated) showed a similar response, and their activation was significantly different from basal Ca2+ levels (Table 1). Moreover, coapplication of raclopride (300 μM) blocked the increase in intracellular Ca2+ (Fig. 6b and Table 1). A decrease in intracellular Ca2 + concentration after raclopride application suggests that the D2 receptor associated signaling pathway was probably already stimulated to a certain degree (Table 1). Direct involvement of D2 receptors in the activation of Ca2+ in isolated astrocytes was also evident from analysis of the D2-deficient mice. Mice lacking the dopamine D2 receptors failed to show Ca2+ elevation after quinpirole stimulation (Fig. 6b and Table 1), whereas wild-type mice retained this activity (Table 1).
Figure 6.
D2 receptor agonist-dependent Ca2+ elevation in isolated astrocytes. (a) A typical response for a single cell, showing baseline Ca2+ level (buffer), and an increase in Ca2+ concentration after stimulation with 150 μM quinpirole at 3 s and 6 s. (Scale bar = 10 μm.) (b) Traces from individual experiments showing the calcium concentration in single astrocytes over time. (Upper) Responses to the application of buffer, 150 μM quinpirole, or 300 μM raclopride followed by 150 μM quinpirole, and 50 mM potassium chloride. No effect was observed in astrocytes isolated from D2 receptor-deficient mice.
Table 1.
D2 receptor dependent Ca2+ elevations in astrocytes
| Treatment | Number of cells | F/F0 | Significance, p > 0.001 |
|---|---|---|---|
| Rat | |||
| Buffer | 268 | 1.00 ± 0.04 | |
| Quinpirole | 643 | 1.25 ± 0.07 | ** |
| Raclopride + quinpirole | 206 | 0.89 ± 0.09 | |
| Quinpirole after washout of raclopride | 69 | 1.36 ± 0.20 | ** |
| KCl in buffer | 152 | 2.55 ± 0.85 | ** |
| Wild-type mice | |||
| Buffer | 96 | 1.00 ± 0.03 | |
| Quinpirole | 96 | 1.31 ± 0.27 | ** |
| D2-deficient mice | |||
| Quinpirole | 184 | 1.00 ± 0.03 | No effect |
| KCl in buffer | 184 | 1.79 ± 0.63 | ** |
Changes in Ca2+ concentration are represented as the ratio of fluorescence intensity after drug application to baseline (F/F0).
Discussion
In line with our previous light microscopic observations in the prefrontal cortex of nonhuman primates (4), immunoreactivity of D2 receptors was mainly localized in the interneurons (Fig 1 a–c). As far as we know, this is the first ultrastructural evidence for the presence of D2 receptors in a subset of cortical interneurons. About 60% of these cells showed labeling in soma (Fig. 3 a and b) and dendritic shafts (4). We also found that a subset (≈40%) of these neurons were devoid of intracellular reaction product and instead showed immunolabeling in astrocytic processes that enwrapped their cell body (Fig. 2a) and we have focused on this finding in the present report. We estimate that approximately 35% of the total D2 receptor binding activity in the cortex may be associated with astrocytes (Fig. 5). Our electron microscopic analysis shows that D2 receptors in this class of glial cells make close anatomical association with interneurons in the cerebral cortex and suggest that this association might provide a substrate for functional interaction between the two cell types. Astrocytic processes that surround neurons have been shown to express other G-protein coupled receptors, including glutamate receptors (18).
There is accumulating evidence that astrocytes may play an active role in neural communication (19–25). Ca2+ elevation in astrocytes evokes a glutamate-dependent inward current in adjacent neurons (19–21) and modulates action–potential-mediated synaptic transmission between cultured hippocampal cells (21, 23). We detected a 25% increase in intracellular Ca2+ concentration induced by quinpirole, a D2-receptor-specific agonist (Fig. 6 a and b and Table 1), and reversibly blocked this response with the D2 antagonist, raclopride. This finding suggests that D2 receptors may trigger Ca2+ waves on activation, analogous to other neurotransmitter receptors (21, 23, 26). Therefore, it is possible that D2-facilitated Ca2+ excitation in astrocytes may increase neuronal excitability (19–24) and, ultimately, synaptic transmission (25), whereas neuronal D2 receptors inhibit the action of GABAergic neurons (3), similar to D4 receptors.
Typical neuroleptic drugs are thought to achieve their antipsychotic efficacy by 70–80% occupancy at D2 receptors (27, 28). Administration of these drugs in clinically effective doses to psychiatric patients also produces extrapyramidal side effects (29, 30). Our results suggest a dual mode of action for D2 receptors in the cerebral cortex, by which they may act directly through GABAergic neurons or indirectly via glial processes. In contrast, D4 receptors might only participate in a direct mode of inhibition, because this receptor is mainly localized in GABAergic neurons and not in glia (6). These findings raise the question of whether some portion of the difference between typical and atypical neuroleptic drugs with respect to relief of clinical symptoms or production of side-effects might account for therapeutic actions at glial targets.
Acknowledgments
We thank Dr. Susan Sesack for sharing acrolein-perfused rat brain sections; and Mary Pappy, K. Szigeti, and J. Musco for expert technical assistance. This study was supported by Grants DA10160–02 (to P.G.R.) and DA12062 (to D.K.G.) from the National Institute on Drug Abuse; Grant MH44866 (to P.G.R) from the National Institute of Mental Health; Grant GM51480 (to B. Ehrlich) from the National Institutes of Health; and a BASF/German National Merit Scholarship Foundation fellowship (to P.K.).
Abbreviation
- GFAP
glial fibrillary acidic protein
References
- 1.Goldman-Rakic P S. Ann NY Acad Sci. 1999;868:13–26. doi: 10.1111/j.1749-6632.1999.tb11270.x. [DOI] [PubMed] [Google Scholar]
- 2.Rao S G, William G V, Goldman-Rakic P S. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunney B S, Aghajanian G K. Life Sci. 1976;19:1783–1789. doi: 10.1016/0024-3205(76)90087-4. [DOI] [PubMed] [Google Scholar]
- 4.Khan Z U, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic P S. Proc Natl Acad Sci USA. 1998;95:7731–7736. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan Z U, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A. J Comp Neurol. 1998;402:353–371. doi: 10.1002/(sici)1096-9861(19981221)402:3<353::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic P S. Nature (London) 1995;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 7.Farooq M, Norton W T. J Neurochem. 1978;31:887–894. doi: 10.1111/j.1471-4159.1978.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelly M A, Rubinstein M, Asa S L, Zhang G, Saez C, Bunzow J R, Allen R G, Hnasko R, Ben-Jonathan N, Grandy D K, et al. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 9.Koulen P, Kuhn R, Wassle H, Helmut-Brandstatter J. Proc Natl Acad Sci USA. 1999;96:9909–9914. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiye A. J Neurosci. 1992;12:781–792. [Google Scholar]
- 11.Chiye A, Joh T H, Pickel V M. Brain Res. 1987;437:264–282. doi: 10.1016/0006-8993(87)91642-8. [DOI] [PubMed] [Google Scholar]
- 12.Henn F A, Anderson D J, Sellstrom A. Nature (London) 1977;266:637–638. doi: 10.1038/266637a0. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer S E, Herschman H R, Lightbody J, Sato G. J Cell Physiol. 1970;75:329–340. doi: 10.1002/jcp.1040750309. [DOI] [PubMed] [Google Scholar]
- 14.Bal A. Mol Brain Res. 1994;23:204–212. doi: 10.1016/0169-328x(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 15.Henn F A. Lancet. 1978;5:293–295. doi: 10.1016/s0140-6736(78)91693-8. [DOI] [PubMed] [Google Scholar]
- 16.Hosli L, Hosli E. In: Glial Cell Receptors. Kimelberg H K, editor. New York: Raven; 1988. pp. 77–93. [Google Scholar]
- 17.Seleman L D, Lidow M S, Goldman-Rakic P S. Biol Psychiatry. 1999;46:161–172. doi: 10.1016/s0006-3223(99)00113-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Muñoz A, Jones E G. J Comp Neurol. 1998;395:450–465. doi: 10.1002/(sici)1096-9861(19980615)395:4<450::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Parpura V, Basarsky T A, Liu F, Jeftinija K, Jeftinija S, Haydon P G. Nature (London) 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 20.Pasti L, Volterra A, Pozzan T, Carmignoto G. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araque A, Parpura V, Sanzgiri R P, Haydon P G. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Lodi Rizzini B, Pozzan T, Volterra A. Nature (London) 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 23.Araque A, Sanzgiri R P, Parpura V, Haydon P G. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mudrick-Donnon L A, Williams P J, Pittman Q J, MacVicar B A. J Neurosci. 1993;13:4660–4666. doi: 10.1523/JNEUROSCI.13-11-04660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theodosis D T, MacVicar B. Trends Neurosci. 1996;19:363–367. doi: 10.1016/0166-2236(96)10055-2. [DOI] [PubMed] [Google Scholar]
- 26.Thorlin T, Eriksson P S, Ronnback L, Hansson E. J Neurosci Res. 1998;54:390–401. doi: 10.1002/(SICI)1097-4547(19981101)54:3<390::AID-JNR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Kapur S. Mol Psychiatry. 1998;3:135–140. doi: 10.1038/sj.mp.4000327. [DOI] [PubMed] [Google Scholar]
- 28.Seeman P, Tallerico T. Mol Psychiatry. 1998;3:123–134. doi: 10.1038/sj.mp.4000336. [DOI] [PubMed] [Google Scholar]
- 29.Farde L. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 30.Meltzer H Y, Stahl H M. Schizophr Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]