Abstract
Objective
To evalute whether a formula could be derived using oxygen saturation (Spo2) to replace Pao2 that would allow identification of children with acute lung injury and acute respiratory distress syndrome. Definitions of acute lung injury and acute respiratory distress syndrome require arterial blood gases to determine the Pao2/Fio2 ratio of 300 (acute lung injury) and 200 (acute respiratory distress syndrome).
Design
Post hoc data analysis of measurements abstracted from two prospective databases of randomized controlled trials.
Setting
Academic pediatric intensive care units.
Patients
A total of 255 children enrolled in two large prospective trials of therapeutic intervention for acute lung disease: calfactant and prone positioning.
Interventions
Data were abstracted including Pao2, Paco2, pH, Fio2, and mean airway pressure. Repeated-measures analyses, using linear mixed-effects models, were used to build separate prediction equations for the Spo2/Fio2 ratio, oxygenation index [(Fio2 × Mean Airway Pressure)/Pao2], and oxygen saturation index [(Fio2 × Mean Airway Pressure)/Spo2]. A generalization of R2 was used to measure goodness-of-fit. Generalized estimating equations with a logit link were used to calculate the sensitivity and specificity for the cutoffs of Pao2/Fio2 ratio of 200 and 300 and equivalent values of Spo2/Fio2 ratio, oxygenation index, and oxygen saturation index.
Measurements and Main Results
An Spo2/Fio2 ratio of 253 and 212 would equal criteria for acute lung injury and acute respiratory distress syndrome, respectively. An oxygenation index of 5.3 would equal acute lung injury criteria, and an oxygenation index of 8.1 would qualify for acute respiratory distress syndrome. An oxygen saturation index, which includes the mean airway pressure and the noninvasive measure of oxygenation, of 6.5 would be equivalent to the acute lung injury criteria, and an oxygen saturation index of 7.8 would equal acute respiratory distress syndrome criteria.
Conclusions
Noninvasive methods of assessing oxygenation may be utilized with reasonable sensitivity and specificity to define acute lung injury and acute respiratory distress syndrome, and, with prospective validation, have the potential to increase the number of children enrolled into clinical trials.
Keywords: acute lung injury, acute respiratory distress syndrome, pediatrics, oxygenation index
Patients who develop acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) and require critical care resources have a major impact on public health. The incidence of ALI has been described recently in the adult population, with the estimate that, each year in the United States, there are >190,000 cases of ALI, with a mortality rate of almost 40% (1). Data presented by Rubenfeld et al suggested that ALI occurs in children at a rate of 12.8 per 100,000 children (1). However, these estimates have been difficult to obtain due to the lack of validated criteria to define ALI in children.
Defining ALI and ARDS has historically been difficult, both for use in clinical practice as well as defining entry criteria for clinical research studies. In 1994, the American-European Consensus Conference on ARDS agreed to standardized definitions in adults that continue to be used presently (2). These well-accepted definitions require: a) an acute onset of the process; b) bilateral infiltrates on chest radiograph; c) no evidence of left atrial hypertension; and d) a defined degree of hypoxia. This extent of hypoxia, which was based on expert opinion of this consensus group, required a partial pressure of oxygen/fraction of inspired oxygen, or Pao2/Fio2 (P/F) ratio, of ≤300 to meet the definitions of ALI, and a P/F ratio of ≤200 to meet the ARDS definition. These definitions do not include any measure of mean airway pressure (Paw), which can have a profound effect on oxygenation. Although they have been adopted by clinicians caring for critically ill children and are commonly utilized in clinical trials, many consider oxygenation index (OI) [Fio2 × Paw/Pao2] to be a better indicator of lung injury. To date, no investigation has been undertaken to define ALI and ARDS criteria based on OI, despite this calculation being utilized for clinical trial entry (3).
Due to the use of the P/F ratio and OI to both diagnose ALI and ARDS and enroll critically ill adults and children in randomized controlled clinical trials, it is conceivable that many subjects who would meet entry criteria are not enrolled due to the lack of the required one, or more often serial, arterial blood gases. With the advancements in pulse oximetry in recent years, this noninvasive measure of systemic oxygenation has become the fifth vital sign (4), and has likely led to a decrease in arterial blood gas measurements. Therefore, children with moderate hypoxemia that would likely meet ALI or even ARDS criteria are not eligible for enrollment due to the lack of a documented Pao2. This has potentiated the recruitment difficulties in two of the largest ALI intervention trials ever completed in children (3, 5). Furthermore, despite both of these trials utilizing entry criteria that targeted patients with moderate, ALI-level, respiratory failure, both trials enrolled subjects with a much more severe lung disease than targeted. Therefore, we undertook the present study to determine diagnostic hypoxemia criteria that could be utilized to diagnose children with severe lung disease and also allow enrollment of children in ALI clinical trials in the absence of arterial blood gas monitoring. We hypothesized that a formula could be derived, utilizing oxygen saturation (Spo2) instead of Pao2, that would allow identification of eligible children to be enrolled in future clinical trials. We further hypothesized that an oxygenation index equivalent to the ALI and ARDS hypoxemia criteria could be developed with adequate sensitivity and specificity to allow the use of this as an equivalent marker of hypoxemia. After prospective validation, it is plausible that this method could be utilized as entry criteria in clinical trails of pediatric ALI and ARDS.
METHODS
The prospective data obtained during the completion of two randomized clinical trials (3, 5) were collected under Institutional Review Board approval at each enrolling institution. The protocol for the use of previously prospectively collected data to develop novel formulas related to the degree of hypoxemia was reviewed by the Human Subjects Protection Office of Pennsylvania State University College of Medicine and declared exempt from Institutional Review Board review, as no personal identifiers were utilized in these data sets. Data were abstracted from two data sets. These data sets were constructed prospectively during the execution of two large randomized controlled trials of interventions aimed at reducing mortality and days of mechanical ventilation in children with ALI and ARDS (3, 5).
The first study was a multicentered, randomized, masked trial that compared intratracheal installation of up to two doses of a calf s lung surfactant (calfactant) with placebo in 153 infants, children, and adolescents with ALI conducted from July 2000 to July 2003. Twenty-one tertiary care pediatric intensive care units participated, and entry criteria included age <21 yrs of age, enrollment within 48 hrs of endotracheal intubation, bilateral lung disease, and an OI of >7 (3). The second study was a multicentered, randomized, controlled, unmasked clinical trial conducted from August 2001 to April 2004. A total of 102 pediatric patients with ALI from seven pediatric intensive care units <18 yrs of age were treated with supine vs. prone positioning. Patients were randomized to either supine or prone positioning within 48 hrs of meeting ALI criteria, with those patients in the prone group remaining prone for 20 hrs each day during the acute phase of their illness for a maximum of 7 days. Study protocols included mechanical ventilation, extubation readiness testing, and the use of sedation (5).
The two data sets were stripped of all personal identifiers, and then abstracted data were collected into a distinct data set for the proposed study. Data collected included Pao2, Paco2, pH, Fio2, Spo2 and Paw. All available data were collected from each patient where a complete set of data was present for analysis. Due to the nonlinear nature of the relationship between Pao2 and Spo2 at higher SpO2 values, patient data for each time point were only utilized if the Spo2 was ≤97%.
Repeated-measures analyses via linear mixed-effects models, utilizing log-transformed data, were used to build separate prediction equations for the Spo2/Fio2 ratio (S/F), oxygenation index, and a novel measure termed “oxygen saturation index” (OSI) [(Fio2 × Paw)/Spo2] from P/F. A generalization of R2, the model concordance correlation coefficient, was used to measure goodness-of-fit for these prediction models (6), with values approaching 1 being that approaches 1 is preferable. The fitted models were used to compute values of the S/F, OI, and OSI that correspond to the P/F cutoffs of 200 and 300. As an independent validation, these cutoffs of S/F, OI, and OSI derived from the calfactant study data set were applied to the prone study data set to calculate the sensitivity, specificity, and corresponding 95% confidence intervals, using generalized estimating equations with a logit link. The calfactant and prone studies were conducted coincidentally, and the calfactant study was chosen for model development as it was the larger of the two studies. All methods account for the repeated observations per child.
Receiver operator characteristic curves were generated based on the prone study data, and the areas under the receiver operator characteristic curves were computed using the trapezoidal rule. These areas measure discrimination, i.e., the ability of the test to separate those with the condition (ARDS or ALI) from those without the condition. The general rule for interpreting these levels of discrimination is: excellent = 0.9–1; good = 0.8–0.9; fair = 0.7–0.8.
RESULTS
A total of 255 subjects had data available that were accessed to determine eligibility for use in this analysis. This included 153 subjects from the calfactant study, and 102 subjects from the prone study. The demographics of the children included in these studies are detailed in Table 1. The data from the calfactant study were utilized to develop the model for each variable. There were a total of 1548 observations from 130 children that were available for use. To build the model for each variable, the following observations were used from this data set: the S/F ratio model was built using 1159 observations from 112 subjects; the OI model was built using 1376 observations from 128 subjects; and the OSI model was built using 1142 observations from 112 subjects. The data from the prone study were utilized to test the models that were developed. There were a total of 1291 observations in data set from 80 subjects; for each of the three models tested, 648 observations from 78 subjects were utilized. A description of the variables utilized in these models is outlined in Table 2.
Table 1.
Demographics of the 255 children enrolled in both studies
| Calfactant | Placebo | Prone | Supine | |
|---|---|---|---|---|
| Age, median (IQR), yr | 6.6 (0.8–13.4) | 4.3 (1.0–11.5) | 2.0 (0.3–11.0) | 2.1 (0.3–8.2) |
| Male sex, % | 65 | 55 | 47 | 59 |
| White | 56 | 55 | 54 | 56 |
| Black | 26 | 23 | 10 | 12 |
| Hispanic | 8 | 19 | 28 | 20 |
| Other | 10 | 4 | 8 | 12 |
| PRISM III score, mean (SD) | 15 (99.4) | 14.1 (7.9) | 11 (9) | 11 (8) |
| Pao2/Fio2 ratio, mean (SD) | 128 (54) | 126 (73) | 94 (41) | 105 (48) |
| Oxygenation index, mean (SD) | 20.0 (12.9) | 20.5 (14.7) | 18 (18) | 15 (12) |
IQR, interquartile range; PRISM, Pediatric Risk of Mortality; SD, standard deviation.
Table 2.
Description of the diagnostic variables utilized in both the model development (calfactant study) and the model validation (prone study)
| Study | Variable | n | Median (25%ile–75%ile) |
|---|---|---|---|
| Calfactant | P/F ratio | 1393 | 146.7 (107.3–204.0) |
| Calfactant | S/F ratio | 1276 | 180.0 (153.3–236.3) |
| Calfactant | OI | 1376 | 12.8 (6.9–19.8) |
| Calfactant | OSI | 1259 | 10.0 (5.9–15.2) |
| Prone | P/F ratio | 648 | 133.3 (106.2–170.7) |
| Prone | S/F ratio | 655 | 184.0 (147.5–227.5) |
| Prone | OI | 648 | 12.5 (7.4–21.2) |
| Prone | OSI | 655 | 9.3 (5.7–15.6) |
P/F ratio, Pao2/Fio2 ratio; S/F ratio, Spo2/Fio2 ratio; OI, oxygenation index; OSI, oxygen saturation index.
The linear mixed-effects model concordance coefficients for the calfactant study data set were: 0.77 for the S/F ratio, 0.83 for the OI, and 0.44 for the OSI. The model concordance coefficients for the validation using the prone data set were: 0.70 for the S/F ratio, 0.74 for the OI, and 0.35 for the OSI.
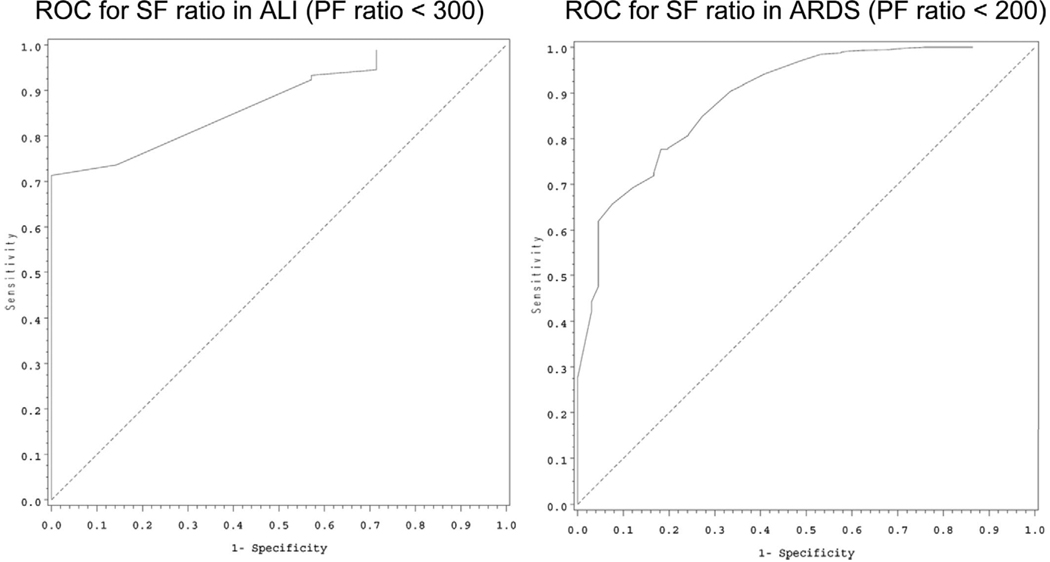
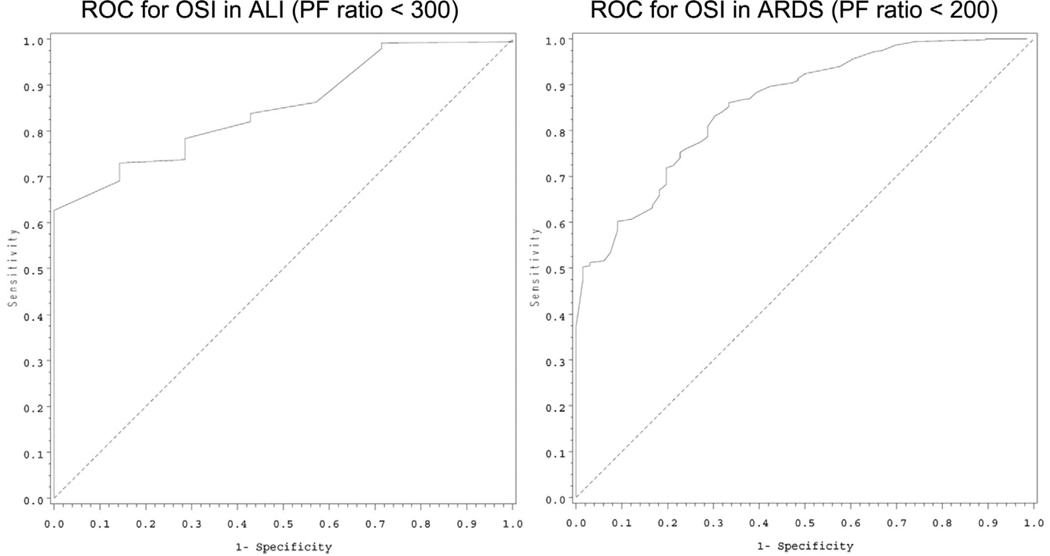
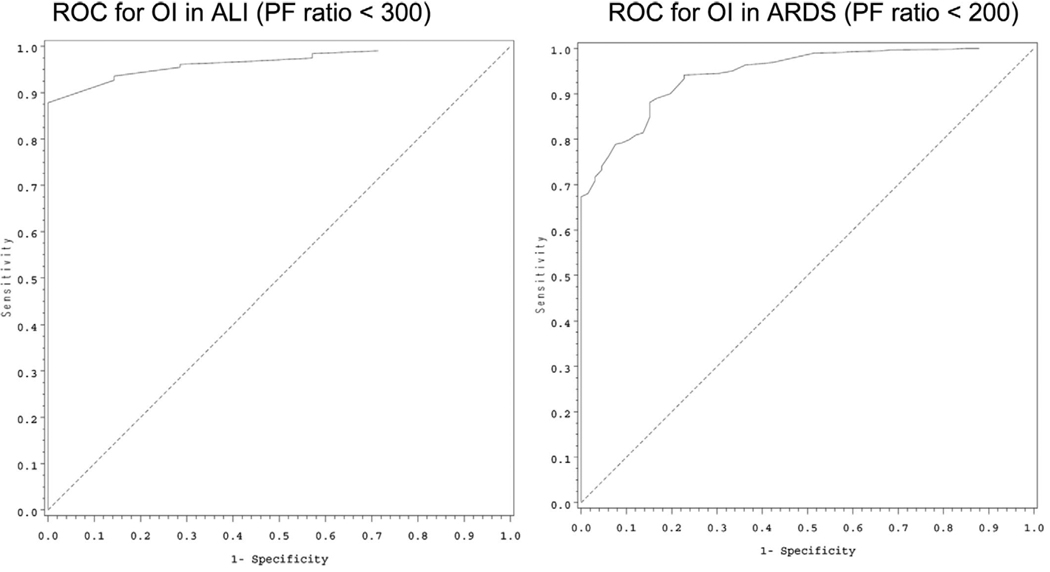
Based on the methods described, patients with an S/F ratio of 253 would meet criteria for ALI, and an S/F ratio of 212 would be equivalent to the ARDS criteria of a P/F ratio of 300. To include the Paw as a general measure of the amount of ventilatory support required to maintain the P/F ratio, an OI of 5.3 would equal ALI criteria, and a child with an OI of 8.1 would qualify for ARDS. Of note, the entry criteria for the calfactant trial (3) was an OI of at least 7. Table 3 provides more detail related to the above calculations as well as the results of the novel method OSI; an OSI of 6.5 would be equivalent to the ALI P/F ratio criteria, and an OSI of 7.8 would equal ARDS criteria. Receiver operator characteristic curves for the above measures are provided in Figures 1 to 3. The discrimination values, calculated from the areas under the receiver operator characteristic curves, demonstrate excellent or good discrimination of all values:
S/F ratio: P/F ratio <200: 0.88; P/F ratio <300: 0.87
OI: P/F ratio <200: 0.93; P/F ratio <300: 0.95
OSI: P/F ratio <200: 0.84; OSI P/F ratio <300: 0.84
Table 3.
Estimates of the calculated values for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
| ALI P/F Ratio 300 | ARDS P/F Ratio 200 | |||||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |||
| S/F ratio (95% CI) | 253 (245.3–261.0) | 93% (87%–96%) | 43% (17%–73%) | 212 (207.0–217.3) | 76% (68%–83%) | 83% (69%–92%) |
| OI (95% CI) | 5.3 (5.0–5.6) | 92% (88%–94%) | 86% (50%–97%) | 8.1 (7.7–8.6) | 79% (71%–85%) | 92% (81%–97%) |
| OSI (95% CI) | 6.5 (6.0–7.1) | 70% (61%–78%) | 86% (50%–97%) | 7.8 (7.2–8.4) | 64% (53%–74%) | 82% (66%–91%) |
P/F ratio, Pao2/Fio2 ratio; CI, confidence interval; S/F ratio, Spo2/Fio2 ratio; OI, oxygenation index; OSI, oxygen saturation index.
Figure 1.
Receiver operator characteristic (ROC) curves for Spo2/Fio2 (SF) ratio for acute lung injury (ALI) (left) and acute respiratory distress syndrome (ARDS) (right) based on the accepted Pao2/Fio2 (PF) ratio definitions of each category of acute lung disease.
Figure 3.
Receiver operator characteristic (ROC) curves for oxygen saturation index (OSI) for acute lung injury (ALI) (left) and acute respiratory distress syndrome (ARDS) (right) based on the accepted Pao2/Fio2 (PF) ratio definitions of each category of acute lung disease.
DISCUSSION
The results of this study demonstrate that noninvasive methods of oxygenation assessment, utilizing pulse oximetry as a substitute for Pao2, can be calculated and used as a surrogate for the diagnosis of ALI and ARDS in children. Additionally, because mechanical ventilation can largely influence the resultant oxygenation for a delivered percentage of oxygen, the OSI likely represents a more robust measure of lung disease severity. These parameters may be used potentially in the clinical diagnosis of ALI and ARDS and, after prospective validation, may serve as entry criteria for lung injury studies to assure that the study population mimics the target population.
The use of these parameters in recruitment of pediatric patients in interventional and epidemiologic studies of ALI and ARDS has the potential to reduce or even eliminate one of the major obstacles of pediatric lung injury clinical trialists: the failed enrollment of the target population of children with both ALI and ARDS. In two of the largest ALI/ARDS trials in children, both studies enrolled children with a much more severe level of lung injury than was targeted. In a study of exogenous calfactant administration (3), the entry criteria stated that subjects only required a persistent OI of >7, yet the study population demonstrated severe lung disease, represented by an OI of >20, and a P/F ratio of <130. Similarly, in the study of prone positioning for lung injury in children (5), the target entry criteria of P/F ratio of <300 (ALI criteria) was not represented in the study group, which demonstrated a P/F ratio of 100. Therefore, it is conceivable that a large number of subjects who had severe enough hypoxia to be eligible and potentially benefit from the intervention were not eligible due to lack of the Pao2 measurement. As our endeavors into larger ALI/ARDS studies continue to grow, it is clear that a new method to diagnose these potential subjects, without the need for Pao2 measurement, is needed. Based on the results described in this report, the OSI is a tool that, if prospectively validated, can achieve these goals.
There are other issues that require study in the pediatric ALI/ARDS population. Presently, pediatric critical care practitioners have adopted the consensus criteria definitions of ALI and ARDS, even though it is plausible that the pathophysiology may be different in children. It has been suggested that severity of hypoxia is correlated with mortality in children (7), with a stepwise increase in mortality with decreasing P/F ratio. Therefore, the use of hypoxia measures seems to be a reliable indicator of severity of illness, and likely a good target for use in the diagnosis of ALI and ARDS. Because the OI takes into account a general measure of respiratory system support (Paw), many practitioners in pediatrics feel it more clearly defines the degree of hypoxia in children with lung injury. Further prospective studies aimed at the validity and accuracy of OI in predicting outcome from lung injury are required. However, even the substitution of OI, instead of P/F ratio, for diagnosis does not alter the requirement for arterial blood sampling and the resultant underdiagnosis of ALI and ARDS in children. The use of the OSI, however, will alleviate both issues of the P/F ratio described above. This tool has not been described in either pediatric or adult lung injury subjects to date, and will require well-designed, prospective validation before legitimate use in clinical diagnosis as well as entry criteria into clinical trials.
There are limitations of the data presented in this report which deserve discussion. First, although the data elements collected in both the development and the validation data sets were collected prospectively, the analysis was done post hoc. Although this post hoc analysis likely does not affect the mathematical modeling or the validity of the calculations, it is possible that the most accurate measurements were not made during the collection of these data. This concern is lessened by the prospective nature of the data collection as well as the rigor of the study protocols (8). A prospective study of data collection, with close attention to the variance of the pulse oximeter and the exact timing of the arterial blood gas measurement with the recording of the Spo2, is required in future validation studies of these measures. Another limitation is that, again due to the use of Spo2 data that were gathered in the conduct of large-scale clinical trials, it is conceivable that issues, such as poor oximeter waveform, unclean oximetry probe, and patient movement, may have affected the accuracy of the measurement. However, as these patients were enrolled in large, academic pediatric intensive care units with a wealth of experience with oximetry, this is less likely to be a concern. Third, the oxygen-hemoglobin dissociation curve and thus the relationship between Pao2 and Spo2 are known to be affected by a variety of variables, including pH, temperature, Paco2, and concentration of 2,3 diphosphoglycerate (9). These variables were measured but not linked to Spo2 or arterial blood gas data and, therefore, were not used in the modeling; these measurements will require close monitoring during the prospective validation of this model. However, despite this, the correlation between the Pao2 and Fio2 seems to be acceptable in these coupled measurements. Again, prospective data collection with collection of these potential confounding variables is necessary to validate the values derived in this present study. Finally, our ALI and ARDS diagnostic variables were derived and validated from a data set that only included samples ≤97% saturated. This was due to the flattening of the oxygen-hemoglobin dissociation curve at the upper end of saturation. Therefore, the S/F ratio and the OSI can only be utilized in patients <98% saturated. As these variables will be used to diagnose lung disease with significant hypoxemia by definition, this should not limit the clinical utility of these measures.
In conclusion, the data presented in this study determine that an OSI of 6.5 and 7.8 may be substituted for P/F ratio for defining ALI and ARDS in children with satisfactory, but not optimal, sensitivity and specificity. Further prospective research is required in this area of study to determine whether values can be calculated with higher sensitivity and specificity and still maintain the clinical usefulness. Compared with the other models, OSI would be the preferable method, as it has the advantage of including the Paw and also utilizing the noninvasive measure of oxygenation (Spo2). Although these values require validation in a prospective trial, utilizing this new criterion clinically has the potential to allow a more accurate diagnosis of ALI and ARDS in children and allow investigators to gain a true incidence of these lung diseases in pediatric populations. From a clinical trial perspective, use of the OSI as entry criteria in clinical trials is likely to increase the number of eligible children enrolled and allow investigators to enroll a more reasonable target population. This has the potential to impact the study of acute lung disease in children.
Figure 2.
Receiver operator characteristic (ROC) curves for oxygenation index (OI) for acute lung injury (ALI) (left) and acute respiratory distress syndrome (ARDS) (right) based on the accepted Pao2/Fio2 (PF) ratio definitions of each category of acute lung disease.
Acknowledgments
This work was supported, in part, by research grants (NINR R01NR05336) from National Institutes of Health and Children’s Miracle Network of Penn State Hershey Children’s Hospital.
Dr. Thomas is an advisory board and scientific committe member for Discovery Laboratories. Dr. Willson has received grant support from Pneuma Pharmaceuticals (formally ONY) for an ongoing trial. Dr. Shaffer has consulted for Discovery Laboratories Data Safety and Monitoring Board.
Footnotes
The work was presented, in part, at the American Thoracic Society 2007 International Conference, San Francisco, CA, May 18–23, 2007.
The remaining authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: A randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 4.Mower WR, Sachs C, Nicklin EL, et al. Pulse oximetry as a fifth pediatric vital sign. Pediatrics. 1997;99:681–686. doi: 10.1542/peds.99.5.681. [DOI] [PubMed] [Google Scholar]
- 5.Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: A randomized controlled trial. JAMA. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonesh EF, Chinchilli VM, Pu K. Goodness-of-fit in generalized nonlinear mixed-effects models. Biometrics. 1996;52:572–587. [PubMed] [Google Scholar]
- 7.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 8.Curley MA, Arnold JH, Thompson JE, et al. Clinical trial design—Effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. J Crit Care. 2006;21:23–32. doi: 10.1016/j.jcrc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerbaux T, Detry B, Reynaert M, et al. Right shift of the oxyhemoglobin dissociation curve in acute respiratory distress syndrome. Pathol Biol (Paris) 1997;45:269–273. [PubMed] [Google Scholar]