Abstract
Burn injury destroys skin, the second largest innate immune organ in the body, and triggers chaotic immune and inflammatory responses. The pattern recognition molecule, mannan-binding lectin (MBL), plays an important role in the first-line host defense against infectious agents. MBL initiates the lectin complement pathway and acts as an opsonin. Recent studies suggest that MBL also modulates inflammatory responses. We report that local responses after burn in MBL null mice differ from those found in wild-type (WT) mice in the following important biological markers: spontaneous eschar separation, thinned epidermis and dermis, upregulation of soluble factors including cytokines, chemokines, cell adhesion molecules, a growth factor-binding protein, and matrix metalloproteinases. Mice lacking C1q, C4, or C3 did not show the lack of eschar separation seen in MBL null-burn phenotype. These findings implicate MBL as an important molecule in the maintenance of the homeostatic balance.
INTRODUCTION
The skin is the second largest innate immune organ in the body providing not only a physical barrier but also serving as an immune-surveillance system (Allgower et al., 1995). The skin comprises the epidermis, which is packed with keratinocytes forming a mechanical barrier as well as an immune sentinel (Nickoloff and Turka, 1994), and the dermis, which consists of a variety of immune cells. Thermal injuries damage this organ and trigger chaotic immune and inflammatory responses, both locally and systemically (Allgower et al., 1995; Sparkes, 1997; Pruitt et al., 1998). Eschar, a scab-formed following burn, may spontaneously slough off, otherwise it has to be surgically removed to improve the prognosis of burn victims (Quinby et al., 1981). The spontaneous shedding process involves matrix metalloproteinases (MMPs) as extracellular matrix has to be enzymatically digested (Soo et al., 2000; Armstrong and Jude, 2002; Dasu et al., 2003; Burke, 2004). In fact, MMPs in blood increase temporally in burn victims (Stricklin et al., 1993; Young and Grinnell, 1994; Dasu et al., 2003; Ulrich et al., 2003).
Mannan-binding lectin, a pattern-recognition molecule of the innate immune system, plays an important role as a first-line host defense mechanism against infection by recognizing pathogen-associated molecular patterns of a variety of pathogens (Takahashi et al., 2006). MBL is primarily synthesized in liver and circulates in blood (Uemura et al., 2002). MBL acts as an opsonin (Kuhlman et al., 1989; Sumiya et al., 1991) and initiates the lectin pathway of the complement system (Ikeda et al., 1987) in cooperation with MBL-associated serine proteases (MASPs) (Matsushita and Fujita, 1992; Thiel et al., 1997). Recent studies have demonstrated that MBL also recognizes altered-self in the context of apoptosis or necrosis and participates in the removal of such dying cells (Lokitz et al., 2005; Takahashi et al., 2006). MBL recognizes these targets through a carbohydrate recognition domain (CRD). The carbohydrate recognition domain is followed by a neck region and a collagen-like domain that multimerizes MBL (Ng et al., 1996). The multimerization and serum concentration of MBL is reduced by single amino-acid substitutions owing to single nucleotide polymorphisms in exon 1, encoding the collagen-like domain. These single nucleotide polymorphisms results in MBL levels that vary from undetectable to 10 µg/ml (Christiansen et al., 1999).
All complement pathways activate C3 to generate complement opsonins, the membrane attack complex, and anaphylactic factors that attract inflammatory cells (Ward, 1980; Taylor, 1992; Cunnion et al., 2004). The aberrant MBL proteins may still have the opsonic function (Super et al., 1992), while being unable to activate the lectin pathway (Matsushita et al., 1995; Wallis and Dodd, 2000).
Recent animal infection studies using MBL null and wild-type (WT mice) provided evidence that MBL is involved in the regulation of inflammatory responses (Takahashi et al., 2002; Shi et al., 2004; Moller-Kristensen et al., 2006). Several studies suggest that proinflammatory cytokines modulate synthesis and induction of MMPs (Park et al., 2004; Distler et al., 2005), thus, raise the question whether MBL modulates MMP activities. We observed striking differences between MBL null and WT mice in eschar formation after burn injury, and now present investigation on the possible cause of this difference.
RESULTS
MBL null mice fail to separate spontaneously eschar following burn injury
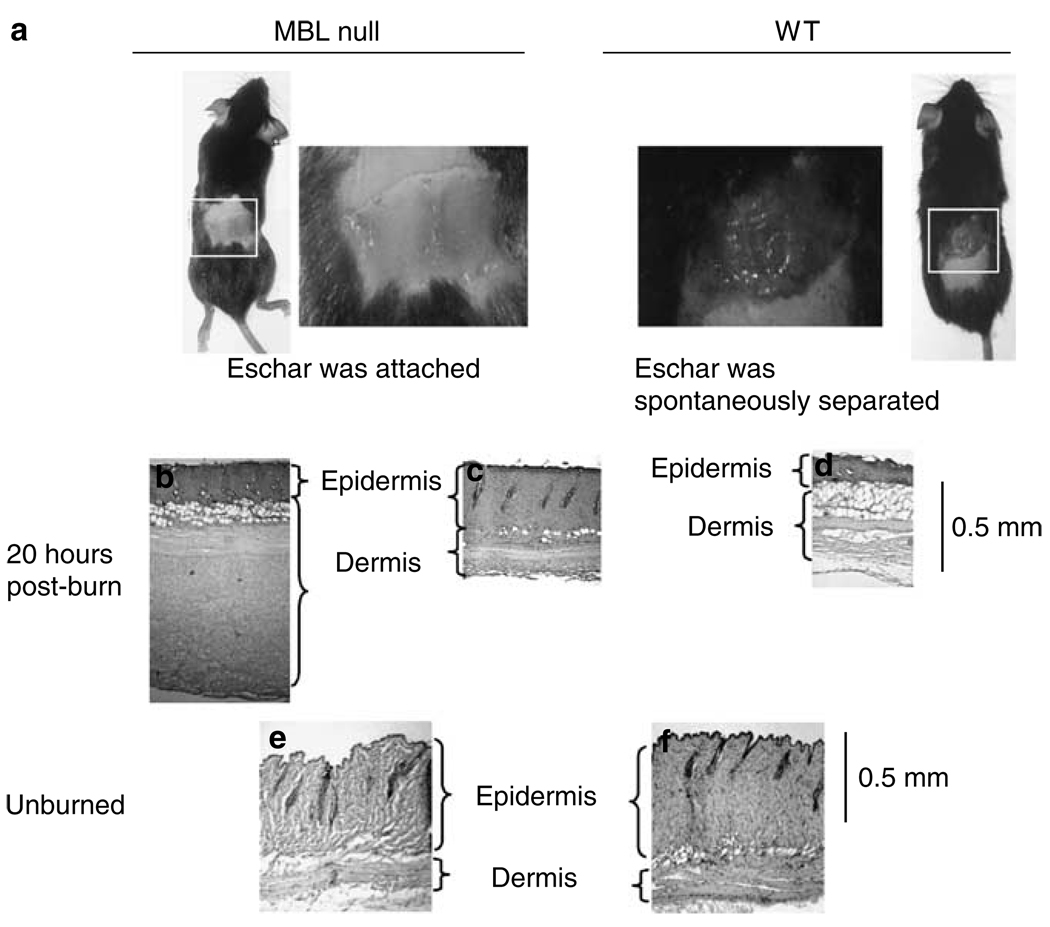
To test our hypothesis that MBL plays a role in response to burn injury, we subjected MBL null and WT mice to full-thickness burn corresponding to 5% TBSA. We examined changes in the burned and the surrounding nonburned areas. Within 24 hours after burn, the eschar of WT mice was spontaneously lost, whereas the eschar remained attached in MBL null mice (Figure 1a), even a week after burn (data not shown). Neither burn injury nor eschar separation resulted in changes in weight, behavior, or overall health (data not shown). Twenty-one out of 23 WT mice lost the eschar by 24 hours after burn, whereas only three out of 18 MBL null mice showed signs of eschar separation (P<0.00001, Table 1). The phenotype was reversed by reconstituting MBL null mice with recombinant human MBL (rhMBL), as five out of six reconstituted MBL null mice showed spontaneous eschar separation (P<0.005 against MBL null mice, Table 1) confirming that the phenotype was MBL-dependent.
Figure 1. Eschars in WT and MBL null mice after burn.
(a) Phenotype of MBL null mice and WT mice 20 hours postburn. Histological examination of eschars after 20 hours by hematoxylin and eosin staining; (b) and (c) MBL null mice; (d) WT mice. Normal skin without burn; (e) MBL null mice; and (f) WT mice.
Table 1.
Summary of spontaneous eschar separation at 24 h after burn
| WT | MBL null | MBL null + rhMBL | C4 null | C1q null | C3 null | |
|---|---|---|---|---|---|---|
| Eschar separation | 21 | 3 | 5 | 4 | 4 | 5 |
| Total | 23 | 18 | 6 | 4 | 6 | 6 |
| P-values vs MBL null | <0.0001 | <0.005 |
MBL, mannan-binding lectin; WT, wild-type.
Histological examination revealed that eschars from MBL null mice had two different histological appearances, thickened dermis (Figure 1b) and epidermis (Figure 1c) compared with that of WT mice (Figure 1d). In WT mice, a mesh-like structure was observed underneath the dermis suggesting enzymatic digestion of extracellular matrix (Figure 1d). The difference in thickness and structures was the result of different responses to the thermal insults in WT and MBL null mice, as there was no difference between WT and MBL null mice without burn (Figure 1e and f).
Next, we investigated whether the mechanism of the spontaneous eschar separation was involved with complement activation as MBL activates the lectin pathway and complement is believed to play a major role in inflammation and tissue damage (Ward and Till, 1990; Schmid et al., 1997; Laufer et al., 2001; Monsinjon et al., 2001; Hart et al., 2005; Moller-Kristensen et al., 2005; Walsh et al., 2005). We tested mice lacking C4, C1q, or C3 for the following reasons: C4, the first cleaved by the MBL–MASP complex in the lectin pathway; C1q, the initiator of the classical pathway; C3, the essential to all complement pathways. All C4 null mice, four out of six C1q null mice, and five out of six C3 null mice spontaneously lost eschar in 24 hours following burn (Table 1), suggesting that the process of the eschar separation does not require complement activation.
Low MMP activities after burn in skin of MBL null mice
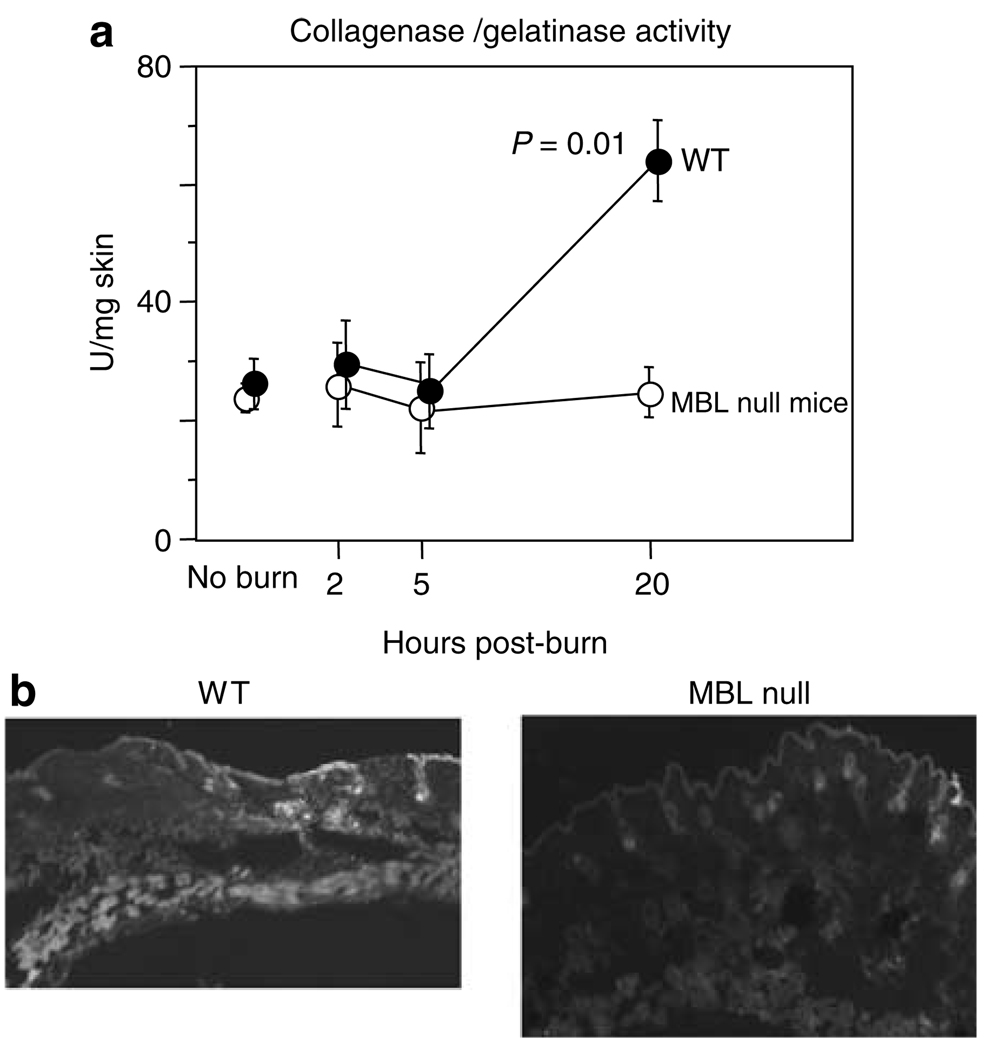
We next hypothesized that the mechanism of spontaneous eschar separation could be partly attributed to locally increased MMP activities in WT mice. MMP activities were determined in skin homogenates at various time points following burn. At 20 hours after burn injury, MMP activities increased almost 2-fold in WT mice compared with MBL null mice (P = 0.01, Figure 2), whereas nonburned WT and MBL null mice had similar activities (Figure 2a). MMP activities were baseline at 2 and 5 hours following burn in both WT and MBL null mice (Figure 2a) even though the eschar separation in WT mice could be observed as early as 6 hours following burn. To localize MMP activities in the skin, cryosections were incubated with fluorescein-labeled gelatin. The intense FITC signal, reflecting MMP activity, was observed in subcutaneous layer in WT mice (Figure 2b), whereas it was almost undetectable in MBL null mice (Figure 2b).
Figure 2. MMP activities in skin after burn.
(a) MMP activities (collagenase/gelatinase) were determined at various time points. Numbers of mice used were 4, 6, 6, and 5 for WT mice and 5, 5, 6, and 5 for MBL null mice at no burn and after 2, 5, and 20 hours, respectively. (b) Localization of MMP activity in skin adjacent to burned skin after 20 hours. Original magnification ×20.
Reduced local inflammatory responses in MBL null mice compared with WT mice
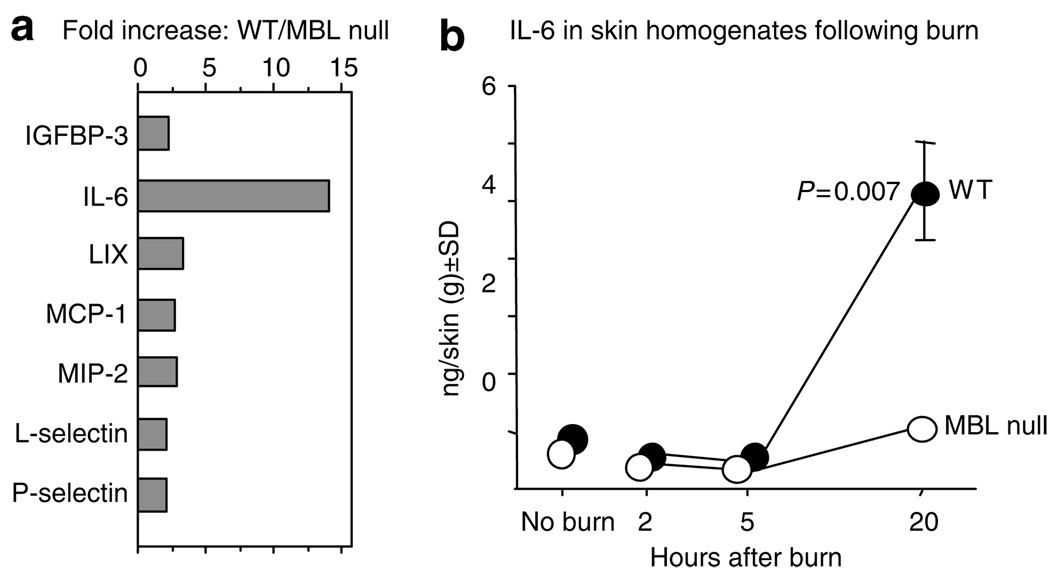
We have previously shown that IL-6 in skin was significantly increased in WT mice compared with MBL null mice at 20 hours following burn (Moller-Kristensen et al., 2006). In this study, we examined local responses by analyzing multiple factors at 20 hours after burn. The analysis was performed using the cytokine antibody array designed to detect 32 factors (Figure S1). Among positively identified factors, those that increased more than 2-fold in WT mice over MBL null mice were plotted in Figure 3a. The largest increase was observed for IL-6 (14-fold) followed by 2 to 3-fold increase in chemokines, lipopolysaccharide-induced CXC-chemokine (LIX), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-2 (MIP-2); the cell adhesion molecules, l-selectin and P-selectin; and insulin-like growth factor-binding protein-3 (IGFBP-3, Figure 3a). The marked increase of IL-6 at 20 hours in WT compared with MBL null mice is in agreement with our previous study (Moller-Kristensen et al., 2006). However, we did not examine the kinetics of IL-6 in the previous study. Therefore, we also investigated early time points. IL-6 was undetectable at 2 and 5 hours as it remained similar to the levels in nonburned animals (Figure 3b). These results indicate that MBL is involved with the modulation of not only cytokines but also chemokines, cell adhesion molecules, and growth factor-binding protein.
Figure 3. Local biological response in burned skin.
(a) The increase of molecules in WT as compared with MBL null mice. (b) IL-6 levels in WT and MBL null mice following burn injury. Levels of IL-6 were determined in skin extracts at different time points after the burn injury. Numbers of mice used were 5, 6, 7, and 7 for WT and 5, 3, 5, and 6 for MBL null mice at no burn and after 2, 5 and 20 hours, respectively. Two experiments were combined. The data at 20 hours after burn injury were reported in our previous study (Møller-Kristensen et al., 2006).
DISCUSSION
In this study, we demonstrate that spontaneous eschar separation following burn injury is inhibited in MBL null mice compared with WT mice (Figure 1). This was confirmed to be MBL-dependent as the reconstitution of MBL null mice with rhMBL reversed the phenotype similar to WT mice (Table 1). Thus, our findings show for the first time that MBL plays a role in the phenotypic local response to burn. Investigation of mice lacking other complement components (a) C4 is first cleaved by the MBL–MASP complex in the lectin pathway; (b) C1q initiates the classical pathway that could have been influenced by the absence of the lectin pathway in burned hosts, although there is no difference in naïve mice (Shi et al., 2004); (c) C3 is essential to all three complement pathways: the classical, the lectin, and the alternative pathway (Fujita, 2002) showed a phenotype similar to WT mice. MBL activates the lectin pathway and complement is believed to play a major role in inflammation and tissue damage (Ward and Till, 1990; Schmid et al., 1997; Laufer et al., 2001; Monsinjon et al., 2001; Hart et al., 2005; Moller-Kristensen et al., 2005; Walsh et al., 2005). Although we did not directly investigate deposition of complement factors on the eschar in vivo, the results strongly suggest that the separation is MBL-dependent and does not require the complement activation.
Histological examination of excised eschar revealed the thickening of both dermis and epidermis in MBL null mice (Figure 1b and c), unlike that observed in WT mice (Figure 1d). The difference in skin thickness in response to thermal insult is most likely affected by MBL as there is no difference in the thickness between MBL null and WT mice without burn (Figure 1e and f). Of interest, four out of six MBL null mice presented epidermal acanthosis, whereas only one out of four WT mice developed a mild pathology in our previous study on mice aged 16–18 months (Stuart et al., 2005). These data suggest that MBL may have a role in maintaining physically healthy skin in addition to modulating local response to thermal insults.
A logical hypothesis is that MMPs are involved in spontaneous eschar separation by enzymatically digesting extracellular matrix. We found that MMP activities were reduced in skin homogenates of eschar and surrounding skin of MBL null mice at 20 hours after burn compared with WT mice (Figure 2a). Intense MMP staining was localized to subcutaneous layers of WT mice, whereas it was undetectable in MBL null mice (Figure 2b). Although we did not identify which of the MMPs were responsible, multiple MMPs including MMP-1, -2, -3, and -9, have been associated with burn wounds (Stricklin et al., 1994; Young and Grinnell, 1994; Soo et al., 2000; Dasu et al., 2003; Ulrich et al., 2003, 2005; Mwaura et al., 2006). Interestingly, MMP-2 in endothelial cells can be activated by a plant lectin, Ulex europaeus agglutinin-I (Gomez et al., 1995), which binds to l-fucose, a favored carbohydrate target of MBL (Holmskov et al., 1994) and is expressed on endothelial cells (Gomez et al., 1995). Conversely, MMP-2 and MMP-9 can be inhibited by l-fucose (Gomez et al., 1995; Isnard et al., 2002). One can speculate that these MMPs were inhibited by free l-fucose in the MBL-deficient host while MBL binds to l-fucose on endothelial cells and activates MMPs like Ulex europaeus agglutinin-I in WT mice. On the contrary, MBL has been shown to inhibit the activation of meprin-α and -β that are members of MMP family, though their presence in skin has not been examined (Hirano et al., 2005). Although we did not investigate them in this study, tissue inhibitors of metalloproteinases (TIMPs), which are specific inhibitors of MMPs, have been detected in burned hosts (Dasu et al., 2003; Ulrich et al., 2003). Therefore, there is a possibility that tissue inhibitors of metalloproteinases may be upregulated in hosts lacking MBL. Thus, further studies are required to elucidate the mechanisms as to precisely how MBL modulates MMPs and possibly tissue inhibitors of metalloproteinases.
The local increase of MMP activity in WT mice was associated with locally upregulated inflammatory responses illustrated by increased chemokines, such as LIX, MIP-2 and MCP-1, and proinflammatory cytokine, IL-6 (Figure 3a). The latter two proteins have been detected systemically (Kataranovski et al., 1999; Furukawa et al., 2002) and locally (Ono et al., 1995; Gibran et al., 1997; Shallo et al., 2003) following thermal injuries. MIP-2 was reported to be increased in lungs in response to burn (Chen et al., 2006), whereas LIX has not been seen in relation to thermal injury. Interestingly, the binding of MBL induces production of MIP-2 from macrophages in vitro (Nadesalingam et al., 2005). Our results demonstrate that MBL modulates multiple cytokines and chemokines in response to burn insults, supporting previous observations by us and others (Jack et al., 2001; Shi et al., 2004). Each factor may have different kinetics, although we only followed the kinetics of IL-6 in the skin homogenates that was undetectable until 20 hours (Figure 3b), correlating with MMP activities (Figure 2).
Interplay between proinflammatory factors and MMPs has been shown by other groups. MCP-1 and IL-6 induce and activate MMPs from fibroblasts, endothelial cells, and macrophages that could be either resident macrophages or macrophages recruited to sites of inflammation (Yamamoto et al., 2000; Robinson et al., 2002; Werle et al., 2002; Park et al., 2004). Interestingly, MCPs enzymatically truncated by MMPs have been shown to act as MCP antagonists (Mcquibban et al., 2002), suggesting that chemokines and MMPs may tightly regulate each other. In this regard, insulin-like growth factors and IGFBPs, in particular IGFBP-3 have been reported to attenuate the acute inflammatory response in burned patients (Lang et al., 1996; Jeschke et al., 2000a, 2000b, 2002). Other molecules upregulated in burn wounds of WT mice compared to MBL null mice are cell adhesion molecules, l- and P-selectin. Selectins have been suggested to play a role in vascular injury by recruiting inflammatory leukocytes following burn (Mulligan et al., 1994; Hansbrough et al., 1996; Katahira et al., 2002). Vascular cell adhesion molecule-1, another adhesion molecule suggested to play a role in burn (Chen et al., 2004), was strongly detected in both WT and MBL null mice (Figure S1). Of note, when neutrophil elastase null mice that are reportedly impaired in neutrophil recruitment were tested in this burn model, the spontaneous eschar separation was reduced comparable to MBL null mice (unpublished observation). Taken together, these results suggest that the initial step of spontaneous eschar separation may be triggered by the vascular injury caused by pathologically increased cell adhesion molecules. MBL may somehow locally modulate the production and/or function of cell adhesion molecules after burn.
Our data also demonstrate that the burn phenotype is mediated by an MBL-dependent mechanism distinct from the complement pathway, as the phenotype in mice lacking C1q, C4, or C3 was similar to that of WT mice (Table 1). These results suggest that the local tissue destruction from thermal injury is not caused by complement activation even though this possibility has been previously proposed (Radke et al., 2000). However, the mechanisms may have tissue and species dependency as complement activation was implicated in lung tissue destruction following burn of rat skin (Schmid et al., 1997).
Lastly, MBL does not seem to be synthesized in the skin, as no messenger RNA is detected in normal skin (Uemura et al., 2002), although induction of messenger RNA in acutely injured and inflamed tissues cannot be ruled out. The most likely source of MBL would be plasma, where MBL naturally circulates, because endothelial cell permeability has been reported to increase after burn injuries (Arturson, 1979; Ward and Till, 1990). Whether MBL messenger RNA or protein increase upon burn injury as observed following UV irradiation (Lokitz et al., 2005) and whether the presence of MBL in situ is required for the spontaneous eschar separation remains a matter for further investigation.
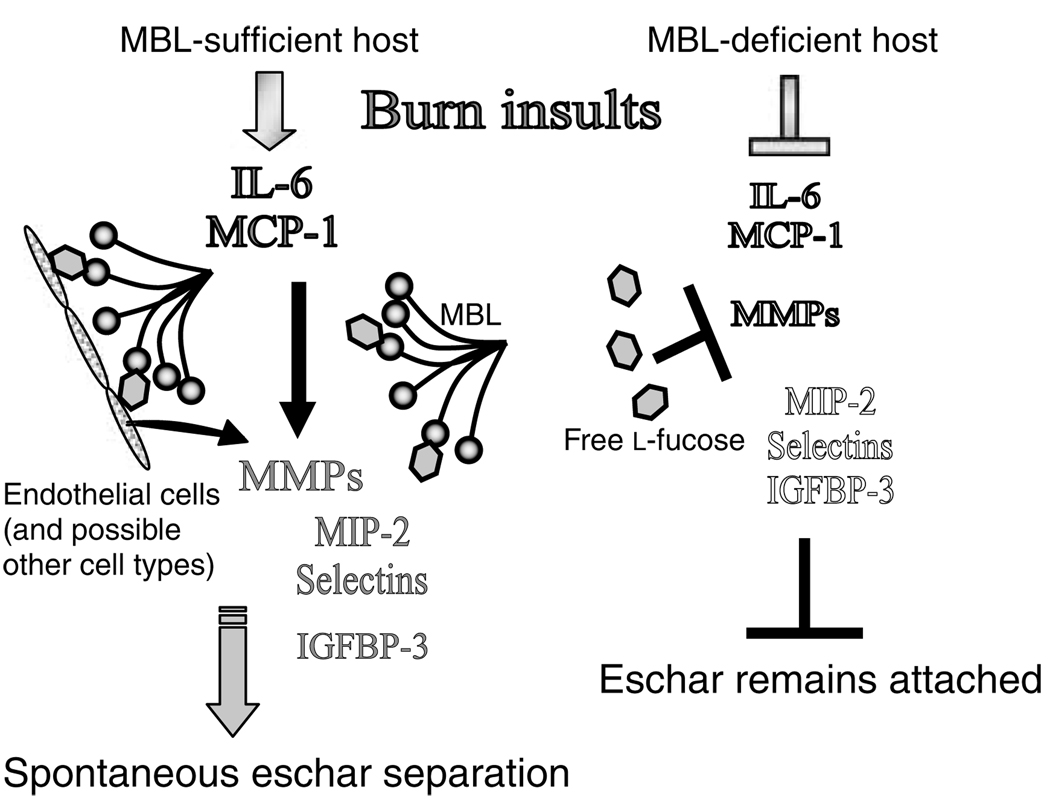
In conclusion, our data demonstrate that MBL modulates not only inflammatory factors, such as cytokines and chemokines, but also cell adhesion molecules, growth factor-binding protein, and particularly MMPs that are the most likely direct effectors in the eschar separation. Figure 4 schematically summarizes our speculation that there is likely a complex interaction between the molecules discussed above in MBL-sufficient and -deficient hosts after thermal insults. However, the detailed mechanisms as to how MBL regulates these molecules will have to be investigated in future. We propose that MBL plays a key role in modulating a wide range of molecules beyond infection and inflammation, and suggest that MBL is an important molecule in maintenance of homeostatic balance.
Figure 4. Proposed roles of MBL against burn insults.
Arrowheads indicate activation and induction. Arrows with blunt heads indicate inhibition and blocking. Names of factors in solid and outlined letters represent inflammatory and non-inflammatory states, respectively.
MATERIALS AND METHODS
Mice
MBL null mice were generated as described previously (Shi et al., 2004) and were backcrossed for seven generations onto C57B/6J. The C57B/6J genetic background was monitored by microsatellite analysis at Charles River Laboratories. Less than 4% of the genetic background, a part of chromosomes used for gene targeting, was not on C57B/6J. The C1q null mice were kindly provided by M Botto (Botto et al., 1998). Both C3 and C4 null mice were kindly provided by M Carroll (Wessels et al., 1995). All mice were on the C57BL/6J background. Age- and gender-matched mice (8–10 weeks) were used. All animal experiments were performed under a protocol approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital (Boston, MA).
Burn model
Mice were subjected to a dry non-lethal burn injury as described previously (Moller-Kristensen et al., 2006). Briefly, mice were anesthetized and dorsal-side skin was burned using two healed brass bars in boiling water. Routine observation, including weight and behavior was followed up to 4 weeks.
Imaging
To record burn wound progression, digital photographs were taken immediately after burn injury, after 12 and 24 hours, and daily thereafter.
Reconstitution of MBL null mice with rhMBL
Clinical grade rhMBL (Vorup-Jensen et al., 2001) was provided by NatImmune A/S (Copenhagen, Denmark). MBL null mice were injected intraperitoneal with 75 µg rhMBL (Shi et al., 2004) in 0.2 ml saline per mouse at 12 and 2 hours before and 24 hours after the burn injury.
Histology
Skin samples including burned skin and unburned skin adjacent to the burned area (3 mm surrounding edges) were obtained 20 hours post-burn. Healthy dorsal skin was also obtained from unburned WT and MBL null mice. For paraffin sections, the samples were fixed in neutral 3.7% formalin, dehydrated through serial dilution of ethanol followed by xylene and embedded in paraffin. For cryosections, the skin samples were embedded in tissue-freezing media (Triangle Biomedical Sciences, Durham, NC) and snap-frozen in liquid nitrogen. Paraffin sections in 4 µm thickness were stained with hematoxylin and eosin. Cryosections in 6 µm thickness were stained for gelatinase activity using fluorescein-labeled DQ gelatin (Molecular Probes, Eugene, OR) (Oh et al., 1999) and examined under a fluorescence microscopy (NIKON TE2000-U) operated by Openlab system (Inprovision Inc., Lexington, MA).
MMP activity
Skin samples (burned skin and the surrounding edges, 3 × 15 mm) were weighed and then homogenized in 0.5 ml saline using a tissue homogenizer (Dremel model 395, MS). Supernatants were stored at −80°C until assays were performed. Gelatinase/collagenase activity was assayed according to the manufacturer’s instructions (Molecular Probes, Invitrogen, Carlsbad, CA).
Cytokine assay
Pooled skin extracts of WT and MBL null mice were analyzed for 32 molecules (Figure S1a) using a cytokine antibody array (RayBiotech Inc., Norcross, GA) according to the manufacturer’s instructions. The reaction was visualized by chemiluminescence exposing to an X-ray film. Intensities of positive factors identified by darkened spots on X-ray film were analyzed using a GS-700 imaging densitometer (Bio-Rad, Hercules, CA). Results were expressed as the fold-increase in WT relative to MBL null mice. A 2-fold increase was defined as positive. IL-6 in skin homogenate was individually determined at various time points using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical analysis
Differences between groups were analyzed using the nonparametric Wilcoxon test (using the program JMP (SAS Institute, Cary, NC). The number of mice used in each experiment are indicated in figure legends.
Supplementary Material
Local biological response in burned skin.
ACKNOWLEDGMENTS
We thank R. Alan B. Ezekowitz and the members of Development Immunology, Massachusetts General Hospital for helpful discussions. We thank Edward A Carter for advice on the burn model. We also acknowledge Marina Botto for providing us C1q null mice. We thank NatImmune A/S for providing rhMBL.
Abbreviations
- IGFBP-3
insulin-like growth factor-binding protein-3
- LIX
lipopolysaccharide-induced CXC-chemokine
- MASP
MBL-associated serine protease
- MBL
mannan-binding lectin
- MIP-2
macrophage inflammatory protein-2
- MMP
matrix metalloproteinase
- rhMBL
recombinant human MBL
- SNP
single-nucleotide polymorphism
- TBSA
total body surface area
- WT
wild type
Footnotes
CONFLICT OF INTEREST
J.C. Jensenius and S. Thiel have a financial interest in NatImmune A/S, Copenhagen, the company providing the rhMBL.
REFERENCES
- Allgower M, Schoenenberger GA, Sparkes BG. Burning the largest immune organ. Burns. 1995;21:S7–S47. doi: 10.1016/0305-4179(95)90028-4. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. J Am Podiatr Med Assoc. 2002;92:12–18. doi: 10.7547/87507315-92-1-12. [DOI] [PubMed] [Google Scholar]
- Arturson G. Microvascular permeability to macromolecules in thermal injury. Acta Physiol Scand Suppl. 1979;463:111–122. [PubMed] [Google Scholar]
- Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Burke B. The role of matrix metalloproteinase 7 in innate immunity. Immunobiology. 2004;209:51–56. doi: 10.1016/j.imbio.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Chen LW, Chang WJ, Wang JS, Hsu CM. Thermal injury-induced peroxynitrite production and pulmonary inducible nitric oxide synthase expression depend on JNK/AP-1 signaling. Crit Care Med. 2006;34:142–150. doi: 10.1097/01.ccm.0000190621.48720.8c. [DOI] [PubMed] [Google Scholar]
- Chen XL, Xia ZF, Wei D, Liao HG, Ben DF, Wang GQ. Expression and regulation of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells induced by sera from severely burned patients. Crit Care Med. 2004;32:77–82. doi: 10.1097/01.CCM.0000104220.68149.DA. [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Kilpatrick DC, Souter V, Varming K, Thiel S, Jensenius JC. Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand J Immunol. 1999;49:193–196. doi: 10.1046/j.1365-3083.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- Cunnion KM, Hair PS, Buescher ES. Cleavage of complement C3b to iC3b on the surface of Staphylococcus aureus is mediated by serum complement factor I. Infect Immunol. 2004;72:2858–2863. doi: 10.1128/IAI.72.5.2858-2863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Spies M, Barrow RE, Herndon DN. Matrix metalloproteinases and their tissue inhibitors in severely burned children. Wound Repair Regen. 2003;11:177–180. doi: 10.1046/j.1524-475x.2003.11305.x. [DOI] [PubMed] [Google Scholar]
- Distler JH, Jungel A, Huber LC, Seemayer CA, Reich CF, III, Gay RE, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA. 2005;102:2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Kobayashi M, Herndon DN, Pollard RB, Suzuki F. Appearance of monocyte chemoattractant protein 1 (MCP-1) early after thermal injury: role in the subsequent development of burn-associated type 2 T-cell responses. Ann Surg. 2002;236:112–119. doi: 10.1097/00000658-200207000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibran NS, Ferguson M, Heimbach DM, Isik FF. Monocyte chemoattractant protein-1 mRNA expression in the human burn wound. J Surg Res. 1997;70:1–6. doi: 10.1006/jsre.1997.5017. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Yoshiji H, Kim JC, Thorgeirsson UP. Ulex europaeus I lectin induces activation of matrix-metalloproteinase-2 in endothelial cells. Biochem Biophys Res Commun. 1995;216:177–182. doi: 10.1006/bbrc.1995.2607. [DOI] [PubMed] [Google Scholar]
- Hansbrough JF, Wikstrom T, Braide M, Tenenhaus M, Rennekampff OH, Kiessig V, et al. Effects of E-selectin and P-selectin blockade on neutrophil sequestration in tissues and neutrophil oxidative burst in burned rats. Crit Care Med. 1996;24:1366–1372. doi: 10.1097/00003246-199608000-00016. [DOI] [PubMed] [Google Scholar]
- Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, et al. Gastrointestinal ischemia–reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- Hirano M, Ma BY, Kawasaki N, Okimura K, Baba M, Nakagawa T, et al. Mannan-binding protein blocks the activation of metalloproteases meprin alpha and beta. J Immunol. 2005;175:3177–3185. doi: 10.4049/jimmunol.175.5.3177. [DOI] [PubMed] [Google Scholar]
- Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987;262:7451–7454. [PubMed] [Google Scholar]
- Isnard N, Peterszegi G, Robert AM, Robert L. Regulation of elastase-type endopeptidase activity, MMP-2 and MMP-9 expression and activation in human dermal fibroblasts by fucose and a fucose-rich polysaccharide. Biomed Pharmacother. 2002;56:258–264. doi: 10.1016/s0753-3322(02)00196-8. [DOI] [PubMed] [Google Scholar]
- Jack DL, Read RC, Tenner AJ, Frosch M, Turner MW, Klein NJ. Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis. 2001;184:1152–1162. doi: 10.1086/323803. [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Barrow RE, Herndon DN. Insulinlike growth factor I plus insulinlike growth factor binding protein 3 attenuates the proinflammatory acute phase response in severely burned children. Ann Surg. 2000a;231:246–252. doi: 10.1097/00000658-200002000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, Barrow RE, Suzuki F, Rai J, Benjamin D, Herndon DN. IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med. 2002;8:238–246. [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, Herndon DN, Barrow RE. Insulin-like growth factor I in combination with insulin-like growth factor binding protein 3 affects the hepatic acute phase response and hepatic morphology in thermally injured rats. Ann Surg. 2000b;231:408–416. doi: 10.1097/00000658-200003000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira J, Murakami K, Schmalstieg FC, Cox R, Hawkins H, Traber LD, et al. Role of anti-l-selectin antibody in burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1043–L1050. doi: 10.1152/ajplung.00305.2001. [DOI] [PubMed] [Google Scholar]
- Kataranovski M, Magic Z, Pejnovic N. Early inflammatory cytokine and acute phase protein response under the stress of thermal injury in rats. Physiol Res. 1999;48:473–482. [PubMed] [Google Scholar]
- Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Fan J, Frost RA, Gelato MC, Sakurai Y, Herndon DN, et al. Regulation of the insulin-like growth factor system by insulin in burn patients. J Clin Endocrinol Metab. 1996;81:2474–2480. doi: 10.1210/jcem.81.7.8675563. [DOI] [PubMed] [Google Scholar]
- Laufer J, Katz Y, Passwell JH. Extrahepatic synthesis of complement proteins in inflammation. Mol Immunol. 2001;38:221–229. doi: 10.1016/s0161-5890(01)00044-x. [DOI] [PubMed] [Google Scholar]
- Lokitz ML, Zhang W, Bashir M, Sullivan KE, Ang G, Kwon EJ, et al. Ultraviolet-B recruits mannose-binding lectin into skin from noncutaneous sources. J Invest Dermatol. 2005;125:166–173. doi: 10.1111/j.0022-202X.2005.23794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Ezekowitz RA, Fujita T. The Gly-54– >Asp allelic form of human mannose-binding protein (MBP) fails to bind MBP-associated serine protease. Biochem J. 1995;311:1021–1023. doi: 10.1042/bj3111021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–1502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- Moller-Kristensen M, Ip WK, Shi L, Gowda LD, Hamblin MR, Thiel S, et al. Deficiency of mannose-binding lectin greatly increases susceptibility to postburn infection with Pseudomonas aeruginosa. J Immunol. 2006;176:1769–1775. doi: 10.4049/jimmunol.176.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller-Kristensen M, Wang W, Ruseva M, Thiel S, Nielsen S, Takahashi K, et al. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61:426–434. doi: 10.1111/j.1365-3083.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- Monsinjon T, Richard V, Fontaine M. Complement and its implications in cardiac ischemia/reperfusion: strategies to inhibit complement. Fundam Clin Pharmacol. 2001;15:293–306. doi: 10.1046/j.1472-8206.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- Mulligan MS, Till GO, Smith CW, Anderson DC, Miyasaka M, Tamatani T, et al. Role of leukocyte adhesion molecules in lung and dermal vascular injury after thermal trauma of skin. Am J Pathol. 1994;144:1008–1015. [PMC free article] [PubMed] [Google Scholar]
- Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, et al. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31:306–310. doi: 10.1016/j.ejvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Nadesalingam J, Dodds AW, Reid KB, Palaniyar N. Mannose-binding lectin recognizes peptidoglycan via the N-acetyl glucosamine moiety, and inhibits ligand-induced proinflammatory effect and promotes chemokine production by macrophages. J Immunol. 2005;175:1785–1794. doi: 10.4049/jimmunol.175.3.1785. [DOI] [PubMed] [Google Scholar]
- Ng KK, Drickamer K, Weis WI. Structural analysis of monosaccharide recognition by rat liver mannose-binding protein. J Biol Chem. 1996;271:663–674. doi: 10.1074/jbc.271.2.663. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Turka LA. Immunological functions of non-professional antigen-presenting cells: new insights from studies of T-cell interactions with keratinocytes. Immunol Today. 1994;15:464–469. doi: 10.1016/0167-5699(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, et al. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. A study of cytokines in burn blister fluid related to wound healing. Burns. 1995;21:352–355. doi: 10.1016/0305-4179(95)00005-4. [DOI] [PubMed] [Google Scholar]
- Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, et al. Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J Invest Dermatol. 2004;123:1012–1019. doi: 10.1111/j.0022-202X.2004.23487.x. [DOI] [PubMed] [Google Scholar]
- Pruitt BA, Jr, McManus AT, Kim SH, Goodwin CW. Burn wound infections: current status. World J Surg. 1998;22:135–145. doi: 10.1007/s002689900361. [DOI] [PubMed] [Google Scholar]
- Quinby WC, Jr, Burke JF, Bondoc CC. Primary excision and immediate wound closure. Intensive Care Med. 1981;7:71–76. doi: 10.1007/BF01687263. [DOI] [PubMed] [Google Scholar]
- Radke A, Mottaghy K, Goldmann C, Khorram-Sefat R, Kovacs B, Janssen A, et al. C1 inhibitor prevents capillary leakage after thermal trauma. Crit Care Med. 2000;28:3224–3232. doi: 10.1097/00003246-200009000-00018. [DOI] [PubMed] [Google Scholar]
- Robinson SC, Scott KA, Balkwill FR. Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-alpha. Eur J Immunol. 2002;32:404–412. doi: 10.1002/1521-4141(200202)32:2<404::AID-IMMU404>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Schmid E, Piccolo MT, Friedl HP, Warner RL, Mulligan MS, Hugli TE, et al. Requirement for C5a in lung vascular injury following thermal trauma to rat skin. Shock. 1997;8:119–124. doi: 10.1097/00024382-199708000-00010. [DOI] [PubMed] [Google Scholar]
- Shallo H, Plackett TP, Heinrich SA, Kovacs EJ. Monocyte chemoattractant protein-1 (MCP-1) and macrophage infiltration into the skin after burn injury in aged mice. Burns. 2003;29:641–647. doi: 10.1016/s0305-4179(03)00070-6. [DOI] [PubMed] [Google Scholar]
- Shi L, Takahashi K, Dundee J, Shahroor-Karni S, Thiel S, Jensenius JC, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199:1379–1390. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo C, Shaw WW, Zhang X, Longaker MT, Howard EW, Ting K. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg. 2000;105:638–647. doi: 10.1097/00006534-200002000-00024. [DOI] [PubMed] [Google Scholar]
- Sparkes BG. Immunological responses to thermal injury. Burns. 1997;23:106–113. doi: 10.1016/s0305-4179(96)00089-7. [DOI] [PubMed] [Google Scholar]
- Stricklin GP, Li L, Jancic V, Wenczak BA, Nanney LB. Localization of mRNAs representing collagenase and TIMP in sections of healing human burn wounds. Am J Pathol. 1993;143:1657–1666. [PMC free article] [PubMed] [Google Scholar]
- Stricklin GP, Li L, Nanney LB. Localization of mRNAs representing interstitial collagenase, 72-kda gelatinase, and TIMP in healing porcine burn wounds. J Invest Dermatol. 1994;103:352–358. doi: 10.1111/1523-1747.ep12394926. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- Sumiya M, Super M, Tabona P, Levinsky RJ, Arai T, Turner MW, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- Super M, Gillies SD, Foley S, Sastry K, Schweinle JE, Silverman VJ, et al. Distinct and overlapping functions of allelic forms of human mannose binding protein. Nat Genet. 1992;2:50–55. doi: 10.1038/ng0992-50. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Gordon J, Liu H, Sastry KN, Epstein JE, Motwani M, et al. Lack of mannose-binding lectin-A enhances survival in a mouse model of acute septic peritonitis. Microbes Infect. 2002;4:773–784. doi: 10.1016/s1286-4579(02)01597-6. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ip WE, Michelow IC, Ezekowitz RA. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PW. Complement-mediated killing of susceptible Gram-negative bacteria: an elusive mechanism. Exp Clin Immunogenet. 1992;9:48–56. [PubMed] [Google Scholar]
- Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- Uemura K, Saka M, Nakagawa T, Kawasaki N, Thiel S, Jensenius JC, et al. MBP is expressed in epithelial cells of mouse small intestine. J Immunol. 2002;169:6945–6950. doi: 10.4049/jimmunol.169.12.6945. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg. 2005;116:539–545. doi: 10.1097/01.prs.0000173447.81513.7a. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Noah EM, von Heimburg D, Pallua N. TIMP-1, MMP-2, MMP-9, and PIIINP as serum markers for skin fibrosis in patients following severe burn trauma. Plast Reconstr Surg. 2003;111:1423–1431. doi: 10.1097/01.PRS.0000049450.95669.07. [DOI] [PubMed] [Google Scholar]
- Vorup-Jensen T, Jensen UB, Liu H, Kawasaki T, Uemura K, Thiel S, et al. Tail-vein injection of mannan-binding lectin DNA leads to high expression levels of multimeric protein in liver. Mol Ther. 2001;3:867–874. doi: 10.1006/mthe.2001.0335. [DOI] [PubMed] [Google Scholar]
- Wallis R, Dodd RB. Interaction of mannose-binding protein with associated serine proteases: effects of naturally occurring mutations. J Biol Chem. 2000;275:30962–30969. doi: 10.1074/jbc.M004030200. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, et al. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- Ward PA. Inflammatory proteins: chemical and biological aspects. Clin Biochem. 1980;13:187–190. doi: 10.1016/s0009-9120(80)80021-x. [DOI] [PubMed] [Google Scholar]
- Ward PA, Till GO. Pathophysiologic events related to thermal injury of skin. J Trauma. 1990;30:S75–S79. doi: 10.1097/00005373-199012001-00018. [DOI] [PubMed] [Google Scholar]
- Werle M, Schmal U, Hanna K, Kreuzer J. MCP-1 induces activation of MAP-kinases ERK, JNK and p38 MAPK in human endothelial cells. Cardiovasc Res. 2002;56:284–292. doi: 10.1016/s0008-6363(02)00600-4. [DOI] [PubMed] [Google Scholar]
- Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Eckes B, Mauch C, Hartmann K, Krieg T. Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol. 2000;164:6174–6179. doi: 10.4049/jimmunol.164.12.6174. [DOI] [PubMed] [Google Scholar]
- Young PK, Grinnell F. Metalloproteinase activation cascade after burn injury: a longitudinal analysis of the human wound environment. J Invest Dermatol. 1994;103:660–664. doi: 10.1111/1523-1747.ep12398424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Local biological response in burned skin.