Abstract
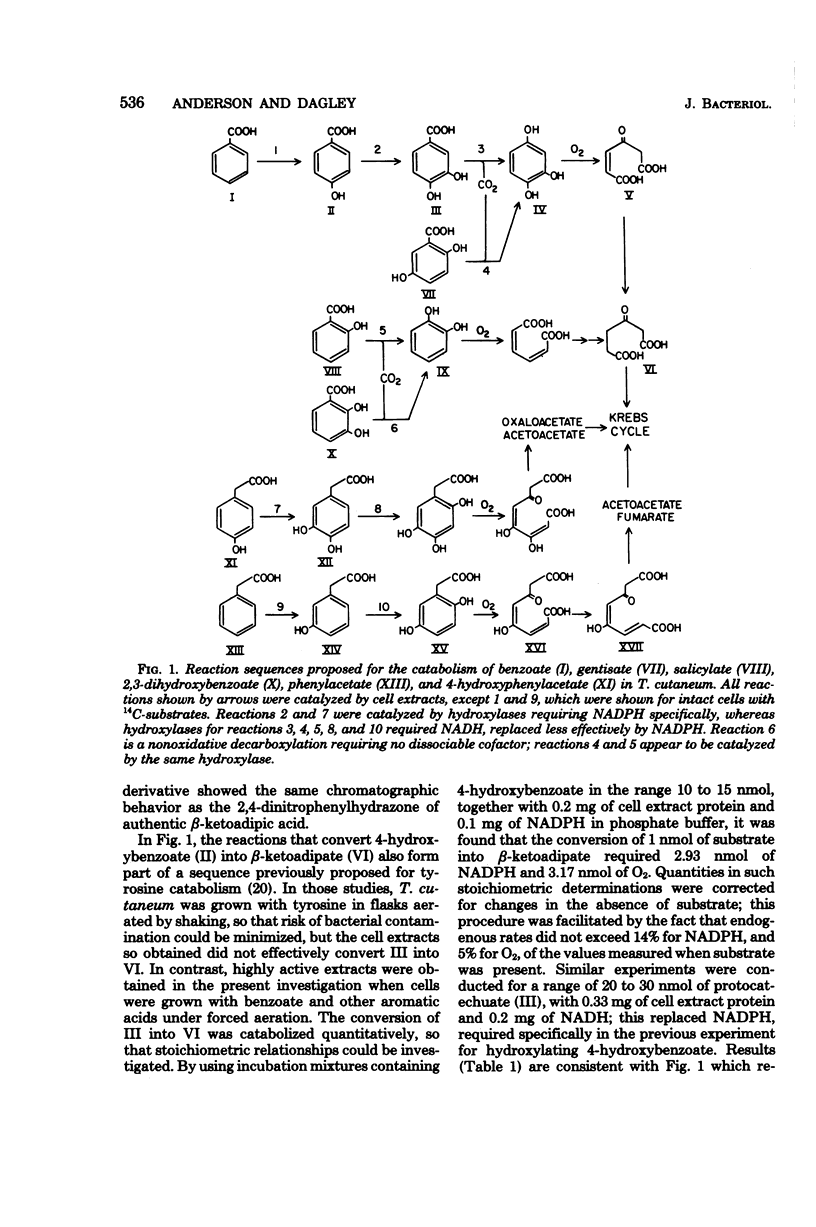
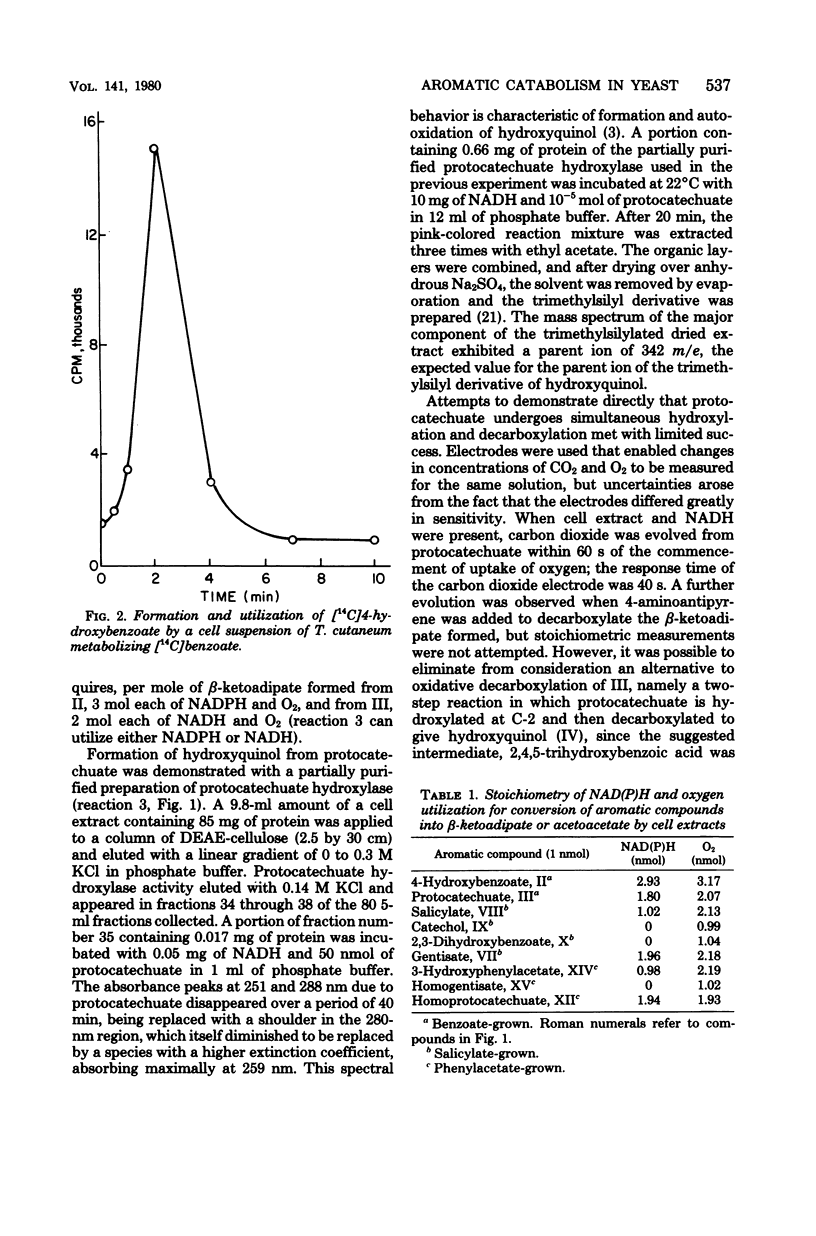
Trichosporon cutaneum readily metabolized protocatechuate, homoprotocatechuate, and gentisate, but lacked ring fission dioxygenases for these compounds. Benzoic, salicylic, 2,3-dihydroxybenzoic, and gentisic acids were converted into beta-ketoadipic acid before entry into the Krebs cycle. Benzoic acid gave rise successively to 4-hydroxybenzoic acid, protocatechuic acid, and hydroxyquinol (1,3,4-trihydroxybenzene), which underwent ring fission to maleylacetic acid. Salicylate and 2,3-dihydroxybenzoate were both initially metabolized to give catechol. 2,3-Dihydroxybenzoate was the substrate for a specific nonoxidative decarboxylase induced by salicylate, although 2,3-dihydroxybenzoate was not a catabolite of salicylate. Gentisate was metabolized to maleylacetic acid and was also readily attacked by salicylate hydroxylase at each stage of a partial purification procedure. Phenylacetic acid was degraded through 3-hydroxyphenylacetic, homogentisic, and maleylacetoacetic acids to acetoacetic and fumaric acids. All the reactions of these catabolic sequences were catalyzed by cell extracts, supplemented with reduced pyridine nucleotide coenzymes where necessary, except for the hydroxylations of benzoic and phenylacetic acids which were demonstrated with cell suspensions and isotopically labeled substrates.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPMAN P. J., DAGLEY S. Oxidation of homogentistic acid by cell-free extracts of a vibrio. J Gen Microbiol. 1962 Jun;28:251–256. doi: 10.1099/00221287-28-2-251. [DOI] [PubMed] [Google Scholar]
- Cain R. B., Bilton R. F., Darrah J. A. The metabolism of aromatic acids by micro-organisms. Metabolic pathways in the fungi. Biochem J. 1968 Aug;108(5):797–828. doi: 10.1042/bj1080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. J., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. J Bacteriol. 1976 Mar;125(3):985–998. doi: 10.1128/jb.125.3.985-998.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S. A biochemical approach to some problems of environmental pollution. Essays Biochem. 1975;11:81–138. [PubMed] [Google Scholar]
- Dagley S. Determinants of biodegradability. Q Rev Biophys. 1978 Nov;11(4):577–602. doi: 10.1017/s0033583500005679. [DOI] [PubMed] [Google Scholar]
- Dagley S. Microbial catabolism, the carbon cycle and environmental pollution. Naturwissenschaften. 1978 Feb;65(2):85–95. doi: 10.1007/BF00440546. [DOI] [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. Metabolism of phenol and resorcinol in Trichosporon cutaneum. J Bacteriol. 1979 Jan;137(1):13–21. doi: 10.1128/jb.137.1.13-21.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hareland W. A., Crawford R. L., Chapman P. J., Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975 Jan;121(1):272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. L., Dagley S. Comparison of two dioxygenases from Pseudomonas putida. J Bacteriol. 1977 Sep;131(3):1016–1017. doi: 10.1128/jb.131.3.1016-1017.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozinov A. B., Eremina S. S., Sokolov G. V. Vliianie kontsentratsii kisloroda i fiziologicheskogo sostoianiia kletok na dykhatel'nuiu tsep' drozhzhei Candida mycoderma. Mikrobiologiia. 1977 Sep-Oct;46(5):878–884. [PubMed] [Google Scholar]
- Mills S. C., Child J. J., Spencer J. F. The utilization of aromatic compounds by yeasts. Antonie Van Leeuwenhoek. 1971;37(3):281–287. doi: 10.1007/BF02218497. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Gaal A. Phenol hydroxylase from yeast. Purification and properties of the enzyme from Trichosporon cutaneum. Eur J Biochem. 1973 Jun;35(2):386–400. doi: 10.1111/j.1432-1033.1973.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Gaal A. Phenol hydroxylase from yeast. Sulfhydryl groups in phenol hydroxylase from Trichosporon cutaneum. Eur J Biochem. 1975 Oct 15;58(2):351–357. doi: 10.1111/j.1432-1033.1975.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Varga J. M. Degradation of phenols by intact cells and cell-free preparations of Trichosporon cutaneum. Eur J Biochem. 1970 Mar 1;13(1):37–44. doi: 10.1111/j.1432-1033.1970.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. O-pyrocatechiuc acid carboxy-lyase from Aspergillus niger. Arch Biochem Biophys. 1967 Nov;122(2):466–473. doi: 10.1016/0003-9861(67)90220-2. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W., Senior P. J. 2,3-dihydroxybenzoate 3,4-oxygenase from Pseudomonas fluorescens--oxidation of a substrate analog. Arch Biochem Biophys. 1970 Jun;138(2):557–565. doi: 10.1016/0003-9861(70)90381-4. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Anderson J. J., Omans J., Dagley S. Degradation of 4-hydroxyphenylacetic acid by Trichosporon cutaneum. J Bacteriol. 1978 Oct;136(1):449–451. doi: 10.1128/jb.136.1.449-451.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Burbee D. G., Dagley S. Catabolism of L-tyrosine in Trichosporon cutaneum. J Bacteriol. 1979 May;138(2):425–430. doi: 10.1128/jb.138.2.425-430.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J., Dagley S. Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J Bacteriol. 1974 Oct;120(1):159–167. doi: 10.1128/jb.120.1.159-167.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J. M., Neujahr H. Y. Purification and properties of catechol 1,2-oxygenase from Trichosporon cutaneum. Eur J Biochem. 1970 Feb;12(3):427–434. doi: 10.1111/j.1432-1033.1970.tb00869.x. [DOI] [PubMed] [Google Scholar]


