Abstract
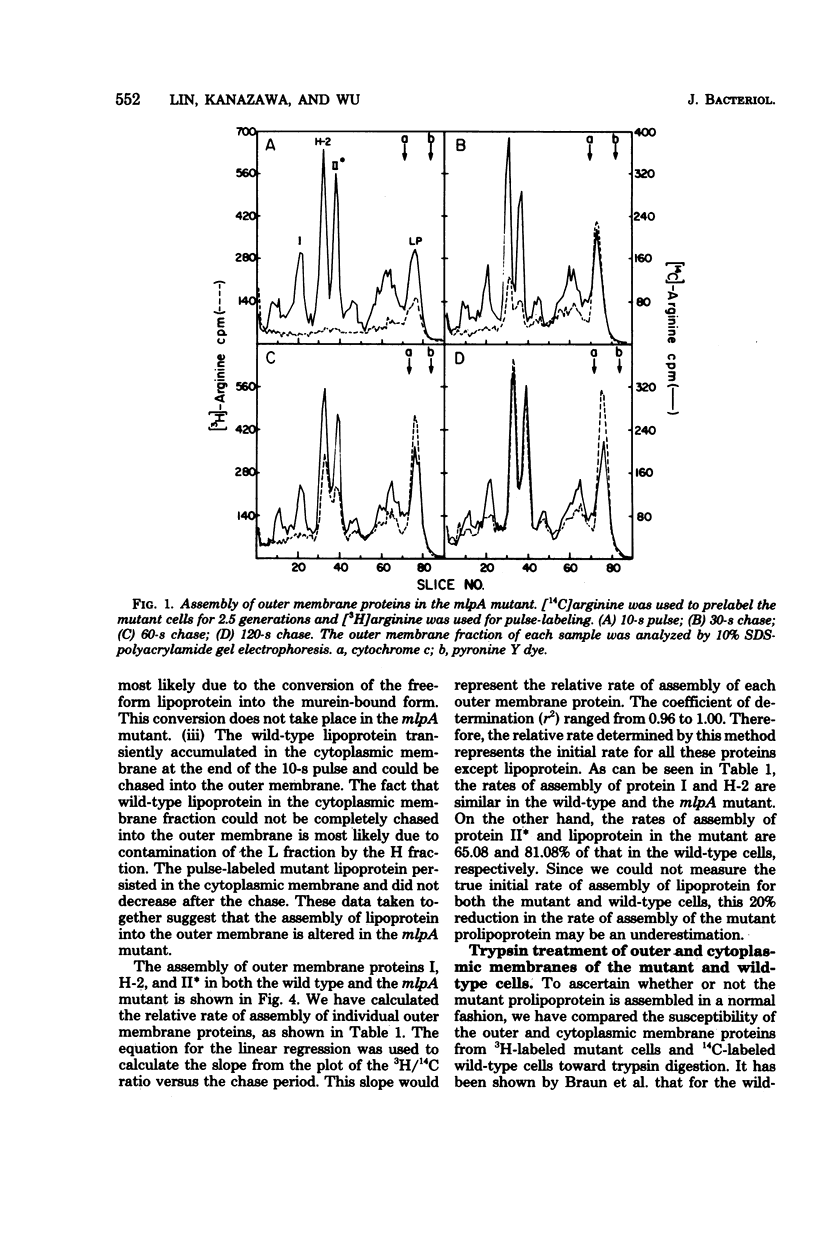
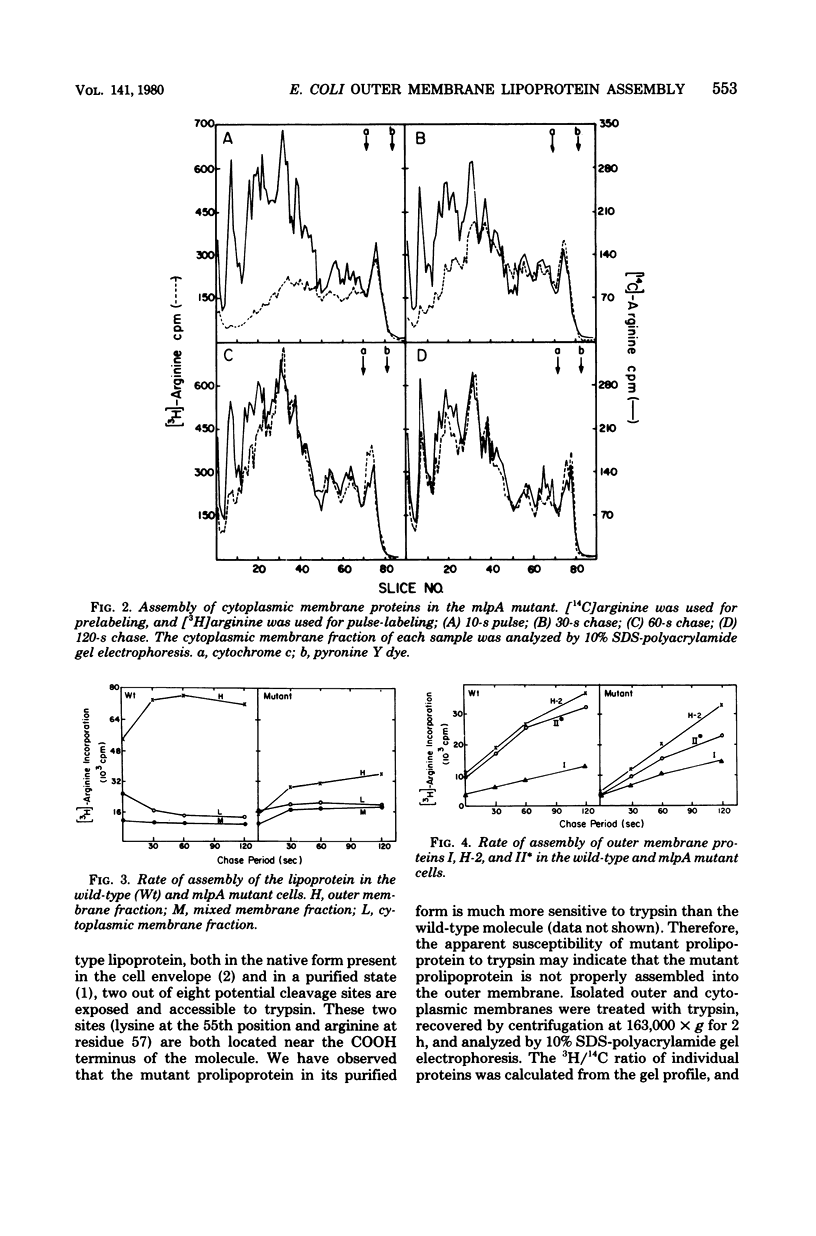
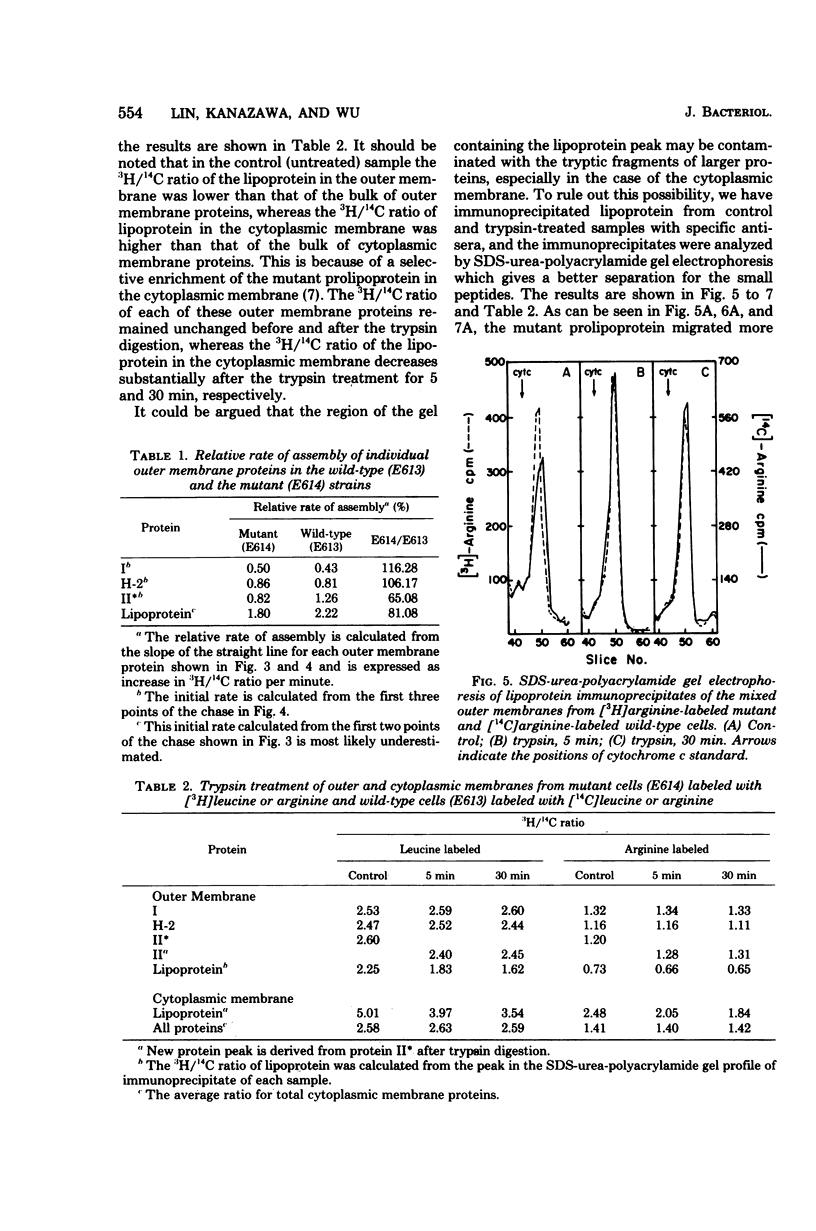
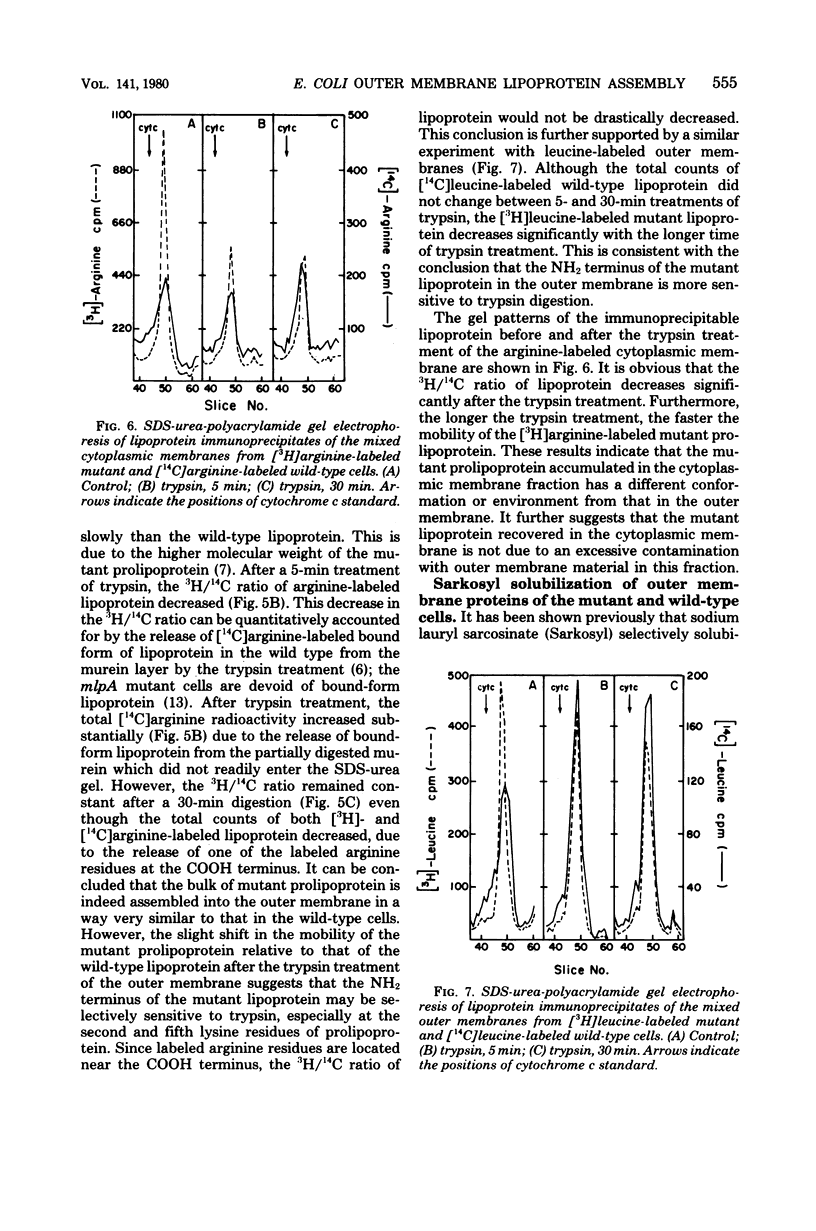
We have compared the rate of assembly of outer membrane proteins including the lipoprotein in a pair of isogenic mlpA+ (lpp+) and mlpA (lpp) strains by pulse-chase experiments. The rate of assembly of the mutant prolipoprotein into the outer membrane was slightly slower than that of the wild-type lipoprotein. The rate of assembly of protein I and protein H-2 was similar in the wild type and the mutant, whereas the rate of assembly of protein II into the outer membrane was slightly reduced in the mutant strain. The organization of outer membrane was slightly reduced in the mutant strain. The organization of outer membrane proteins in the mutant cells appeared not to be grossly altered, based on the apparent resistance (or susceptibility) of these proteins toward trypsin treatment and their resistance to solubilization by Sarkosyl. Like the wild-type lipoprotein, the mutant prolipoprotein in the outer membrane was resistant to trypsin. On the other hand, the prolipoprotein in the cytoplasmic membrane fraction of the mutant cell envelope was susceptible to trypsin digestion. We conclude from these data that proteolytic cleavage of prolipoprotein is not essential for the translocation and proper assembly of lipoprotein into outer membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Endermann R., Krämer C., Henning U. Major outer membrane proteins of Escherichia coli K-12: evidence for protein II being a transmembrane protein. FEBS Lett. 1978 Feb 1;86(1):21–24. doi: 10.1016/0014-5793(78)80089-1. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Yee M. L. Specific removal of proteins from the envelope of Escherichia coli by protease treatments. J Bacteriol. 1972 Oct;112(1):585–592. doi: 10.1128/jb.112.1.585-592.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Kanazawa H., Ozols J., Wu H. C. An Escherichia coli mutant with an amino acid alteration within the signal sequence of outer membrane prolipoprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4891–4895. doi: 10.1073/pnas.75.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Palva E. T. Protein interactions in the outer membrane of Escherichia coli. Eur J Biochem. 1979 Feb 1;93(3):495–503. doi: 10.1111/j.1432-1033.1979.tb12848.x. [DOI] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Hou C., Lin J. J., Yem D. W. Biochemical characterization of a mutant lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1388–1392. doi: 10.1073/pnas.74.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Lin J. J. Escherichia coli mutants altered in murein lipoprotein. J Bacteriol. 1976 Apr;126(1):147–156. doi: 10.1128/jb.126.1.147-156.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Genetic characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1977 Sep;131(3):759–764. doi: 10.1128/jb.131.3.759-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1978 Mar;133(3):1419–1426. doi: 10.1128/jb.133.3.1419-1426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


