Abstract
The numbers and proportion of neurons in areas and regions of cortex were determined for a single cortical hemisphere from two prosimian galagos, one New World owl monkey, one Old World macaque monkey, and one baboon. The results suggest that there is a common plan of cortical organization across the species examined here and also differences that suggest greater specializations in the Old World monkeys. In all primates examined, primary visual cortex (V1) was the most neuron-dense cortical area and the secondary visual areas had higher-than-average densities. Primary auditory and somatosensory areas tended to have high densities in the Old World macaque and baboon. Neuronal density varies less across cortical areas in prosimian galagos than in the Old World monkeys. Thus, cortical architecture varies greatly within and across primate species, but cell density is greater in cortex devoted to the early stages of sensory processing.
Keywords: cell density, cortex, isotropic fractionator, neuron number
The basic building blocks of information-processing circuits, the neurons and nonneuron cells of the cerebral cortex, have never been quantified in relation to identified cortical areas and regions across the entire cortical sheet. Several studies have reported total numbers of cells and neurons for the entire cortex (1–4). These reports are useful for examining cortical scaling principles across species, but because the cerebral cortex is such a heterogeneous structure with multiple parallel sensory and motor processing systems, whole-cortex cell number data have limited utility for understanding cortical information-processing circuits. Studies that have analyzed the cellular composition of particular cortical areas generally focused on a single cortical area or examined a limited number of areas. These studies (e.g., ref. 5) provide valuable comparative data, and a recent review thoughtfully considers differences between species (6). However, a global examination of the cellular composition of the cortical expanse with attention to cortical areas is clearly lacking in the published literature. A cellular and neuronal density map of the cortex of a number of species of primates and other mammals would contribute to interpretations of neuroimaging data in clinical and in cognitive neuroscience experimental settings. The density map also would improve our knowledge of processing circuits in the cortex and provide a foundation on which to build a cortical connection map (7) and on which to base neural network models of cortical function. The data presented here begin to fill this gap in our knowledge of cellular distribution patterns in the cortex.
Although it seems obvious that areas and regions of the cortex vary in neuronal densities according to information-processing demands, there currently is little data on cell numbers and distribution across the cortex within and across species. Small data sets examining a few cortical areas often have generated erroneous conclusions. For example, results from one study are pervasive throughout the literature and argue that the cerebral cortex is a uniform structure, with a fixed number of neurons distributed in columns of fixed size across the cortical expanse of all mammalian species (8). Rockel et al. (8) further reported that the only area in the cortex that defied neuronal uniformity was the primary visual cortex (V1) of macaque monkeys and humans. These strong conclusions were based on sampling six areas of the cortex in five mammalian species, including humans. Several later studies have disagreed with the conclusions of Rockel et al. (8), but no study to date has sampled the cortical sheet comprehensively for cell number distribution to refute their conclusions directly. Skoglund et al. (9) surveyed three cortical areas in the rat brain and found significant differences in neuron number within a fixed cortical surface area and significant differences in neuronal density across areas. Beaulieu and Colonnier (10) compared neuron number across six cortical areas in cats. Their results did not confirm those reported by Rockel et al. (8) and suggest higher numbers of neurons in sensory areas than in motor areas. Beaulieu and Colonier attribute these differences largely to the thickness of cortical layer 4 and the density of neurons in that layer. Data in support of cortical uniformity come from one study which reported a uniform density of neurons in three cortical areas in mice (11). More recently, Herculano-Houzel et al. (3) evaluated the number of neurons in the cerebral cortex in nine species of primates and in a tree shrew. This study revealed as much as threefold variation across species in the ratio of total neuron number to total cortical surface area. These findings suggest a nonuniform distribution of neurons in the cortex across species but still do not take into account areal and regional variations in neuronal density within the cortex. Our results suggest that cortical areas vary greatly in neuronal density and that homologous areas vary across species. Our results strongly support our working hypothesis that areas across the cortical sheet vary in neuron density and also vary internally according to the representational zone, i.e., the foveal representation in V1 is expected to have a higher neuron density than the representation of peripheral parts of the visual field.
Results
In all cases, neuron density and the ratio of neurons to nonneuron cells (mainly glia) varied greatly across cortical areas and regions. The highest values were obtained from V1 in each species, but other sensory areas also had high values in the Old World macaque monkey and baboon. Repeated counts from the same tissue were highly consistent, rarely varying by more than 10% (12). Values for areal and species differences are presented below.
Prosimian Galagos.
Cortex from one hemisphere from each of two galagos was divided into sectors for counts in two ways. In galago #1 (case 07–104), cortex was flattened and viewed on a light box so that the borders of densely myelinated areas V1, MT, primary auditory cortex (A1), and primary somatosensory cortex (S1; area 3b) could be identified (Fig. 1A). The flattened cortex then was cut into a grid of 46 tissue pieces, each measuring ≈5 × 5 mm (pieces along the edges of the hemisphere were smaller), and the total number of cells, the percentage of neurons, and number of neurons per square millimeter of cortical surface were determined for each piece or group of pieces. The tissue was processed as 36 samples; smaller pieces of cortex along the edges of the flat hemisphere were processed together or with larger neighboring pieces. Contour plots of total cells and neurons per millimeter are shown in Fig. 1 C and D.
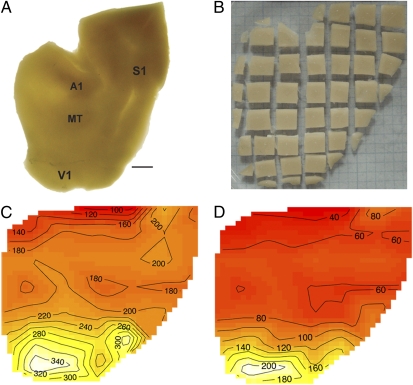
Fig. 1.
(A) Flat left cortex from a galago (Otolemur garnetti) backlit to reveal approximate borders of heavily myelinated areas such as A1 and S1. The V1/V2 border is visible. (B) The flat hemisphere shown in A cut into a grid of 46 tissue pieces. Each piece is assigned to a cortical area before processing. (C and D) Contour plots of cellular density (C) and neuronal density (D) in each tissue cube. Surface areas of each tissue piece were measured using National Institutes of Health ImageJ software, and x and y centroids were determined for each piece to produce a contour plot. The lightest areas on the plot indicate the highest cellular and neuronal densities. Primary sensory areas such as V1 and S1 stand out as the most cell-dense. The central representation in V1 (white) appears to be the most neuron-dense and the boundary between V1 and V2 can be distinguished by reduced neuron density in V2. The raw data are given in Dataset S1.
For galago #1, the total cortical surface area of the hemisphere was 2,261 mm2. The estimated total number of cells in the cortex from this hemisphere was ≈326 million, and the total number of neurons was 127 million (Dataset S1). The average percent of neurons across the cortical expanse was 39%; the range across tissue pieces was 27–60% neurons. Cortex in the caudal part of the cerebral hemisphere, corresponding largely to V1 and extrastriate areas such as the second visual area, V2, had the highest percentages of neurons, and frontal cortex had the lowest.
V1 cortex was not uniform in neuron density. The values in V1 appear to be highest in the location of the central visual representation (13) and reduced in more peripheral representations. The neuronal density contour plot (Fig. 1D) reveals the approximate boundaries of V1, V2, and other extrastriate areas and makes it possible to distinguish somatosensory cortex from surrounding areas. Regions of somatosensory and auditory cortex had somewhat elevated neuron densities.
Results for specific cortical areas were obtained from the cortex of a second galago (#2; case 08–07; Fig. S1). After the flattened cortex was viewed on a light box, V1 was easily identified, dissected from the rest of the cortex, and processed separately. By using the V1/V2 border as a landmark, the widths and locations of V2 and the third visual area, V3, were estimated from previous descriptions (13–15) and were dissected. The visual area MT and primary auditory cortex (A1) plus the rostral primary auditory area, R, were visualized directly and dissected, as was S1, corresponding to densely myelinated area 3b (16, 17). The location of the primary motor cortex, M1, was estimated from previous descriptions, and this block of tissue probably included parts of area 3a and possibly some of premotor cortex. Each identifiable cortical area was processed as a single sample. The remaining cortex was processed as a single, large sample. For this flat hemisphere, the total surface area of the cortex was 1,849 mm2, containing 243 million total cells and 107 million total neurons, with an average of 44% neurons across the cortical expanse (range, 30–69%; Fig. S1). Thus, this hemisphere was somewhat smaller than galago #1, but the numbers of cells and neurons are in proportion to those from the first galago hemisphere. Here, V1 as a whole contained 35 million neurons, 69% of the total of 51 million cells. The average V1 neuronal density was 119 million neurons/g of cortex. Thus, when V1 was processed separately, neuron densities were higher than in all other parts of cortex. Nevertheless, other visual areas had higher-than-average neuron densities. V2 contained nearly 10 million neurons, 58% of the 17 million total cells. The dorsal (V3d) and ventral (V3v) halves of V3 were processed separately but had similar values of 37% (V3d) and 41% (V3v) neurons, corresponding to ≈2 million neurons and 5.5 million total cells in each dorsal and ventral half of V3. In comparison, MT had a lower density of neurons, at 33% neurons (1.6 million neurons, 4.8 million total MT cells). Similar values were obtained for visual cortex between MT and V3, largely corresponding to the dorsolateral visual area (DL or V4) (34% neurons). A1 was more cell- and neuron-dense than surrounding cortex with 35% neurons (1.6 million neurons, 4.6 million total cells). Surprisingly, tissue blocks identified as S1 (3b) and M1 were similar in neuron percentages (41%), each containing ≈11 million cells and 4.5 million neurons. Cortex ventral to S1 and M1 had the least dense distribution of neurons (30%). Other cortex averaged 35% neurons. Overall, the cortex of nocturnal prosimian galagos, with limited architectonic differentiation of cortex, nevertheless demonstrates marked regional differences in neuron densities, with visual areas V1, V2, and V3 having the highest values.
New World Owl Monkey.
For comparison with nocturnal prosimian galagos, we processed a single flat cortical hemisphere from an owl monkey, a New World monkey that is the only known nocturnal monkey (Fig. S2). Visual and other areas and regions of cortex were processed separately, similar to prosimian galago #2 (case 08–07). The entire cortical sheet contained more than 553 million cells, of which 212 million were neurons. Neurons comprised 38% of the cells in the cortical sheet, very similar to the percentage reported for both galago hemispheres above. Again, V1 and, to a lesser extent, other visual areas had the highest neuron densities and percentages of neurons. V1 contained more than 81 million cells, of which 48 million (59%) were neurons; V2 contained about 47 million cells with 24 million neurons (∼51%). Both V3v and V3d contained 15–16 million total cells, of which 39% (V3v) and 40% (V3d) were neurons (∼6 million neurons each, for 12 million neurons total). Neuron densities in visual areas MT (62 million neurons/g) and DL (50 million neurons/g) were higher than the average values for cortex but not as high as in the auditory cortex (A1; 68 million neurons/g) and exceeded that in S1 (42 million neurons/g). As in galago #2, S1 (area 3b) was similar to M1 (including area 3a). Dorsal premotor plus prefrontal cortex had a lower-than-average percentage of neurons (31%). Overall, data from the owl monkey cortex resembled that from the galago cortex: Neuron ratios and densities were highest in V1 and, to a lesser extent, in V2, V3, and other extrastriate cortical areas. Although other regions of cortex varied, their values were closer to the average.
Old World Macaque Monkey.
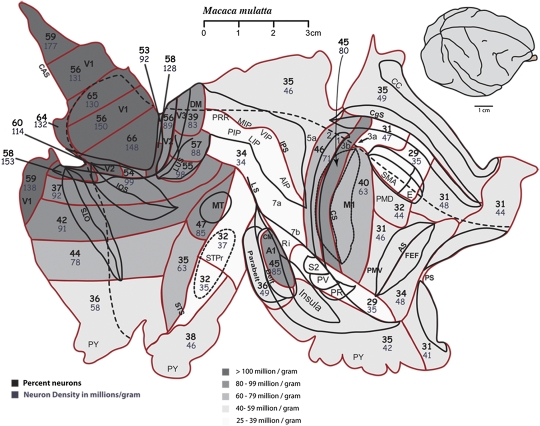
We also examined one hemisphere each from the brains of two Old World monkeys—a rhesus macaque and a baboon. The hemisphere from the macaque was not flattened, but it was dissected into a grid of tissue pieces. The pieces were larger than those dissected from galago #1 above. The results are portrayed on a surface view of flattened cortex for ease of presentation, but surface areas were not determined, and surface view extents are approximate. Nevertheless, the locations of primary sensory areas were identified for dissection, and values for these and other locations were obtained (Fig. 2). Of the nearly 3.5 billion cells in the macaque cortex, 40% (1.4 billion) were neurons, a percentage similar to that in the New World owl monkey (38%) and prosimian galagos (38%; 44%). In these Old World monkeys, neuron percentages were highest for V1, and as determined for galago #1, were highest in portions of V1 near the representation of central vision, ranging from 56–66%. Neuron density was variable across the V1 retinotopic map, ranging from 99–176 million neurons/g in various parts of the representation of the visual field (Fig. 2). Other visual areas were not considered separately, but extrastriate areas and the MT region had high neuronal densities (85 million neurons/g) relative to surrounding areas, values well above the overall average of 37 million neurons/g. Neuronal density was higher in S1, area 3b, (80 million neurons/g) than in the combined somatosensory areas 1 and 2 (71 million neurons/g) or in the M1 plus part of 3a (63 million neurons/g). In this dissection, a cut was made in the depth of the central sulcus, effectively dividing area 3a into two small pieces, one of which was processed with 3b and the other with M1. A1 plus R had a high neuronal density, 85 million neurons/g. As in the other primates, neuron densities were highest in V1 and also were high in early extrastriate visual areas. In addition, macaque cortex was characterized by high densities in the S1 and A1 areas.
Fig. 2.
Flat cortex from the right hemisphere of a macaque monkey (08-59). Red lines indicate the locations of cuts between tissue pieces that were processed for cell numbers. V1 is cut into seven pieces that were analyzed separately. The darkest shades of gray indicate the highest neuronal densities (>100 million neurons/g of tissue). V1 is the most neuron-dense (up to 177 million neurons/g), followed by extrastriate cortical areas and nonvisual primary sensory areas, S1 and A1. Motor cortex is less dense (63 million neurons/g). This case was dissected in most detail in the visual cortical areas and in much less detail in the nonvisual areas. Area 3b (primary somatosensory cortex) was processed as a single tissue sample. The raw data are given in Dataset S1.
Old World Baboon.
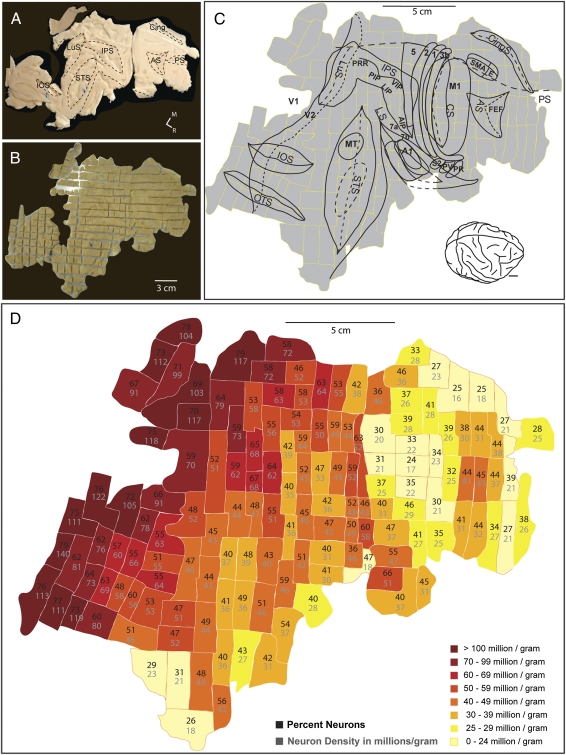
Finally, the cortex of one Old World baboon (Papio cynocephalus anubis) hemisphere was flattened (Fig. 3A) and dissected into a grid of 268 tissue pieces (5 × 5 mm; Fig. 3B), similar to the cortex from galago #1 (07-104). The cortex was processed as 142 samples, because some adjacent pieces were combined. The baboon flat hemisphere encompassed 18,577 mm2 of cortical surface area and contained a total of 4.67 billion cells, of which 2.36 billion (51%) were neurons. Thus, the cortical sheet was larger and had more neurons than that of the macaque and had a slightly higher overall percentage of neurons (51% vs. 40%). When the regional differences in percentages of neurons and neuron densities were compared with the estimated locations of sensory and motor areas (Fig. 3C), cortex in the regions of early visual areas, (V1, V2, V3) had the highest values, as in the other primates. In the S1 (area 3b) and auditory cortex, high neuronal densities were apparent, whereas much of frontal cortex had low neuron densities. Interestingly, some of prefrontal cortex, just rostral to the expected location of the frontal eye field had higher neuron densities than other regions of frontal cortex.
Fig. 3.
The flattened cortex from baboon 09–27 (A) was dissected into 268 tissue cubes (B) and processed as 142 samples (C). AS, arcuate sulcus; CingS, cingulate sulcus; CS, central sulcus; IOS, inferior occipital sulcus; IPS, intraparietal sulcus; LS, lateral sulcus; LuS, lunate sulcus; OTS, occipital temporal sulcus; STS, superior temporal sulcus. (D) The resulting cortical neuron density map made from the flat right hemisphere. The area of highest neuronal density is V1, at the caudal edge of the hemisphere. Other areas that stand out as higher density are extrastriate cortical areas. S1 is located in the caudal bank of the central sulcus, which stands out as more neuron dense here. The raw data are given in Dataset S1.
Discussion
Here we provide estimates of the total number and density of neurons for all of the cortex and for areas and regions within the cortex for four species of primates. Neuron numbers for cortical areas and subcortical structures are considered important because neurons constitute the basic processing units of neuronal networks. Thus, V1 has more neurons than the dorsal lateral geniculate nucleus, because cortex evaluates more stimulus dimensions (18, 19). Our results indicate that neuron densities vary as much as five times across cortical areas within a species. Although the results presented here are limited in the number of individuals and species examined, they have major implications.
First, a single neuronal architecture cannot be assumed in models of cortical function. Not only does the neuronal density and the number of neurons per unit of cortical surface vary for primates (3, 20), but neuron density also varies greatly across cortical areas in the same species and across homologous cortical areas in different species.
Second, sensory areas and especially visual areas in primates are favored by higher-than-average neuronal density. Previously, higher neuronal density was reported for V1 of Old World macaques and humans (8); now we know that higher neuronal densities exist in a range of primate species, including prosimian galagos and New World monkeys. Also, higher values are found in the parts of visual cortex representing central vision. In addition, higher values are found for extrastriate visual areas, auditory cortex, and somatosensory cortex. These results indicate that cortical areas involved in the first stage and sometimes higher stages of cortical processing have a distinctively different architecture that is based on small neurons with restricted receptive fields.
Third, we have the intriguing suggestion that prosimian galagos, and perhaps other prosimian primates, differ from most monkeys and possibly other anthropoid primates by showing less variability in neuron densities across cortical areas and regions. This suggestion is consistent with the less pronounced cytoarchitectonic differences across cortical areas in prosimian primates (21), which also have less neocortex in proportion to the rest of the brain compared with other primates (22). We suggest that high neuron density represents a cortical specialization that emerged more strongly in some lines of primate evolution.
Fourth, neuron densities across cortical areas in the owl monkey cortex, the only nocturnal monkey, more closely resemble those of nocturnal prosimian galagos than those of the other anthropoid primates examined here. Thus, ethological factors appear to have a large role in how cortical architecture evolves. Because the New World species of monkeys vary greatly in brain size and behavior, broad conclusions about New World monkeys are premature, but similarities in neuron distribution in large-brained, behaviorally advanced New World cebus monkeys and Old World macaque monkeys are anticipated.
Finally, neuron densities across cortical areas appear to be fairly similar across members of two radiations of Old World simians: the macaques and the baboons. Further study is clearly needed. Most importantly, comparable studies of ape and human cortices are lacking, but there are reasons to suppose even greater variability across cortical areas in these taxa and perhaps higher neuron densities in areas other than those involved in the early stages of sensory processing.
It is impossible to discuss all of our present results and conclusions in this brief report. Here, our primary goals were to demonstrate that variability across species and cortical areas can be extensive and to indicate the richness of information that can be obtained by examining cortex, piece by piece, across species. There is great value in examining further species, and more individuals of each species, with this approach. It will be especially important to identify more cortical areas for separate evaluation and to have improved criteria for outlining and dissecting cortical areas and parts of areas. Unfortunately, even for the most intensively studied mammalian species, there are considerable uncertainties about how cortex is subdivided into functionally distinct areas. However, one advantage of our approach of dividing cortex into a large number of separately analyzed tissue pieces is that any scheme of cortical organization can be related at least roughly to the cell-count results to provide additional information about possible differences in internal cellular architecture for proposed subdivisions. Comparisons of cell-count results with differing proposals of functional organization would help evaluations of such theories, because certain consistencies, including gradients of change, are expected within a field, and (except perhaps between intrinsic modules for distinctively different functions) sharp discontinuities in cell-count results would not be expected. Here, for example, we provide evidence that dorsal and ventral halves of the visual area we defined as V3 have similar neuronal densities, providing further evidence that these two regions belong to the same visual area (23).
For now, at least two topics especially require further discussion. First, given the important implications of our results, it is necessary to consider their validity. The isotropic fractionator method now has been used in many studies, and there is general agreement that the NeuN antibody reliably labels cortical neurons in adult brains (e.g, ref. 3). In our own suspensions of cellular nuclei, successive counts from the same sample varied little, typically by much less than 10% from microscope counts by a trained observer and even less when counts were done using a flow cytometer (12). Comparisons of results using present methods and those using traditional stereological methods and mounted brain sections have been limited because of the great investment involved in such comparisons, but neuron counts in human brains and parts of brains have been similar with both methods. Using stereological methods, Pakkenberg and Gundersen (4) estimated adult human cortex to contain ≈19–23 billion neurons, and a similar estimate of 16 billion neurons was reported by Herculano-Houzel (2) using the isotropic fractionator method. A stereology study recently reported the total number of neurons in the macaque monkey cortex to be 1.38 billion (1), and our results using the isotropic fractionator estimated 1.36 billion neurons in the macaque cortex.
Perhaps a more interesting issue is why visual cortex has been especially favored with high neuronal density. Part of the answer may be that vision is especially important in primates. In addition, object vision at high spatial resolution, a feature of the visual systems of diurnal primates, would seem to require not only a large primary visual area but one with high neuron density. As has been long recognized, the neurons of layer 4 of primary sensory areas, the koniocellular areas (24), are unusually small in primates, especially in V1. In addition, the apical dendritic arbors of layer 3 pyramidal cells are smaller in V1, at least in macaque monkeys, than in any other area examined (25). These earlier results are consistent with the present finding that the highest neuronal densities characterize V1 in primates, because high neuronal densities implies a decrease in average neuronal size (26). Small neurons at a high density across a large cortical area, V1, allow a visual scene to be reconstructed in great detail, limited by the number of hypercolumns (pixels) and feature-analysis channels within each hypercolumn. High densities of smaller neurons allow a larger number of hypercolumns, or their equivalents, to be crowded in the space of a cortical area. Object detail is preserved and extracted at the cost of global comparisons, a condition that is especially useful in primary and even secondary sensory areas but not in higher areas involved in multisensory integration and decision making (27).
Methods
All brain tissue for these experiments was obtained from ongoing experiments of other investigators at Vanderbilt University or was purchased, so tissue preparation methods varied across cases but not in any way that would affect our cell counts. The baboon brain was purchased from the tissue program at the University of Washington National Primate Research Center. All brains were perfused with PBS, followed by 2–4% paraformaldehyde fixative. The baboon brain was perfused with PBS only and then was fixed by immersion in 4% paraformaldehyde after flattening and dissection. Identifiable visual, somatosensory, auditory, and motor areas or regions were dissected after the flat cortex was viewed on a light box, so that myelin-dense sensory areas appear dark relative to surrounding cortex (Fig. 1A). Flat hemispheres were dissected into a grid of 5 × 5 mm pieces. Each piece was weighed, and, where possible, pieces were assigned to a cortical area. Some pieces were processed individually, and some were combined with smaller neighboring pieces. The locations of numbered tissue samples on the cortical sheet is given in Fig. S3. The isotropic fractionator method (28) was used to determine the number of neurons and nonneuron cells in each sample. Details of processing steps are detailed in previous publications (e.g., ref. 12). Percentages of neurons relative to nonneuron cells and neuron densities were calculated for all tissue samples.
Additional details are given in SI Methods.
Supplementary Material
Acknowledgments
This work was funded by a grant from the G. Harold and Leila Y. Mathers Foundation to J.H.K. and C.E.C.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010356107/-/DCSupplemental.
References
- 1.Christensen JR, et al. Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 2007;290:330–340. doi: 10.1002/ar.20504. [DOI] [PubMed] [Google Scholar]
- 2.Herculano-Houzel S. The human brain in numbers: A linearly scaled-up primate brain. Frontiers in Human Neuroscience. 2009 doi: 10.3389/neuro.09.031.2009. 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herculano-Houzel S, Collins CE, Wong P, Kaas JH, Lent R. The basic nonuniformity of the cerebral cortex. Proc Natl Acad Sci USA. 2008;105:12593–12598. doi: 10.1073/pnas.0805417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 5.Cheung AF, et al. The subventricular zone is the developmental milestone of a 6-layered neocortex: Comparisons in metatherian and eutherian mammals. Cereb Cortex. 2010;20:1071–1081. doi: 10.1093/cercor/bhp168. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AF, Pollen AA, Tavare A, DeProto J, Molnár Z. Comparative aspects of cortical neurogenesis in vertebrates. J Anat. 2007;211:164–176. doi: 10.1111/j.1469-7580.2007.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohland JW, et al. A proposal for a coordinated effort for the determination of brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale. PLOS Comput Biol. 2009;5:e1000334. doi: 10.1371/journal.pcbi.1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockel AJ, Hiorns RW, Powell TP. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- 9.Skoglund TS, Pascher R, Berthold CH. Heterogeneity in the columnar number of neurons in different neocortical areas in the rat. Neurosci Lett. 1996;208:97–100. doi: 10.1016/0304-3940(96)12569-6. [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu C, Colonnier M. Number of neurons in individual laminae of areas 3B, 4 gamma, and 6a alpha of the cat cerebral cortex: A comparison with major visual areas. J Comp Neurol. 1989;279:228–234. doi: 10.1002/cne.902790206. [DOI] [PubMed] [Google Scholar]
- 11.Schüz A, Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurol. 1989;286:442–455. doi: 10.1002/cne.902860404. [DOI] [PubMed] [Google Scholar]
- 12.Collins CE, Young NA, Flaherty DK, Airey DC, Kaas JH. A rapid and reliable method of counting neurons and other cells in brain tissue: A comparison of flow cytometry and manual counting methods. Frontiers in Neuroanatomy. 2010 doi: 10.3389/neuro.05.005.2010. 10.3389/neuro.05.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosa MG, Casagrande VA, Preuss T, Kaas JH. Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti) J Neurophysiol. 1997;77:3193–3217. doi: 10.1152/jn.1997.77.6.3193. [DOI] [PubMed] [Google Scholar]
- 14.Collins CE, Stepniewska I, Kaas JH. Topographic patterns of v2 cortical connections in a prosimian primate (Galago garnetti) J Comp Neurol. 2001;431:155–167. [PubMed] [Google Scholar]
- 15.Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav Evol. 2002;59:114–129. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- 16.Sur M, Nelson RJ, Kaas JH. Representation of the body surface in somatic koniocortex in the prosimian Galago. J Comp Neurol. 1980;189:381–402. doi: 10.1002/cne.901890211. [DOI] [PubMed] [Google Scholar]
- 17.Wu CW, Kaas JH. Somatosensory cortex of prosimian Galagos: Physiological recording, cytoarchitecture, and corticocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol. 2003;457:263–292. doi: 10.1002/cne.10542. [DOI] [PubMed] [Google Scholar]
- 18.Stevens CF. An evolutionary scaling law for the primate visual system and its basis in cortical function. Nature. 2001;411:193–195. doi: 10.1038/35075572. [DOI] [PubMed] [Google Scholar]
- 19.Stevens CF. Predicting functional properties of visual cortex from an evolutionary scaling law. Neuron. 2002;36:139–142. doi: 10.1016/s0896-6273(02)00902-9. [DOI] [PubMed] [Google Scholar]
- 20.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong P, Kaas JH. Architectonic subdivisions of neocortex in the galago (Otolemur garnetti) Anat Rec (Hoboken) 2010;293(6):1033–1069. doi: 10.1002/ar.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radinsky L. Primate brain evolution. Am Sci. 1975;63:656–663. [PubMed] [Google Scholar]
- 23.Kaas JH, Lyon DC. Visual cortex organization in primates: Theories of V3 and adjoining visual areas. Prog Brain Res. 2001;134:285–295. doi: 10.1016/s0079-6123(01)34019-0. [DOI] [PubMed] [Google Scholar]
- 24.Sanides F, Krishnamurti A. Cytoarchitectonic subdivisions of sensorimotor and prefrontal regions and of bordering insular and limbic fields in slow loris (Nycticebus coucang coucang) J Hirnforsch. 1967;9:225–252. [PubMed] [Google Scholar]
- 25.Elston GN. Cortical heterogeneity: Implications for visual processing and polysensory integration. J Neurocytol. 2002;31:317–335. doi: 10.1023/a:1024182228103. [DOI] [PubMed] [Google Scholar]
- 26.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci USA. 2006;103:12138–12143. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain and Mind. 2000;1:7–23. [Google Scholar]
- 28.Herculano-Houzel S, Lent R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.