Abstract
The A2AR is largely coexpressed with D2Rs and enkephalin mRNA in the striatum where it modulates dopaminergic activity. Activation of the A2AR antagonizes D2R-mediated behavioral and neurochemical effects in the basal ganglia through a mechanism that may involve direct A2AR–D2R interaction. However, whether the D2R is required for the A2AR to exert its neural function is an open question. In this study, we examined the role of D2Rs in A2AR-induced behavioral and cellular responses, by using genetic knockout (KO) models (mice deficient in A2ARs or D2Rs or both). Behavioral analysis shows that the A2AR agonist 2–4-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine reduced spontaneous as well as amphetamine-induced locomotion in both D2 KO and wild-type mice. Conversely, the nonselective adenosine antagonist caffeine and the A2AR antagonist 8-(3-chlorostyryl)caffeine produced motor stimulation in mice lacking the D2R, although the stimulation was significantly attentuated. At the cellular level, A2AR inactivation counteracted the increase in enkephalin expression in striatopallidal neurons caused by D2R deficiency. Consistent with the D2 KO phenotype, A2AR inactivation partially reversed both acute D2R antagonist (haloperidol)-induced catalepsy and chronic haloperidol-induced enkephalin mRNA expression. Together, these results demonstrate that A2ARs elicit behavioral and cellular responses despite either the genetic deficiency or pharmacological blockade of D2Rs. Thus, A2AR-mediated neural functions are partially independent of D2Rs. Moreover, endogenous adenosine acting at striatal A2ARs may be most accurately viewed as a facilitative modulator of striatal neuronal activity rather than simply as an inhibitory modulator of D2R neurotransmission.
A2ARs are highly concentrated in the basal ganglia where they modulate dopaminergic activity (1–3). Within the striatum, A2AR mRNA is largely coexpressed with D2R as well as enkephalin mRNA in striatopallidal neurons (4, 5) (although the expression of A2AR mRNA also has been detected in striatal cholinergic interneurons; ref. 6). For example, in situ hybridization studies reveal that 93% of D2R mRNA-bearing cells contain A2AR mRNA, and 95% of A2AR mRNA-bearing cells have D2R mRNA in striatum (4, 5). This colocalization of A2AR and D2R mRNAs suggests that the striatal efferent system is an important site for the integration of adenosine and dopamine signaling in brain. Indeed, behavioral analyses show that the nonselective adenosine antagonists caffeine and theophylline as well as the more selective A2AR antagonists SCH58261 {7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-c]-1,2,4-triazolo-[1,5,-c]-pyrimidine} and KW6002 [(E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dihydro-1H-purine-2,6-dione] potentiate dopamine-mediated psychomotor stimulant effects (2, 7, 8) whereas the A2AR agonists 2–4-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS21680) and APEC inhibit the psychomotor effects induced by dopamine agonists (9, 10). This antagonism between A2A and D2 receptors is further supported by the neurochemical demonstration that activation of the A2AR antagonizes the D2R agonist-mediated inhibition of acetylcholine release in the striatum (11, 12) and γ-aminobutyric acid (GABA) release in the striatum and globus pallidus (13), and potentiates D2R antagonist-induced expression of the immediate early gene c-fos in striatum (1, 3, 14).
The antagonistic interaction between A2A and D2 receptors has been explained by a model of receptor–receptor interaction, i.e., postsynaptic inhibition of D2Rs by A2ARs in striatum (15). This model is based not only on the colocalization of A2ARs and D2Rs in striatopallidal neurons, but also on pharmacological findings that some psychomotor effects of adenosine agonists and antagonists depend on an intact nigrostriatal dopaminergic system (1). In addition, neurochemical studies have shown that activation of A2ARs reduces the binding affinity of D2 agonists to their receptors. This A2A–D2 receptor–receptor interaction has been demonstrated in striatal membrane preparations of rats (16) as well as in fibroblast cell lines after cotransfection with A2AR and D2R cDNAs (17, 18). In agreement with an intramembrane interaction, A2A–D2 receptor interactions have been demonstrated in membrane preparations without ATP addition and in transfected cell lines without cotransfection of adenylyl cyclase (1, 15). Furthermore, A2AR-mediated direct inhibition of D2Rs also has been suggested to contribute to A2AR modulation of GABA release in the striatum and globus pallidus (6).
However, the direct receptor–receptor antagonistic model does not adequately explain recent findings that activation of the A2AR exerts a tonic excitatory effect on c-fos expression in dopamine-depleted animals and on D2R antagonist-(haloperidol)-induced phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) in striatum (19). For example, the A2AR agonist CGS21680 induced c-fos expression in the 6-hydroxydopamine (6-OHDA)-lesioned striatum, but failed (at doses up to 50-fold higher) to stimulate c-fos expression in normal striatum (20). Also, the A2AR antagonist 8-(3-chlorostyryl)caffeine (CSC) has been shown to inhibit D2R antagonist-induced c-Fos immunoreactivity in reserpinized rats (21). Furthermore, the D2R antagonist eticlopride induces DARPP-32 phosphorylation in the striatum of wild-type (WT) mice but not A2A knockout (KO) mice (22), suggesting that DARPP-32 phosphorylation requires tonic stimulation of A2ARs, independent of D2R blockade. These results may be best explained by a proposed model of opposing, independent A2A and D2 receptor modulation of cellular responses, i.e., A2AR activation by endogenous adenosine may exert an excitatory influence on striatopallidal neurons by a D2R-independent mechanism (19, 23).
Thus, whether or not striatal A2AR functions depend, in part or entirely, on D2Rs is a central but open question. This is critical to our understanding not only of the cellular mechanisms underlying adenosine–dopamine interaction, but also of the physiology of endogenous adenosine at A2ARs: Endogenous adenosine may act at A2ARs not only as an inhibitory modulator of dopaminergic neurotransmission (as proposed by A2AR–D2R direct interaction model) but also as a tonic excitatory modulator of striatopallidal neurons (opposing D2R function through its independent cellular actions). The current evidence for D2R-independent effects of striatal A2ARs is based on persistent A2AR actions in dopamine-depleted animals or the presence of D2R antagonists as described above. However, these pharmacological studies cannot exclude the possibility of partial depletion of dopamine or partial inhibition of D2Rs, and therefore residual D2R function may account for the observed persistence of A2AR actions. The recent development of KO mice deficient in D2Rs and A2ARs provides excellent models to address the D2R requirement for A2AR-mediated neural function in vivo. In the present study, we have used the approach of genetic inactivation of D2Rs and A2ARs (in D2 KO, A2A KO, and A2A–D2 double KO mice) as well as pharmacological manipulations of these receptors to clarify the role of D2Rs in the behavioral and cellular actions of endogenous adenosine acting at A2ARs in the striatum.
Materials and Methods
Breeding and Genotyping of A2A and D2 Receptor KO Mice.
Generation of A2A-D2 double KO mice.
A2A KO mice were generated by homologous recombination by using a standard replacement targeting vector as described (24). Chimeric A2A KO mice (F0) which were derived from 129-Steel embryonic stem cells (25) were bred to C57BL/6 mice (Taconic Farms), resulting in mice of hybrid C57BL/6 × 129-Steel background. The generation of D2 KO mice has been described (26). Heterozygous D2 KO mice (derived from an N6 near congenic line in C57BL/6 background) were bred to generate D2 KO and their WT littermates. To generate double homozygous mice (A2A−/−, D2−/−), we first obtained mice heterozygous for either the A2A or D2 receptor gene mutation [i.e., (A2A+/−, D2+/+) and (A2A+/+, D2+/−) mice]. These mice were then crossbred to generate double heterozygous mice. These double heterozygous mice were then crossed to produce double homozygous mutant mice (A2A−/−, D2−/−), D2R-deficient mice (A2A+/+, D2−/−), A2AR-deficient mice (A2A−/−, D2+/+), and WT (A2A+/+, D2+/+) mice, all from the same litters.
Genotyping of mutant mice.
The genotype of each mouse was determined by genomic Southern blot analysis as described (24). Briefly, mouse tail DNA was isolated and digested with BamHI (for the A2AR gene) or SacI/NotI (for the D2R gene). The genomic DNA was hybridized to radiolabeled probes (a 560-bp cDNA fragment for the A2AR gene or a 600-bp cDNA fragment for the D2R gene) as described (24, 26).
Behavioral Assessments.
Animal and drug treatments.
The animals were maintained in temperature- and humidity-controlled rooms with a 12-h light/12-h dark cycle. Before drug treatment, all mice were habituated to the testing environment and basal spontaneous locomotion was recorded for 120 min. Mice were monitored during the light phase of the light/dark cycle to obtain low baseline locomotor activity in the studies with A2AR antagonists and dopaminergic agents, or conversely, in the dark phase to obtain high baseline locomotor activity in the studies with A2AR agonists. All drugs were administered i.p. in a volume of 0.1 ml/10 g of body weight, and locomotor behavior was monitored for 120–480 min. WT, A2A KO, D2 KO, and A2A-D2 double KO mice (male and female littermates from 3–8 months old) were used for this study.
Locomotor activity.
Horizontal locomotor activity was assessed in standard polypropylene cages (15 × 25 cm) that were placed into adjustable frames equipped with seven infrared photocell beams (San Diego Instruments, San Diego). Ambulation (sequential breaks in two adjacent beams) were recorded and analyzed on a computer as described (24, 27).
Catalepsy score.
Catalepsy behavior was induced by the D2R antagonist haloperidol (1.5 mg/kg s.c.). Thirty minutes after haloperidol treatment, mice underwent a habituation session (pretest) and then 150 min later, the extent of catalepsy was evaluated by the vertical grid test. Mice were allowed to climb a vertical metal-wire grid (1.3-cm squares). Duration of immobility (descent latency) was taken as the dependent measurement, with an arbitrary maximal cut-off time set at 180 s.
Neurochemical Assessments.
Receptor autoradiography.
Receptor autoradiography of dopamine and adenosine receptors was performed as described (24, 27–30). For adenosine receptors, mouse brain sections were preincubated in Tris buffer containing 2.0 units/ml adenosine deaminase for 30 min and then incubated at room temperature for 60 min with the same buffer containing either an A1R ligand {1.0 nM [3H]cyclohexyladenosine or 1.0 nM [3H]8-cyclopentyl-1,3-dipropylxanthyne (DPCPX) in the presence of 1 μM GTP} or an A2AR ligand (2.0 nM [3H]CGS21680 or 3.0 nM [3H]SCH58261 in the presence of adenosine deaminase. Nonspecific binding of A1 and A2A receptors were determined by coincubated [3H]ligands with 25 μM 2-chloroadenosine. For dopamine receptors, striatal sections were preincubated with ice-cold 50 mM Tris⋅HCl buffer (pH 7.7) for 30 min, and then incubated at room temperature for 60 min with 0.8 or 2.0 nM [3H]2,3,4,5-Tetrahydro-3-methyl-5-phenyl-1H-3-benzapin-7-olhydrochloride (SCH23390), 1.0 nM [3H]quinpirole, or 2.0 nM [3H]raclopride. To define nonspecific binding for the D1- and D2-like receptors, 2.0 μM SCH23390 or 10 μM eticopride, respectively, was coincubated in adjacent sections.
In situ hybridization histochemistry.
In situ hybridization histochemistry with oligonucleotide and cRNA probes was performed according to protocols described (27, 28, 31, 32). Mouse brain sections were postfixed in buffered 4% paraformaldehyde, acetylated in acetic anhydride, and dehydrated in graded ethanols. The sections were then hybridized with about 0.4 nM [35S]oligonucleotide probe (about 1.5 × 106 cpm per 300 μl per slide) in hybridization buffer at 37°C overnight. The slides were washed to a final stringency of 0.5× SSC at 48°C, or the sections were hybridized with a 35S-labeled 423-bp riboprobe for preproenkephalin gene (gift from S. L. Sabol, National Institute of Mental Health, Bethesda, MD) following described protocols (32). Posthybridization treatment included three washes in 0.1× SSC at 70°C and 100 μg/ml RNase A at 37°C.
Receptor autoradiography and in situ hybridization histochemistry were quantified by using the multianalyst program (Bio-Rad) by an observer blind to treatment assignments as described (27). Receptor-binding densities were expressed as fmol/mg tissue after subtracting the nonspecific binding and calibrating with a [3H]receptor-binding standard (24, 28).
Statistical Analysis.
Single statistical comparisons between two groups were performed by using a nonpaired Student's t test. Analysis of receptor-binding densities or enkephalin mRNA levels of four different genotype groups were performed by one-way ANOVA followed by Tukey's post hoc comparisons. For behavioral analysis, we performed two-way ANOVA followed by Tukey's post hoc comparison to determine the effect of genotype, drug treatment, and their interaction.
Results
Effects of Genetic Inactivation of A2A and D2 Receptors on the Expression of Adenosine and Dopamine Receptors in Striatum.
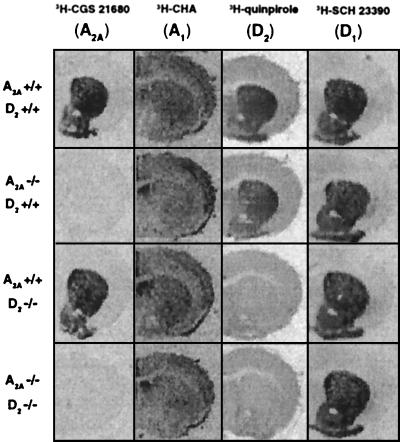
As the first step in characterizing the A2A KO, D2 KO, and A2A-D2 double KO mice, we determined the effects of genetic deletion of A2A and D2 receptors on the expression of adenosine (A1 and A2A) and dopamine (D1- and D2-like) receptors in striatum of drug-naive adult mice by receptor autoradiography. Specific agonist ligands ([3H]quinpirole for D2R, [3H]cyclohexyladenosine for A1R, and [3H]CGS21680 for A2AR; Fig. 1) as well as antagonist ligands {[3H]SCH23390 for D1R (Fig. 1), [3H]raclopride for D2R, [3H]DPCPX for A1R, and [3H]SCH58261 for A2AR; data not shown} were used to determine binding densities for dopamine and adenosine receptors in striatum. A2AR deficiency completely abolished A2AR binding but did not alter binding density for D1- or D2-like dopamine receptors in the striatum (Fig. 1) (24, 27) as well as D1- and D2-like-induced behaviors (27). Consistent with previous results (24), D2R deficiency almost completely abolished D2R binding in the striatum (Fig. 1), and D2 antagonist-induced catalepsy (data not shown). However, D2R deficiency did not alter binding densities for A1Rs or A2ARs in striatum. A2AR and D2R deficiency, nevertheless, produced small but significant opposing effects on striatal A1R binding density [< 8% reduction and increase, respectively, with [3H]cyclohexyladenosine {but not [3H]DPCPX; n = 7, P < 0.05, Student's t test}. A similarly small reduction in D1R binding densities also was observed in D2 KO and A2A-D2 double KO mice compared with their WT littermates (n = 7, P < 0.05, Student's t test), in agreement with previous findings by Kelly et al. (33). However, the functional significance of these modest changes in A1R- and D1R-binding densities in the striatum is not clear.
Figure 1.
Effects of genetic inactivation of A2A and D2 receptors on the expression of adenosine and dopamine receptors in the brain. A2A-D2 double heterozygous mice (A2A+/− D2+/−) were crossbred to generate WT, A2A KO, D2 KO, and A2A-D2 double KO mice, as described in Materials and Methods. Receptor-binding densities for adenosine and dopamine receptors were determined by receptor autoradiography by using specific ligands in coronal brain sections of drug-naive WT (A2A+/+ D2+/+), A2A KO (A2A−/− D2+/+), D2 KO (A2A+/+ D2−/−), and A2A-D2 double KO (A2A−/− D2−/−) mice. Representative autoradiograms show receptor-binding densities for D1-like ([3H]SCH23390), D2-like ([3H]quinpirole), A1 ([3H]cyclohexyladenosine), and A2A ([3H]CGS21680) receptor in mouse brains of the four different genotypes.
Effects of D2R Inactivation on A2A Agonist-Induced Motor Depression in Naive and Amphetamine-Treated Mice.
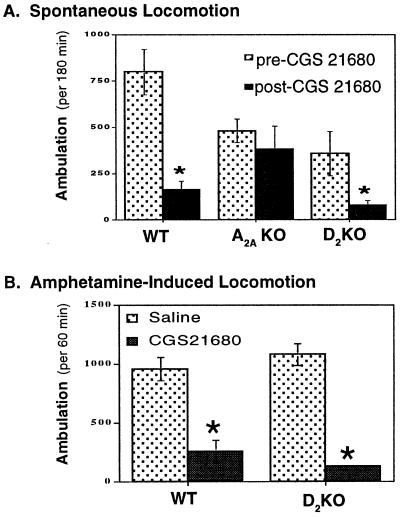
To determine whether or not D2Rs are required for A2AR-mediated motor function, we compared the motor depressant effect of the A2AR agonist CGS21680 on spontaneous as well as amphetamine-induced motor activity in mice lacking the D2R with that in WT mice. Mice were treated with CGS21680 (0.5 mg/kg i.p.) and motor activity was monitored for the 60 min before and after treatment. As expected, CGS21680 produced a significant motor depressant effect in WT mice (Fig. 2A; n = 9; P < 0.05, Tukey's test after two-way ANOVA), but not in A2A KO mice (n = 10), confirming the specificity of CGS21680 for A2ARs. However, D2 KO mice (whose basal locomotion is also lower than that of WT) still displayed significant motor depression in response to CGS21680 (n = 9; P < 0.05, Tukey's test after two-way ANOVA). Two-way ANOVA analysis grouped on genotype and treatment showed that there was genotype–treatment interaction (F(2,56) = 4.20, P = 0.021).
Figure 2.
Effects of D2R inactivation on A2AR agonist-induced motor depression in naive and amphetamine-treated mice. (A) Effects of CGS21680 on spontaneous locomotion of WT, A2A KO, and D2 KO mice. Mice were treated with CGS21680 (0.5 μg/kg i.p.) and their locomotor activities were recorded for 180 min as described in Materials and Methods. (Bars = cumulative ambulation for 180 min before or after CGS21680 treatment.) * indicates a significant reduction by CGS21680 when compared with basal ambulation for the corresponding genotypes (n = 9–10, P < 0.05, Tukey's test after two-way ANOVA). (B) Effects of CGS21680 on amphetamine-induced locomotion of WT and D2 KO mice. Mice were pretreated with CGS21680 (0.5 mg/kg i.p.) or saline 5 min before amphetamine treatment (2.5 mg/kg i.p.). * indicates a significant reduction compared with saline-pretreated group. P < 0.05, Tukey's test after two-way ANOVA.
To further examine the role of the D2R in A2AR agonist-induced motor effect, we studied the motor depressant effect of CGS21680 on amphetamine-induced locomotion in D2 KO mice. Amphetamine (2.5 mg/kg) produced marked locomotion in both WT and D2 KO mice. However, pretreatment with CGS21680 (0.5 mg/kg) almost completely abolished the amphetamine-induced motor stimulation in both WT and D2 KO mice (Fig. 2B; n = 5, P < 0.05, compared with the saline pretreatment group, Student's t test). These results clearly demonstrate that A2A agonist-induced motor-depressant effects on spontaneous as well as amphetamine-induced locomotion can occur in the absence of D2Rs.
Effects of D2R Inactivation on A2A Antagonist-Induced Motor Stimulation in Naive and Reserpinized Mice.
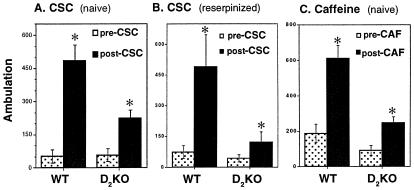
To assess the role of D2Rs in the function of A2ARs stimulated by endogenous adenosine, we also compared the motor stimulant effect of the A2A antagonist CSC and the nonselective adenosine antagonist caffeine on locomotion in D2 KO mice. At the dose of CSC used here, its motor-stimulating effect completely depended on the A2AR, because 5 mg/kg CSC was devoid of activity in A2A KO mice (data not shown). CSC significantly stimulated locomotor activity in WT as well as D2 KO mice, although the absolute level of CSC-induced activity was attenuated in D2 KO mice compared with WT mice (Fig. 3A; n = 7–8, P < 0.05, Tukey's test after two-way ANOVA). Genotype-treatment interaction was found [F(2,36) = 4.78, P = 0.016]. To assess the reliance of the motor stimulant effect of CSC on D2Rs under conditions of dopamine depletion, we treated WT and D2 KO mice with reserpine (5 mg/kg) 20 h before CSC administration. After reserpine treatment, CSC still produced significant motor stimulation in the D2 KO mice, although CSC-induced motor stimulation was again significantly attenuated in D2 KO mice compared with their WT littermates (Fig. 3B; n = 8, P < 0.05, two-way ANOVA followed by Tukey's post hoc comparison). Again, genotype-treatment interaction was found [F(1,36) = 5.98, P = 0.021].
Figure 3.
Effects of the D2R inactivation on A2A antagonist-induced motor stimulation in naive and reserpinized mice. (A) Effect of the A2A antagonist CSC (5 mg/kg i.p.) on locomotor activity was measured in naive WT and D2 KO mice. (B) Effect of CSC (5 mg/kg i.p.) was measured in WT and D2 KO mice after pretreatment with reserpine (5 mg/kg i.p.). Reserpine was administered 20 h before CSC to reduce basal locomotor activity to a similar low baseline level in both groups. (C) WT and D2 KO mice were treated with caffeine (20 mg/kg i.p.). [Bars in A (n = 6–10) and C (n = 7–8) represent the cumulative ambulation for 60 min before or after antagonist treatment; bars in B represent cumulative ambulation over 180-min periods which was used here because of the reduced motor activity in reserpinized mice (n = 8).] * indicates a significant increase after CSC/caffeine when compared with prior basal locomotion (P < 0.05, Tukey's test after two-way ANOVA).
Caffeine has been shown to produce motor stimulation in WT mice through A2AR blockade (7, 34). We also tested the effects of caffeine-induced motor stimulation in A2A KO and D2 KO mice. Caffeine (20 mg/kg) produced motor stimulation in WT but not A2A KO mice (data not shown), confirming that the motor-stimulant effect of caffeine at this dose is mediated by A2ARs. Caffeine-induced motor stimulation persisted in D2 KO mice, although the absolute level of caffeine-induced motor activity was lower in the D2 KO compared with their WT mice (Fig. 3C; n = 6–10, P < 0.05, Tukey's test after two-way ANOVA). These results demonstrate that adenosine antagonists acting specifically at the A2AR produce motor stimulation in a manner partially independent of D2R function.
A2AR Inactivation Partially Reverses D2R Inactivation-Induced Enkephalin Expression in A2A-D2 Double KO Mice.
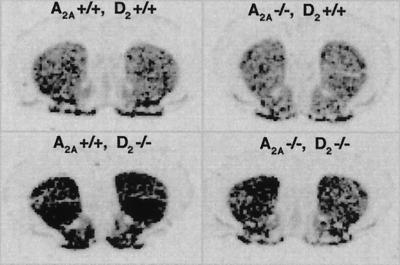
We also investigated the D2R requirement for A2AR action at the cellular level by using striatal enkephalin mRNA levels as a marker of striatopallidal neuron activity (Fig. 4). In situ hybridization histochemistry showed that whereas A2AR deficiency did not significantly reduce enkephalin mRNA expression [comparing A2A−/− D2+/+ (means ± SEM; OD = 0.526 ± 0.014) vs. A2A+/+ D2+/+ (OD = 0.481 ± 0.019; P > 0.05, n = 7], D2R inactivation markedly increased enkephalin mRNA levels in striatum by about 33% [comparing A2A+/+ D2−/− (OD = 0.640 ± 0.031) vs. A2A+/+ D2+/+; n = 7, P < 0.016, Tukey's test after one-way ANOVA]. This D2 receptor KO-induced increase in striatal enkephalin mRNA levels was largely reversed (by about 70%) by A2AR inactivation as seen in A2A-D2 double KO mice [comparing A2A−/− D2−/− (OD = 0.534 ± 0.015) vs. A2A+/+ D2−/−, n = 7, P < 0.016, Tukey's test after one-way ANOVA]. These results suggest that A2ARs and D2Rs exert opposing effects on enkephalin mRNA expression in striatum, with the stimulatory effect of A2AR most apparent when D2R-mediated inhibitory tone is removed.
Figure 4.
A2AR inactivation partially reverses D2R inactivation-induced enkephalin mRNA expression in A2A-D2 double KO mice. Striatal enkephalin mRNA levels were determined by in situ hybridization histochemistry as described in Materials and Methods and quantified in the text. Representative in situ hybridization autoradiograms illustrating enkephalin mRNA expression in coronal sections of drug-naive WT, A2A KO, D2 KO, and A2A-D2 double KO mice.
A2AR Inactivation Counteracts D2R Antagonist-Induced Catalepsy and Striatal Enkephalin mRNA Expression.
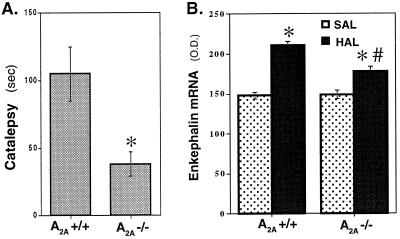
Finally, we also determined the effects of A2AR inactivation on catalepsy and enkephalin mRNA levels in the setting of pharmacological blockade (rather than genetic inactivation) of D2 receptors as induced by using the haloperidol. Catalepsy was scored by the vertical grid test in A2A KO mice and their WT littermates 3 h after haloperidol treatment (1.5 mg/kg s.c.) as described in Materials and Methods. A2A KO mice exhibited significantly less catalepsy compared with their WT littermates (Fig. 5A; n = 9–10; P < 0.01, Student's t test). Selective blockade of D2R by this dose of haloperidol was confirmed by showing that the drug treatment did not produce catalepsy in D2 KO mice (data not shown).
Figure 5.
A2AR inactivation decreases acute haloperidol-induced catalepsy and chronic haloperidol-induced enkephalin mRNA in striatum. (A) Effects of A2AR inactivation on acute haloperidol-induced catalepsy. Mice were treated with haloperidol (1.5 mg/kg s.c.) 180 min before testing. Catalepsy was scored by the vertical grid test (see Materials and Methods) in WT and A2A KO mice. A2A KO mice exhibited significantly less catalepsy compared with their WT littermates (n = 9–10; *, P < 0.01, Student's t test). (B) Effects of A2AR inactivation on the striatal enkephalin mRNA levels induced by the chronic treatment with haloperidol. WT and A2A KO mice were treated with saline or haloperidol (1 mg/kg i.p.) daily for 7 days. Mice were killed 24 h after the last treatment. Striatal enkephalin mRNA levels were determined by in situ hybridization histochemistry and quantified by densitometric analysis as described in Materials and Methods. * indicates significant difference (n = 4, P < 0.05, Student's t test) when comparing haloperidol group to saline controls of the same genotype. # indicates significant difference (n = 4, P < 0.05, Student's t test) when comparing the haloperidol-treated KO group to its WT counterpart.
Enkephalin mRNA levels also were determined in striatum of WT and A2A KO mice after pharmacological blockade of D2Rs with haloperidol (5 mg/kg s.c.) or saline for 7 days. Chronic treatment with haloperidol increased the expression of striatal enkephalin mRNA in WT mice (Fig. 5B, n = 4, P < 0.05, compared with the saline-treated WT group, Student's t test). Although A2AR deficiency again did not alter basal striatal enkephalin mRNA levels, it did partially reverse the haloperidol-induced enkephalin mRNA levels (Fig. 5B, n = 4, P < 0.05 compared with the haloperidol-treated WT group, Student's t test). These results demonstrate that the A2AR inactivation-induced reductions of both catalepsy and enkephalin mRNA levels can occur in the presence of pharmacological blockade of the D2R, consistent with the genetic demonstration that A2AR-mediated motor and cellular effects are at least partially independent of D2Rs.
Discussion
The A2A Adenosine Receptor Exerts Its Neural Effects at Least Partially Independently of D2 Receptors.
The basis for the antagonistic relationship between A2A and D2 receptor function in striatum has not been established. A widely adopted model proposes a direct A2A-D2 receptor–receptor interaction in striatopallidal neurons, and holds that A2AR-mediated effects are based on the inhibition of D2R function (1, 3, 15). This model has been widely used to explain how A2ARs modulate locomotor activity, GABA release, and c-fos expression in the basal ganglia (3). Fuxe et al. (15) have further proposed A2A-D2 receptor heterodimers as a potential mechanism for direct functional intramembrane interactions, a concept that received experimental support from the recent demonstration of heterodimeric interaction between D2 and somatostatin (SST5) receptors in striatal neurons (35).
Implicit in this model that A2ARs exert their effects by modulating D2R activity is the dependence of A2AR actions on the presence and integrity of the D2R. However, the data presented here clearly demonstrate that the A2AR can modulate motor function and striatal cellular activity in a manner that is partly independent of the D2R. The demonstration of A2AR-induced locomotor behavioral and enkephalin mRNA responses in mice lacking D2Rs argues strongly for D2R-independent mechanisms contributing to A2AR actions in the brain. This result is consistent with a very recent finding that the A2A antagonist KW6002 reverses locomotor impairment in D2R KO mice (36). Furthermore, genetic inactivation of the A2AR did not alter enkephalin mRNA expression, but partially reversed the D2 KO-induced enkephalin mRNA in A2A-D2 double KO mice, suggesting that an A2AR-mediated facilitation of enkephalin mRNA expression is best manifested when D2R-mediated inhibitory tone is removed. The demonstration of A2AR modulation of motor activity and enkephalin expression occurring despite pharmacological blockade of D2Rs (as well as genetic inactivation of D2Rs) suggests that D2R-independent effects of A2ARs do not result from developmental adaptations to D2R inactivation.
However, in each of the varied behavioral and cellular experiments performed here, A2AR functions were not completely independent of the D2R. For example, whereas the A2AR agonist CGS21680-induced motor depression in D2 KO mice was comparable to that of their WT littermates, the A2AR antagonists CSC- and caffeine-induced motor stimulation were greatly attenuated in D2 KO mice (Fig. 3; see also ref. 37), indicating the partial D2R dependency of endogenous adenosine acting on the A2AR. Alternatively, attenuation of A2AR antagonist-induced locomotor activity in D2 KO mice may result from adaptive change(s) leading to functional uncoupling of A2ARs in D2 KO mice, as suggested by Zahniser et al. (37). Interestingly, in their study, CGS21680 failed to increase either GABA release or cAMP accumulation in striatopallidal slices from D2 KO mice, despite normal expression of A2AR and its coupled signaling molecules (Gs, Golf, and adenylyl cyclase type 6) (37). In contrast to the behavioral data, their neurochemical results suggest a functional uncoupling of A2ARs in D2 KO mice and indicate a critical role for D2Rs in mediating A2AR-induced GABA release in the striatum. The difference in CGS21680-induced GABA release and motor depression observed by their group and ours, respectively, may be caused by the different preparations (slices versus intact animals) and the different readouts (GABA release versus locomotor behavior). Because A2AR-mediated motor effects may involve multiple neurotransmitter systems (e.g., dopamine, GABA, and acetylcholine), it is possible that the D2R may be critical in A2AR modulation of GABA release but not as essential in A2AR modulation of motor activity or striatal enkephalin mRNA expression. However, electrophysiological and pharmacological studies support GABAergic involvement in A2AR-mediated motor regulation (6, 38). Further studies are needed to determine the exact role of GABA neurotransmission in the A2AR modulation of motor behavior.
The demonstration of D2R-independent effects of A2ARs indicates that neural pathways not associated with striatal D2Rs may contribute to A2AR-mediated behavioral and cellular responses in vivo. In this regard, the A2AR has been shown to interact with D1Rs at a network level (3, 39). Although A2ARs and D1Rs localize to different striatal projection neurons, A2ARs have been shown to indirectly interact with D1Rs to modulate D1R-mediated locomotor activity and c-fos expression (39, 40). This network level interaction has recently been found to involve a synergistic contribution from the A2AR and D1R in their regulation of DARPP-32 phosphorylation and cAMP accumulation in a striatal slice preparation (23). Thus, multiple mechanisms are likely involved in A2A–dopamine receptor interactions.
Endogenous Adenosine Acting at A2ARs Exerts a Tonic Facilitative Influence on the Expression of Enkephalin mRNA in Striatum Independent of D2Rs.
The fundamental aspect of the direct receptor–receptor model is that activation of the A2AR exerts its inhibitory influence on D2Rs, which, in turn, have inhibitory effects on striatopallidal neurons (1, 15, 19). Thus, the A2AR may exert its neuronal function by releasing the inhibitory D2R influence on these neurons, i.e., by disinhibiting them. An alternative model focuses on cellular actions of A2ARs and proposes that A2AR activation exerts an excitatory influence on striatopallidal neurons, independent of D2Rs (19, 41). Consistent with the second model, we noted that D2 KO-induced enkephalin mRNA expression in striatum was reduced in A2A-D2 double KO mice. These results would agree with pharmacological studies showing that A2AR inactivation (Fig. 5) or repeated treatment with the A2AR antagonist KF17387 (6) partially reverses the elevation of enkephalin mRNA expression induced by the repeated treatment with the D2R antagonist eticlopride. The notion of A2AR-mediated facilitation on the striatal cell is supported by a recent study showing that the D2R antagonist haloperidol induces phosphorylation of DARPP-32 in the striatum of WT but not A2A KO mice (22). This result indicates a critical, independent role of A2AR facilitation on DARPP-32 phosphorylation in the striatum. Together, these results strongly support the view that endogenous adenosine acting at the A2ARs exerts a facilitative influence on striatal cellular activity, manifesting best when D2R-mediated inhibitory tone is removed.
The facilitative influence of A2AR on striatal cellular activity may in part be explained by the fact that A2ARs positively couple with Gs protein to stimulate adenylyl cyclase and increase production of cAMP and consequent DARPP-32 phosphorylation (19). Several cAMP responsive elements in the promotor regions of the enkephalin gene (42), and their role in regulating enkephalin gene expression by the cAMP pathway have been demonstrated (42, 43). Thus, the regulation of enkephalin mRNA may result from A2AR inactivation and associated decreased activity of the cAMP-signaling pathway. The demonstration of reversal of D2 KO-induced enkephalin mRNA by A2AR inactivation has implications for the development of A2AR antagonists as a potential therapeutic intervention for Parkinson's disease (PD). It is interesting to note that neither genetic nor pharmacological inactivation of A2ARs alters enkephalin mRNA in naive mice, but both reverse the increase in enkephalin mRNA induced by chronic blockade of D2Rs. The fact that an A2AR-mediated facilitative effect on enkephalin mRNA expression is best observed when the strong inhibitory tone of the D2R is removed (such as in the D2 KO mice) suggests that A2AR antagonists may more efficiently improve PD symptoms when the dopaminergic degeneration is more advanced. This notion is consistent with the previous demonstration that the A2A-D2 receptor interaction is enhanced in the dopamine-depleted animals (1, 3, 19). Furthermore, l-dopa, the mainstay treatment of PD, has been shown to reverse the decrease in substance P but fails to counteract the increase in enkephalin mRNA noted in animal models of PD (44, 45). It has been suggested that one of the reasons that l-dopa fails to fully alleviate the symptoms of PD may be related to its inability to reverse the induction of enkephalin mRNA (44, 45). Thus, the ability of A2AR antagonists to reduce the levels of enkephalin mRNA induced by D2R blockade may prove advantageous in PD treatment.
In summary, we have complemented standard pharmacological methods with a set of genetic KO models to demonstrate that the A2AR exerts its neuronal activity in the striatum in a manner partially independent of D2Rs. The D2R-independent component of A2AR function is demonstrable at the behavioral (motor activity) as well as cellular (enkephalin mRNA expression) levels. Furthermore, A2A and D2 receptors produce opposite effects on enkephalin mRNA expression, with A2AR-mediated stimulation of enkephalin mRNA manifesting best when D2R-mediated inhibition is removed. These results argue strongly for D2R-dependent as well as D2R-independent mechanisms of A2AR neural functions in vivo. Furthermore, they suggest that endogenous adenosine acting at striatal A2ARs may be most accurately viewed as a facilitative modulator of striatal neuronal activity rather than simply as an inhibitory modulator of D2R neurotransmission.
Acknowledgments
We thank Yue-Hang Xu for expert technical assistance. This work was supported by National Institutes of Health Grants DA07496 (M.A.S.), NS373403 (J.F.C.), and DA12062 (D.K.G. and M.J.L.); and grants from the Parkinson's Disease Foundation (J.F.C.); a Cotzias Fellowship from the American Parkinson's Disease Association (M.A.S.); and CAM no. 8.5/43.1/99 and FIS 98/1368 from the Spanish government (R.M.).
Abbreviations
- A2AR
A2A adenosine receptor
- CGS21680
2–4-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine
- CSC
8-(3-chlorostyryl)caffeine
- D2R
D2 dopamine receptor
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of 32 kDa
- DPCPX
8-cyclopentyl-1,3-dipropylxanthyne
- KO
knockout
- PD
Parkinson's disease
- WT
wild type
- GABA
γ-aminobutyric acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ferre S, Fuxe K, VonEuler G, Johansson G, Fredholm B. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- 2.Ongini E, Fredholm B B. Trends Pharmacol Sci. 1996;17:364–372. [PubMed] [Google Scholar]
- 3.Ferre S, Fredholm B B, Morelli M, Popoli P, Fuxe K. Trends Neurosci. 1997;10:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 4.Fink J S, Weaver D R, Rivkees S A, Peterfreund R A, Pollack A E, Adler E A, Reppert S M. Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 5.Schiffmann S N, Vanderhaeghen J-J. J Neurosci. 1993;13:1080–1087. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson P J, Kase H, Jenner P G. Trends Pharmacol Sci. 1997;18:338–344. doi: 10.1016/s0165-6147(97)01096-1. .; 19, 46–48. [DOI] [PubMed] [Google Scholar]
- 7.Fredholm B B, Bättig K, Holmén J, Nehlig A, Zvartau E E. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 8.Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, Kuwana Y. Psychopharmacology. 1999;147:90–95. doi: 10.1007/s002130051146. [DOI] [PubMed] [Google Scholar]
- 9.Green R D, Proudfit H K, Yeung S-M H. Science. 1982;281:58–61. doi: 10.1126/science.7123218. [DOI] [PubMed] [Google Scholar]
- 10.Heffner T G, Wiley J N, Williams A E, Bruns R F, Coughenour L L, Downs D A. Psychopharmacology. 1989;98:31–37. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- 11.Jin S, Johansson B, Fredholm B B. J Pharmacol Exp Ther. 1993;267:801–808. [PubMed] [Google Scholar]
- 12.Kurokawa M, Koga K, Kase H, Nakamura J, Kuwana Y. J Neurochem. 1996;66:1882–1888. doi: 10.1046/j.1471-4159.1996.66051882.x. [DOI] [PubMed] [Google Scholar]
- 13.Mayfield R D, Larson G, Orona R A, Zahniser N R. Synapse. 1996;22:132–138. doi: 10.1002/(SICI)1098-2396(199602)22:2<132::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Pollack A E, Fink J S. Neuroscience. 1995;68:721–728. doi: 10.1016/0306-4522(95)00168-i. [DOI] [PubMed] [Google Scholar]
- 15.Fuxe K, Ferre S, Zoli M, Agnati L F. Brain Res Rev. 1998;26:258–273. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 16.Ferre S, von Euler G, Johansson B, Fredholm B B, Fuxe K. Proc Natl Acad Sci USA. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S N, Dasgupta S, Lledo P M, Vincent J D, Fuxe K. Neuroscience. 1995;68:729–736. doi: 10.1016/0306-4522(95)00171-e. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta S, Ferre S, Kull B, Hedlund P B, Finnman U B, Ahlberg S, Arenas E, Fredholm B B, Fuxe K. Eur J Pharmacol. 1996;316:325–331. doi: 10.1016/s0014-2999(96)00665-6. [DOI] [PubMed] [Google Scholar]
- 19.Svenningsson P, Le Moine C, Fisone G, Fredholm B B. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 20.Morelli M, Pinna A, Wardas J, Di Chiara G. Neuroscience. 1995;67:49–55. doi: 10.1016/0306-4522(94)00602-2. [DOI] [PubMed] [Google Scholar]
- 21.Boegman R J, Vincent S R. Synapse. 1996;22:70–77. doi: 10.1002/(SICI)1098-2396(199601)22:1<70::AID-SYN8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm B B, Fisone G. Proc Natl Acad Sci USA. 2000;97:1856–1860. doi: 10.1073/pnas.97.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svenningsson P, Lindskog M, Rognoni F, Fredholm B B, Greengard P, Fisone G. Neuroscience. 1998;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- 24.Chen J-F, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz M A, Fink J S, Schwarzschild M A. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 26.Kelly M A, Rubinstein M, Asa S L, Zhang G, Saez C, Bunzow J R, Allen R G, Hnasko R, Ben-Jonathan N, Grandy D K, et al. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 27.Chen J-F, Beilstein M, Xu Y-H, Turner T, Moratalla R, Standaert D G, Aloyo V J, Fink J S, Schwarzschild M A. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- 28.Chen J-F, Aloyo V J, Weiss B. Neuroscience. 1993;54:669–680. doi: 10.1016/0306-4522(93)90238-b. [DOI] [PubMed] [Google Scholar]
- 29.Swanson T H, Drazba J A, Rivkees S A. J Comp Neurol. 1999;363:517–531. doi: 10.1002/cne.903630402. [DOI] [PubMed] [Google Scholar]
- 30.Fredholm B B, Lindstrom K, Dionisotti S, Ongini E. J Neurochem. 1998;70:1210–6.31. doi: 10.1046/j.1471-4159.1998.70031210.x. [DOI] [PubMed] [Google Scholar]
- 31.Moratalla R, Xu M, Tonegawa S, Graybiel A M. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chesselet M F. In: In Situ Hybridization Techniques for the Brain. Handbook Series: Methods In the Neurosciences. Henderson B Z, editor. IBRO. West Sussex, U.K.: Wiley; 1996. pp. 141–149. [Google Scholar]
- 33.Kelly M A, Rubinstein M, Phillips T J, Lessov C N, Burkhart-Kasch S, Zhang G, Bunzow J R, Fang Y, Gerhardt G A, Grandy D K, et al. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yacoubi M E, Ledent C, Menard J-F, Parmentier M, Costentin J, Vaugeois J-M. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocheville M, Lange D C, Kumar U, Patel S C, Patel R C, Patel Y C. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 36.Aoyama S, Kase H, Borrelli E. J Neurosci. 2000;20:5848–5852. doi: 10.1523/JNEUROSCI.20-15-05848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahniser N R, Simonsky J K, Mayfield R D, Negri C A, Hanania T, Larson G A, Kelly M A, Grandy D K, Rubinstein M M L, Low M J, et al. J Neurosci. 2000;20:5949–5957. doi: 10.1523/JNEUROSCI.20-16-05949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khisti R T, Chopde C T, Abraham E. Neuropharmacology. 2000;39:1004–1015. doi: 10.1016/s0028-3908(99)00187-2. [DOI] [PubMed] [Google Scholar]
- 39.Pollack A E, Fink J S. Brain Res. 1996;743:124–130. doi: 10.1016/s0006-8993(96)01036-0. [DOI] [PubMed] [Google Scholar]
- 40.Pinna A, Di Chiara G, Wardas J, Morelli M. Eur J Neurosci. 1996;8:1176–1181. doi: 10.1111/j.1460-9568.1996.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 41.Sebastiao A M, Ribeiro J A. Prog Neurobiol. 1996;48:167–189. doi: 10.1016/0301-0082(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 42.Comb M, Mermod N, Hyman S E, Pearlberg J, Ross M E, Goodman H M. EMBO J. 1988;7:3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyman S E, Comb M, Lin Y S, Pearlberg J, Green M R, Goodman H M. Mol Cell Biol. 1988;8:4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engber T M, Susel Z, Kuo A, Gerfen C R, Chase T N. Brain Res. 1991;552:113–118. doi: 10.1016/0006-8993(91)90667-k. [DOI] [PubMed] [Google Scholar]
- 45.Jolkkonen J, Jenner P, Marsden C D. Mol Brain Res. 1995;32:297–307. doi: 10.1016/0169-328x(95)00084-6. [DOI] [PubMed] [Google Scholar]