Abstract
During inner ear morphogenesis, the process of prosensory specification defines the specific regions of the otic epithelium that will give rise to the six separate inner ear organs essential for hearing and balance. The mechanism of prosensory specification is not fully understood, but there is evidence that the Notch intercellular signaling pathway plays a critical role. The Notch ligand Jagged1 (Jag1) is expressed in the prosensory domains, and mutation of Jag1 impairs sensory formation. Furthermore, pharmacological inhibition of Notch in vitro during prosensory specification disrupts the prosensory process. Additionally, activation of Notch by cDNA electroporation in chick otocysts results in formation of ectopic sensory patches. Here we test whether Notch activity is sufficient for prosensory specification in the mouse, using a Cre-/loxP approach to conditionally activate the Notch pathway in nonsensory regions of the inner ear epithelia during different stages of otic vesicle morphogenesis. We find that broad ectopic activation of Notch at very early developmental stages causes induction of prosensory markers throughout the entire otic epithelium. At later stages of development, activation of Notch in nonsensory regions leads to induction of sensory patches that later differentiate to form complete ectopic sensory structures. Activation of Notch in isolated nonsensory cells results in lateral induction of Jag1 expression in neighboring cells and spreading of prosensory specification to the adjacent cells through an intercellular mechanism. These results support a model where activation of Notch and propagation through lateral induction promote prosensory character in specific regions of the developing otocyst.
Keywords: Jagged1, lateral inhibition, otic development, sensory epithelium, sensory induction
The six sensory organs of the mammalian inner ear are derived from the otic vesicle, an invagination of the otic placode. Through a complex series of morphogenetic events, the otic vesicle differentiates into the membranous labyrinth of the inner ear containing the cochlea and five vestibular sensory epithelia: the two macular organs (the utricle and saccule) and the three cristae of the semicircular canals. Although the membranous labyrinth forms a continuous epithelial structure, the six sensory organs within are individually distinct neuroepithelial sensory structures. These inner ear sensory organs are composed of highly ordered mosaics of mechanosensory hair cells, glial-like supporting cells, and peripheral nerve endings. The developmental process of delineation of sensory regions from nonsensory epithelia is termed “prosensory specification.”
The mechanism of prosensory specification is not yet fully understood, but several lines of evidence demonstrate that the Notch pathway plays a critical role: (i) Notch1 is expressed broadly in the developing inner ear and Jag1 expression marks the prosensory domains long before hair cells and supporting cells differentiate (1–3). Notch signaling is active within the prosensory domains, as indicated by detection of the activated Notch intracellular domain (NotchIC) (4) and the downstream Notch effectors Hey1 and Hey2 (5). (ii) Mice deficient in Jag1 have severe defects in sensory epithelia formation (6–9). (iii) Pharmacological or dominant-negative inhibition of Notch in mouse cochlear explants or chicken embryos impairs sensory formation, as indicated by reduced expression of sensory domain markers and defects in hair cell and supporting cell formation (5, 10, 11). These studies strongly suggest that Jag1-Notch signaling is required for prosensory specification in the inner ear. Moreover, there is evidence in the chicken embryo that Notch activation is sufficient for prosensory specification; overexpression of NotchIC in the chick otocyst results in the formation of ectopic sensory structures containing hair cells and supporting cells (12).
In classic Notch “lateral inhibition,” where cells committed to a given fate inhibit their neighbors from adopting the same fate, Notch activation negatively regulates Notch ligand expression so that a cell that produces high levels of ligand instructs its neighbors to produce less ligand, resulting in a salt-and-pepper pattern of ligand expression and eventual neuronal differentiation. This lateral inhibitory type of Notch signaling is used in the later stage of inner ear development when hair cells and supporting cells differentiate (13).
In contrast, there is some evidence that Notch positively regulates expression of Jag1 via “lateral induction” in the early prosensory patches of the ear, and this strengthens and maintains Notch activation and the prosensory state (10–12, 14). Jag1 expression in the ear does not occur in the salt-and-pepper pattern consistent with lateral inhibition but rather is uniform in cells within the sensory patches (2, 3), and deletion of Jag1 impairs sensory formation rather than lateral inhibition (6–9). Additionally, Jag1 expression is severely reduced in chick otic epithelium when Notch is inhibited, suggesting Jag1 is positively regulated by Notch (11). Although these data support the model of Notch-dependent lateral induction put forth by Lewis and co-workers (10, 12, 14), no studies have yet tested this hypothesis with gain of function in the mouse or directly tested whether this lateral induction process actually acts laterally and is propagated from cell to cell.
In the present study, we test whether Notch activity is sufficient for prosensory specification in the mouse, using a Cre-/loxP approach to conditionally activate the Notch pathway in the nonsensory regions of the otic epithelia. We find that broad ectopic expression of NotchIC at very early stages causes induction of prosensory markers throughout the entire otic epithelium. At intermediate stages of otic morphogenesis, activation of Notch in nonsensory regions leads to the induction of ectopic sensory patches containing hair cells and supporting cells. Additionally, we find that activation of Notch in isolated nonsensory cells results in lateral induction of Jag1 in neighboring cells and spreading of prosensory character up to several cell diameters from the source. These results demonstrate that activation of Notch causes lateral induction of Jag1 and is sufficient to induce sensory structures from the nonsensory epithelium of the inner ear during early and intermediate stages of otic morphogenesis.
Results
Ectopic Notch Activation in FoxG1Cre;RosaNotch Embryos Results in Expansion of the Prosensory Domain Throughout the Otic Epithelium.
To determine whether the entire otic vesicle was competent to generate sensory epithelium, we used a transgenic approach to broadly and constitutively activate Notch in the otic vesicles of mouse embryos. The RosaNotch transgenic mouse was previously engineered with an intracellular fragment of mouse Notch1 (NotchIC), an internal ribosome entry sequence (IRES), and nuclear-localized enhanced green fluorescent protein (GFP) in the Rosa26 locus, preceded by a floxed transcriptional STOP cassette (15). In the presence of Cre-recombinase the STOP is deleted, yielding heritable, constitutive coexpression of NotchIC and nuclear GFP. We crossed the RosaNotch transgenic mice with a FoxG1Cre transgenic line, which expresses Cre-recombinase in the early otic vesicle [around embryonic day (E) 8.75], the developing cortex, and several other regions of the embryo (8, 9, 16, 17). The FoxG1Cre transgenic line was produced by knock-in to the FoxG1 locus, so mice carrying this transgene are heterozygous for FoxG1 and manipulations using this line are in the context of reduced FoxG1 (16). This gene is important for inner ear development, but heterozygous animals do not display an abnormal phenotype (18, 19). For all of our experiments involving FoxG1Cre, we used FoxG1Cre/+ as well as wild-type littermate controls and did not observe any differences between control genotypes.
The FoxG1Cre;RosaNotch double transgenic embryos that we generated did not survive past E13.5. Therefore, we carried out analysis of these embryos at early and intermediate stages of otic development, E9.5 and E12.5. FoxG1Cre;RosaNotch embryos displayed a striking phenotype, including neural tube closure defects resulting in externalization of anterior neural tissues (Fig. S1). We confirmed that Notch was activated ectopically in the externalized cortex at E12.5 using in situ hybridization for the downstream Notch effector Hes5 (Fig. S1).
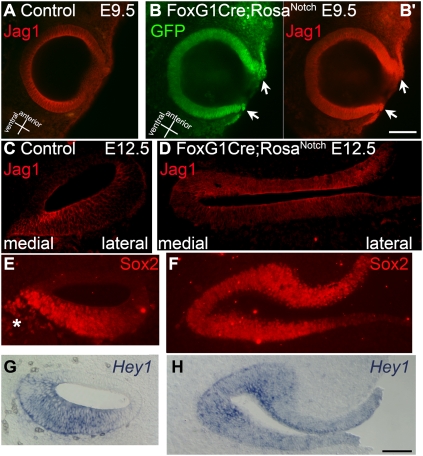
The inner ear development of the FoxG1Cre;RosaNotch embryos was clearly abnormal. The otocyst failed to close properly and the epithelium was externalized and thickened (Fig. 1 and Fig. S1). To determine whether the sensory regions were expanded, we used several markers for the sensory epithelia. At early and middle stages of otic morphogenesis, the FoxG1Cre;RosaNotch embryos displayed ectopic Jag1 labeling throughout the entire externalized otic epithelium (Fig. 1 B, B′, and D and Movie S1). By contrast, Jag1 labeling in control embryos was confined to the ventral otocyst at E9.5 (Fig. 1A) and the distinct cochlear and vestibular prosensory domains at E12.5 (Fig. 1C and Movie S1). Sox2, another early marker of the sensory epithelia, is also restricted to the prosensory domains at E12.5 (Fig. 1E) (20). Notch activation in bigenic embryos caused a similar expansion of Sox2 expression throughout the entire otic epithelium (Fig. 1F). Sox2 also labels delaminating neuroblasts (Fig. 1E, asterisk), which were reduced in bigenic embryos (Fig. 1F). In addition, the Notch effector Hey1 is normally restricted to the prosensory regions, such as the floor of the cochlear duct (Fig. 1G) (5), and is also expressed throughout the entire otic epithelium in bigenic embryos (Fig. 1H). These results suggest that the entire otic vesicle is competent to give rise to sensory epithelia if the domain of Notch signaling is experimentally expanded early in the development of the otocyst.
Fig. 1.
Increased activation of the Notch pathway leads to failed otic vesicle closure and complete expansion of sensory domain markers. (A–B’) Jag1 immunolabeling (red) in E9.5 otic vesicles from control FoxG1Cre/+ (A) and double transgenic FoxG1Cre;RosaNotch (B and B’) embryos. In control embryos, Jag1 is expressed throughout the ventral otic vesicle but is absent from the dorsal side (A). The FoxG1Cre;RosaNotch otic vesicle displays incomplete closure and broad intense labeling of Jag1 (B’, arrows). Expression of GFP (B, green), indicates transgenic Notch activation. (C–H) Cochlear epithelia sections from control FoxG1Cre/+ (C, E, and G) embryos and similar sections through otic epithelia of FoxG1Cre;RosaNotch (D, F, and H) embryos at E12.5. Jag1 immunolabeling is restricted to prosensory domains in control embryos (C) and is ectopically expanded throughout the entire externalized otic epithelia in FoxG1Cre;RosaNotch embryos (D). (E and F) Similarly, Sox2 immunolabeling is restricted to the floor of the cochlear duct in control embryos (E) and expanded throughout the otic epithelium in bigenic embryos (F). Sox2 also labels delaminated neuroblasts (E, asterisk), which are reduced in FoxG1Cre;RosaNotch embryos (F). (G and H) The Notch effector Hey1 is restricted to the prosensory domains in control (G) and is expressed throughout the entire otic epithelium in bigenic embryos (H). [Scale bars, 100 μm (B) and 50 μm (H)].
hGFAPCre Transgenic Mice Exhibit Cre-Mediated Recombination in Sensory and Nonsensory Regions of Developing Vestibular Epithelia.
To complement the above study and to better define the regions of the developing membranous labyrinth that are competent to generate sensory epithelia, we looked for a transgenic Cre driver line that would provide a more restricted pattern of recombination in cells in nonsensory regions of the developing inner ear. One of the Cre lines that we analyzed was the hGFAPCre transgenic line, which was developed earlier with the goal of performing astrocyte-specific modification of genes but was found to have more widespread recombination (21).
To visualize Cre activity in hGFAPCre mice, we crossed the line to two different Cre reporter lines: mT/mG (22) (Fig. S2 A–B′′ and Fig. S3) or R26R-EYFP (23) (Fig. S2 C and C′). In the mT/mG mice, cells that have undergone Cre recombination are labeled by expression of membrane-bound GFP (mGFP, green). In the R26R-EYFP mice, Cre recombination results in the expression of EYFP; the two reporter lines in the inner ears of newborn mice gave essentially identical results. Fig. S2 A and B shows whole-mount views of a postnatal day (P) 0 hGFAPCre;mT/mG vestibular epithelia sample, which was immunolabeled with Sox2 (magenta) to mark the vestibular organs. The hGFAPCre caused recombination (mGFP+) in portions of the utricle and occasional cells within the cristae (Fig. S2 A–B′′ and Fig. S3 A and B). Most importantly for our study, we consistently found numerous mGFP+ cells in the nonsensory regions of the epithelium around the vestibular organs, indicating that the hGFAPCre transgene caused recombination in these nonsensory regions of the labyrinth as well (Fig. S2 A–B′′). In mice examined after birth, all showed recombination in the medial posterior region of the nonsensory vestibular epithelium, and 5/6 showed recombination in the lateral anterior nonsensory epithelium (see diagram and tabulated results in Fig. S4). In the cochlear sensory epithelia, we found hGFAPCre activity was not present in neonates but became expressed in some supporting cells postnatally (Fig. S3 C–F′′).
The pattern of Cre-mediated recombination in the hGFAPCre mice indicated that these mice could be useful for manipulating gene expression in the nonsensory regions of the inner ear. To be useful for our study of prosensory specification, however, the recombination needs to take place early in development. We were first able to detect EYFP expression in portions of the utricle and anterior crista as well as in the nonsensory adjacent regions at E13.5 (Fig. S2 C and C′). In mice examined at later embryonic stages, we found recombination in nonsensory regions similar to that observed in postnatal mice: 4/5 embryos showed recombination in the medial posterior nonsensory epithelium, whereas 2/5 embryos had recombination in the lateral/anterior regions of the nonsensory epithelium (Fig. S4). However, when we examined embryos at E11 or E12.5, we found no evidence of Cre-mediated recombination with the reporters. Taken together, our data indicate that recombination from the hGFAPCre occurs in nonsensory regions between E12.5 and E13.5. Because earlier loss-of-function studies indicated that prosensory specification occurs over a period that extends to this age (5), we concluded that these mice would be useful to test whether ectopic Notch signaling at this stage of development could activate a prosensory process in normally nonsensory epithelium.
Activation of Notch in Nonsensory Regions Leads to Formation of Ectopic Sensory Organs Containing Hair Cells, Supporting Cells, and Afferent Neurites.
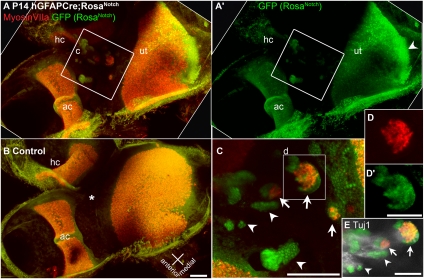
To determine whether ectopic activation of the Notch pathway in nonsensory regions of the inner ear after E12.5 would induce ectopic prosensory specification, we crossed the hGFAPCre mice with the RosaNotch mice described above. The hGFAPCre;RosaNotch mice survive to adulthood, allowing analysis of the fully mature inner ear. In juvenile and adult animals, we consistently found ectopic sensory patches containing cells that express hair cell markers MyosinVI or MyosinVIIa (Figs. 2 and 3 and Fig. S5). Ectopic hair cell patches formed in areas near the utricle and cristae, similar to where we observed recombination in the reporter lines described above. The RosaNotch transgene drives expression of nuclear-localized GFP as well as NotchIC, allowing identification of regions where recombination has occurred. Fig. 2C and Movie S2 show high-magnification views of an hGFAPCre;RosaNotch lateral anterior nonsensory region that contains 12 clusters of GFP+ cells, 3 of which contain MyosinVIIa+ hair cells. As this example shows, ectopic hair cells do not form everywhere NotchIC is activated, but they are not rare. Ectopic hair cell patches always contained GFP+ cells, indicating NotchIC expression, but also contained GFP− cells. Interestingly, the hair cells found in these patches did not express GFP, suggesting that they were induced by neighboring NotchIC-expressing cells (Fig. 2 C and D and Fig. S5 C–E). We also found that vestibular neurites labeled with anti-Tuj1 localize to ectopic patches (Fig. 2E and Movie S2), and Calretinin+ calyceal nerve endings were observed in ectopic hair cell patches (Fig. S5D, arrow).
Fig. 2.
Activation of Notch leads to ectopic patches of hair cells in nonsensory regions. Ectopic clusters of MyosinVIIa-labeled hair cells form in nonsensory regions of the vestibular epithelia of hGFAPCre;RosaNotch mice. Confocal projection views of vestibular epithelia from P14 hGFAPCre;RosaNotch (A and A’) or hGFAPCre/+ control (B) mice, including the utricle (ut), horizontal crista (hc), and anterior crista (ac) immunolabeled with anti-GFP (green) and the hair cell-specific marker anti-MyosinVIIa (red). In the hGFAPCre;RosaNotch epithelium, regions of ectopic hair cell formation are present between the utricle and cristae. In control vestibule, the same region (B, asterisk) is always nonsensory and devoid of hair cells. (C) High-magnification view of the boxed region in A, which contains multiple clusters of GFP+ cells (arrowheads mark examples), three of which contain ectopic MyosinVIIa+ hair cells (arrows and box). (D and D’) Split-channel views of the boxed region in C. (E) Tuj1 immunolabeling (white) shows vestibular neurites projecting to the ectopic patches in the upper region of C, as shown as a z series in Movie S2. [Scale bars, 100 μm (B, C, and E) and 50 μm (D’).]
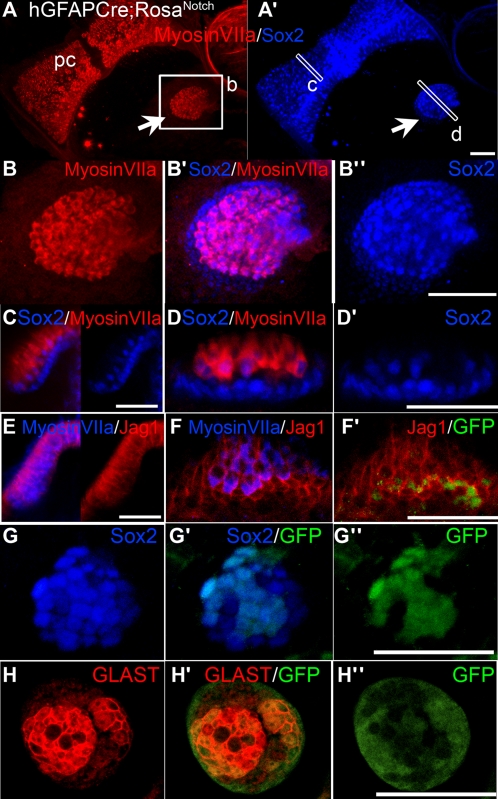
Fig. 3.
Induced ectopic sensory patches in hGFAPCre;RosaNotch mice resemble small vestibular organs and contain hair cells and supporting cells. Examples of ectopic clusters of sensory cells from several different juvenile hGFAPCre;RosaNotch mice are shown. (A and A’) Projection views of an ectopic sensory patch (arrow) located near the posterior crista (pc) labeled with antibodies to MyosinVIIa (red) and the sensory marker Sox2 (blue). (B–B’’) Higher-magnification views of the ectopic patch in A and A’. (C) Transverse view through the boxed region of the posterior crista shown in A and A’ showing the merged Sox2/MyosinVIIa labeling (Left), as well as the Sox2 labeling alone (Right). (D and D’) Transverse views through the boxed region of the ectopic sensory patch shown in A and A’. (E) Transverse view of a crista labeled with anti-MyosinVIIa (blue) and anti-Jag1 (red), showing the merged channels (Left), as well as the Jag1 alone (Right). (F–F’’) Transverse view of an ectopic sensory cluster labeled with anti-MyosinVIIa (blue), anti-Jag1 (red), and anti-GFP (green). (G–G’’) An ectopic cluster colabeled with anti-GFP (green) and anti-Sox2 (blue). (H–H’’) An ectopic cluster colabeled with antibodies to GFP (green) and the mature supporting cell marker GLAST (red). A, A’, and B–B’’ are brightest point projections; all others are single optical sections. (Scale bars, 100 μm.)
Ectopic sensory patches contain supporting cells as well as hair cells and have organization resembling small vestibular sensory organs. Double labeling experiments revealed that expression patterns of MyosinVIIa and Sox2 in ectopic clusters are very similar to those of normal vestibular organs where Sox2 is expressed in all supporting cells and a subset of hair cells (Fig. 3 A–D′) (24). In addition, Jag1 is normally expressed in supporting cell plasma membranes in mature vestibular organs (Fig. 3E and Fig. S5F) (24), and displays a very similar pattern in ectopic patches (Fig. 3F and Fig. S5F). We found that nuclear GFP expression was a common feature of the supporting cells in ectopic sensory patches, based on a high degree of overlap between labeling for GFP and Jag1 (Fig. 3 F and F′), Sox2 (Fig. 3 G and G′), and GLAST (Fig. 3 H and H′). GLAST is normally highly expressed in plasma membranes of mature vestibular supporting cells, but little or no immunolabeling for GLAST is normally found in the nonsensory regions (25). The induction of Sox2, Jag1, and GLAST expression in ectopic sensory clusters and a high degree of overlap between GFP and these markers suggest that constitutive activation of Notch cell-autonomously directs cells toward supporting cell fate.
Notch Activation During Prosensory Specification Leads to Formation of Ectopic Sensory Patches via Lateral Induction.
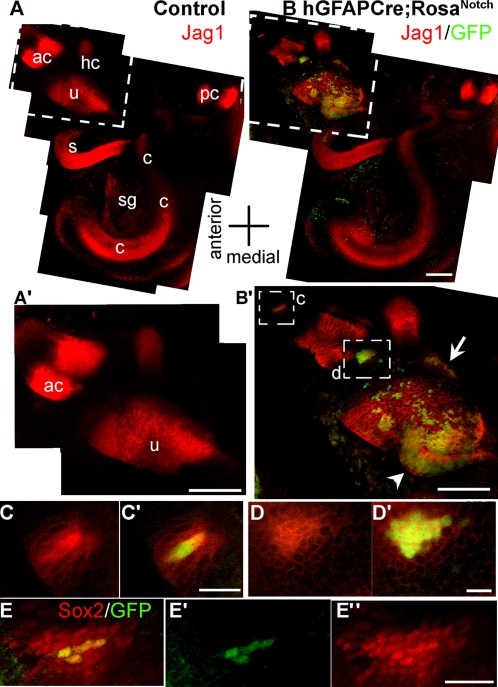
To determine when during development the ectopic patches first appear in hGFAPCre;RosaNotch ears, we imaged intact whole-mount otic capsules at E13.5. At this stage, we saw ectopic expression of the prosensory markers Jag1 and Sox2 in small areas of what would normally be nonsensory epithelium (Fig. 4 B–D′, E, and E′ and Fig. S6 B′–B′′′). These areas of Sox2 and Jag1 expression correlate well with GFP expression, indicating expression of NotchIC. Quantification of the numbers of ectopic patches of Sox2 and Jag1 expression in nonsensory regions relative to the total number of GFP+ patches at this age showed that 91.6% (n = 24) of GFP+ patches express ectopic Sox2 and 96.6% (n = 30) express ectopic Jag1. Interestingly, the few GFP+ patches that lacked ectopic expression of Sox2 or Jag1 were both small in size (≤3 cells) and relatively far away from the endogenous nascent sensory organs (≥70 μm distant). We found ectopic patches of Sox2+ cells in 90% of the hGFAPCre;RosaNotch ears examined at this age (Fig. S4), and never in ears from the control animals. The analysis of the ectopic Jag1 patches revealed another interesting aspect of the phenotype in these mice. The IRES-GFP reports where the NotchIC transgene is expressed, and is clearly seen in the cells expressing Jag1, but Jag1 immunolabeling was also present in neighboring cells up to three cell diameters away (Fig. 4 C and D). Similarly, we found that Sox2 expression was induced in nonsensory cells neighboring GFP+ cells up to several cell diameters away (Fig. 4 E–E′′). This suggests there are nonautonomous effects of Notch activation in recruiting cells to the sensory epithelia. Fig. S4 summarizes the hGFAPCre;RosaNotch embryos analyzed for the presence of ectopic sensory patches. We observed a high correlation between frequency of Cre reporter expression and expression of Sox2 and Jag1 in nonsensory areas. Before E13.5, we saw no ectopic Sox2- or Jag1-positive patches, but between E13.5 and E18.5 we saw the presence of ectopic sensory patches in the medial-posterior nonsensory region in 9/10 animals (Fig. S4A). In addition, in 9/13 animals, we saw Sox2 ectopic patches in the nonsensory region lateral and anterior to the utricle (Fig. S4B). Similar rates of ectopic expression were observed in postnatal and adult animals. These results demonstrate that activation of Notch in the nonsensory regions of the duct as late as E13.5 can induce prosensory specification.
Fig. 4.
Activation of Notch during prosensory specification leads to up-regulation of Jag1 and Sox2 in cis and trans. (A) Jag1 immunolabeling (red) in control E13.5 otic capsules is present in the five vestibular organs as well as the cochlea. (A’) An enlarged view of the boxed region in A showing the Jag1 labeling in the utricle (u) and anterior crista (ac). (B) An E13.5 hGFAPCre;RosaNotch otic capsule immunolabeled for Jag1 (red) and GFP (green, indicating transgenic NotchIC expression). (B’) An enlarged view of the boxed region in B showing the anterior crista and utricle labeled for Jag1 (red) and GFP (green). The pattern of Jag1 labeling in the hGFAPCre;RosaNotch utricle is expanded in a medial posterior protruding region (arrowhead; compare B’ with A’) of GFP+ cells. There are ectopic clusters of Jag1 labeling lateral to the utricle (arrow) and on either side of the anterior crista (boxed regions in B’), all of which contain GFP+ cells. A–A’ and B–B’ are montages of images compiled from brightest point projections of multiphoton excitation micrographs. (C and C’) A single optical section taken from the upper boxed region in B shows two adjacent GFP+ cells brightly labeled for Jag1, and neighboring, GFP−, cells also show up-regulated Jag1 expression. (D and D’) An enlarged view of a single optical section taken from the lower boxed region in B’ shows a cluster of about 15 GFP+ cells that have marked up-regulation of Jag1 expression, whereas surrounding GFP− cells also show up-regulation of Jag1 (D). (E and E’) Another hGFAPCre;RosaNotch sample displaying an ectopic Sox2+ cluster of cells between the utricle and anterior crista. This cluster consists of four GFP+ cells (E’) that are brightly labeled for Sox2 and are surrounded by a perimeter of ectopic Sox2+, GFP−, cells (E–E’’). ac, anterior crista; c, cochlea; hc, horizontal crista; pc, posterior crista; s, saccule; sg, spiral ganglia; u, utricle. [Scale bars, 100 μm (A’, B, and B’) and 20 μm (C’, D’, and E’’).]
Continuous Activation of Notch Within Endogenous Sensory Regions Suppresses Hair Cell Fate.
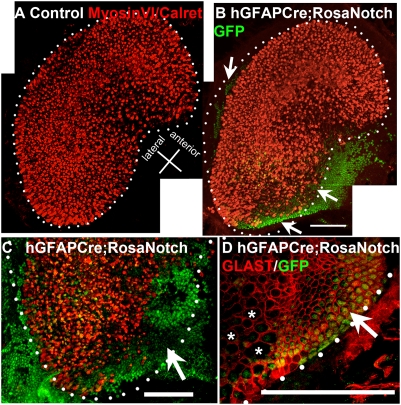
Earlier studies have indicated that, during later stages of sensory epithelium development, Notch signaling mediates the process of lateral inhibition whereby developing hair cells activate Notch in their neighbors, which inhibits hair cell fate in favor of supporting cell fate (13). hGFAPCre;RosaNotch mice have constitutive ectopic Notch activity in portions of the developing utricle starting at E13.5 (Fig. 4 and Fig. S6). To determine the effect of persistent activation of Notch within the sensory organs, we performed double immunolabeling experiments with GFP and markers of hair cells and supporting cells (Fig. 5). In utricles of adult hGFAPCre;RosaNotch mice with widespread GFP expression, we observed a marked reduction or absence of hair cells (Fig. 5 A–C). In contrast, expression of the supporting cell membrane marker GLAST was increased, indicating an increase in supporting cell density in these regions (Fig. 5D′). This suggests that continuous activation of Notch suppresses hair cell development, and is consistent with the well-studied role of Notch in lateral inhibition (13, 14).
Fig. 5.
Notch activation inhibits hair cell formation in vestibular organs. Regions of high-GFP+ cell density in utricles of hGFAPCre;RosaNotch mice contain fewer hair cells and more supporting cells. (A) A montage view of a normal control utricle, stained with an antibody mixture to MyosinVI and Calretinin (both in red) to label hair cells and their associated afferent neurites, displays dense patterning of hair cells within a clearly defined border (dotted line) outlining its characteristic kidney shape. (B) An hGFAPCre;RosaNotch utricle, shown as a montage, displays markedly reduced MyosinVI/Calretinin labeling (red) in areas with a high density of GFP+ cells (arrows, green) and a reduced overall hair cell area lacking a clear border in places. (C) A higher-magnification view of the posterior region of another hGFAPCre;RosaNotch utricle labeled as above shows a large GFP+ region devoid of hair cells (arrow). (D) In an hGFAPCre;RosaNotch utricle, GLAST immunolabeling (specific to supporting cell membranes) displays an abnormally continuous pattern in regions of high-GFP+ cell density, whereas nearby areas with fewer GFP+ cells display the normal pattern of GLAST distribution, where voids in expression of this marker occur in areas containing hair cells (asterisks). The dotted lines in B–D represent what would be the normal border region of the utricle as shown in A. (Scale bars, 100 μm.)
Discussion
To investigate the process of prosensory specification in mice, we have taken a gain-of-function approach using a genetically activated form of Notch and two lines of mice expressing Cre-recombinase under the control of different promoters: the FoxG1Cre, which allowed for activation of Notch in the entire otic vesicle around E9, and the hGFAPCre, which allowed for activation of Notch in nonsensory regions of the inner ear around E13.5. The use of these two lines has allowed us to define the period of development when Notch signaling is sufficient to convert nonsensory epithelium to sensory epithelium. When NotchIC is expressed throughout the otic vesicle early in development, using the FoxG1Cre line, the entire otic vesicle expresses the sensory epithelial markers Sox2, Hey1, and Jag1. The results with the hGFAPCre line showed that when Notch signaling is activated in nonsensory regions as late as E13.5, they are still competent for prosensory specification, which propagates from cell to cell and generates sensory patches that later form both hair cells and supporting cells. We conclude that active Notch is sufficient for prosensory specification from E9 to at least E13.5.
Our results are consistent with those obtained by Lewis and co-workers in the chick that first directly demonstrated the role of Notch activation in the process of prosensory specification (10–12). The ectopic sensory patches we observe in the hGFAPCre;RosaNotch mouse are strikingly similar to those induced by NotchIC transfection in chick otocyst (12). Our data also complement the study of Jayasena et al. (26), who found that activation of Notch in the Pax2+ head ectoderm caused expansion of the otic placode at the expense of epidermis. Taken together with the Jayasena et al. study, it appears that, in the mouse, the nonsensory epithelia can respond to the prosensory Notch signal from the earliest stages of otic placode induction through the time of early morphogenesis of the otic labyrinth. Lewis and colleagues (10–12) also proposed that a process known as lateral induction (mediated by a positive feedback loop in Notch-Serrate signaling) maintains and extends prosensory patches. Our data further support this idea, because ectopic Notch activation leads to high levels of Jag1 cell-autonomously, but in addition we find nonautonomous Jag1 expression in adjacent cells (Fig. 4 C and D).
Although Jag1-Notch-mediated lateral induction may propagate and maintain prosensory character, this model does not explain how the prosensory process is initiated. Daudet and Lewis suggested that factors other than Notch signaling initiate the expression of Jag1 in the otocyst, because expression of Serrate1 was not strongly reduced in the short term by inhibition of Notch (12). FGF signaling, probably through FGF20 and FGFR1, is also required for prosensory induction during inner ear development (17, 27). FGF has been shown to induce Notch-dependent lateral induction of Jag1 in the developing lens (28), and thus could be involved in the initiation of Jag1-Notch signaling in this system. The Wnt signaling pathway is another candidate that could be involved in the initial activation of Jag1, because Wnt signaling has been shown to positively regulate Jag1 in the otic placode and multiple conserved TCF/LEF binding sites have been found within the 5′ region of mammalian Jag1 orthologs (26, 29).
Sox2 is also expressed early in the formation of the sensory epithelia and is critical for their formation (20). In addition, studies performed on cochlear tissue in vitro have indicated that Notch signal activation can drive Sox2 expression and Notch is required for proper Sox2 expression levels (5, 30). Here we show that Notch activation is sufficient to induce ectopic Sox2 expression cell-autonomously as well as in neighboring cells. These results indicate that Notch signaling acts upstream of Sox2 expression and causes intercellular propagation of prosensory character.
Our results indicate that Notch signaling drives the prosensory pathway during development, resulting in expression of the prosensory markers Jag1 and Sox2. This process does not appear to be active in the mature inner ear. Although Jag1 and Sox2 are expressed robustly in adult cochlear supporting cells, Notch may be inactive here because cochlear supporting cells rapidly down-regulate Hes5 during postnatal development (24, 31); however, expression of other Notch effectors has not been fully evaluated in the adult cochlea. Future efforts at defining the interactions between Notch signaling and prosensory specification will no doubt lead to a better understanding of this developmental process, and may allow for the activation of prosensory character in mature cochlea to stimulate regeneration.
Methods
In Situ Hybridization and Immunohistochemistry.
In situ hybridization and immunohistochemistry on paraffin sections and whole-mount postnatal inner ear organs were performed as described previously (5, 31). Immunohistochemistry of whole-mount embryonic otic capsules was carried out based on the same procedures (see SI Methods for more details).
Imaging.
Confocal images were collected using a Nikon A1R or Zeiss LSM Pascal confocal microscope. Multiphoton excitation microscopic images were captured on an Olympus FV1000 MPE BX61 (Spectra Physics).
Supplementary Material
Acknowledgments
We thank Dr. Toshinori Hayashi for generous advice and insight, Cat Ray for help with Hey1 in situ hybridization, Dr. David Raible for helpful advice, Dr. Doug Melton (Harvard University, Cambridge, MA) for providing RosaNotch mice, Linda Robinson for help with animal husbandry, Drs. Ron Seifert and Greg Martin for imaging training, and past and present members of the T.A.R. and O.B.-M. laboratories for support and advice. This research is supported by National Research Service Award Grant T32 HD007183-26A1 (to B.H.H.), National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIH/NIDCD) Grants DC005953 and DC009991 (to O.B.-M.), NIH/National Eye Institute RO1 Grant EY013475 (to T.A.R.), and NIH/NIDCD Research Core Center Grant P30DC004661, and the Lynn and Mike Garvey Cell Imaging Laboratory at the Institute for Stem Cells and Regenerative Medicine, University of Washington.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002827107/-/DCSupplemental.
References
- 1.Adam J, et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: Parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- 2.Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of Notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78:159–163. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- 3.Morrison A, Hodgetts C, Gossler A, Hrabé de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 4.Murata J, Tokunaga A, Okano H, Kubo T. Mapping of Notch activation during cochlear development in mice: Implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497:502–518. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, et al. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiernan AE, et al. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci USA. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrijens K, et al. Ozzy, a Jag1 vestibular mouse mutant, displays characteristics of Alagille syndrome. Neurobiol Dis. 2006;24:28–40. doi: 10.1016/j.nbd.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 10.Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: Lessons from Drosophila. Proc Natl Acad Sci USA. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- 12.Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: Specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- 13.Bryant J, Goodyear RJ, Richardson GP. Sensory organ development in the inner ear: Molecular and cellular mechanisms. Br Med Bull. 2002;63:39–57. doi: 10.1093/bmb/63.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Semin Cell Dev Biol. 1998;9:583–589. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- 15.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hébert JM, McConnell SK. Targeting of Cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 17.Pirvola U, et al. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 18.Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang CH, Simeone A, Lai E, Wu DK. Foxg1 is required for proper separation and formation of sensory cristae during inner ear development. Dev Dyn. 2009;238:2725–2734. doi: 10.1002/dvdy.22111. [DOI] [PubMed] [Google Scholar]
- 20.Kiernan AE, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 21.Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 22.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 23.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takumi Y, et al. Discrete cellular and subcellular localization of glutamine synthetase and the glutamate transporter GLAST in the rat vestibular end organ. Neuroscience. 1997;79:1137–1144. doi: 10.1016/s0306-4522(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 26.Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–2261. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–5999. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev Biol. 2009;332:166–176. doi: 10.1016/j.ydbio.2009.05.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int J Mol Med. 2006;17:681–685. [PubMed] [Google Scholar]
- 30.Dabdoub A, et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman BH, et al. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol. 2009;10:321–340. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.