Abstract
Angiotensin-converting enzyme 2 (ACE2) is a newly discovered homolog of ACE whose actions oppose those of angiotensin II (AngII). However, the underlying mechanisms by which ACE2 effectively suppresses early atherosclerotic lesions remain poorly understood. Here, we show, both in vitro and in vivo, that ACE2 inhibited the development of early atherosclerotic lesions by suppressing the growth of vascular smooth muscle cells (VSMCs) and improving endothelial function. In a relatively large cohort animal study (66 rabbits), aortic segments transfected by Ad-ACE2 showed significantly attenuated fatty streak formation, neointimal macrophage infiltration, and alleviation of impaired endothelial function. Segments also showed decreased expression of monocyte chemoattractant protein 1, lectin-like oxidized low-density lipoprotein receptor 1, and proliferating cell nuclear antigen, which led to the delayed onset of atherosclerotic lesions. At the cellular level, ACE2 significantly modulated AngII-induced growth and migration in human umbilical vein endothelial cells and VSMCs. The antiatherosclerotic effect of ACE2 involved down-regulation of the ERK-p38, JAK-STAT, and AngII-ROS-NF-κB signaling pathways and up-regulation of the PI3K-Akt pathway. These findings revealed the molecular mechanisms of the antiatherosclerotic activity of ACE2 and suggested that modulation of ACE2 could offer a therapeutic option for treating atherosclerosis.
Keywords: atherosclerosis, endothelial cell, gene therapy, smooth muscle cell, signaling pathway
Accumulating evidence indicates that endothelial cell (EC) dysfunction and the proliferation and migration of vascular smooth muscle cells (VSMCs) are salient features of early atherosclerotic lesions, and that the renin-angiotensin system (RAS) plays an important role in the pathogenesis of atherosclerosis (1, 2). Angiotensin II (AngII) promotes EC dysfunction and VSMC proliferation and migration by increasing the expression of monocyte chemoattractant protein 1 (MCP-1) and lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1), leading to aggravation of atherosclerotic lesions (3–5). Delivery of ACE inhibitors or AngII type 1 receptor (AT1R) blockers to limit AngII bioactivity is an effective approach against atherosclerosis.
Recent studies show that endogenous levels of AngII are regulated by the opposing action of two carboxypeptidases, angiotensin-converting enzyme (ACE) and angiotensin-converting enzyme 2 (ACE2). The latter is thought to counterbalance ACE by cleaving AngI into the inactive angiotensin 1–9 and cleaving AngII into the vasodilating and antiproliferative angiotensin 1–7 [Ang(1-7)]. ACE2 is thus considered a potential therapeutic target of RAS for the treatment of cardiovascular diseases by virtue of its key role in the formation of vasoprotective peptides from AngII (6–8). Our recent study using a rabbit atherosclerosis model showed that ACE2 overexpression stabilized aortic plaques at a late stage and attenuated the progression of early atherosclerotic lesions. These therapeutic effects were due to reduced AngII levels and ACE activity as well as increased Ang(1-7) levels in the local RAS (9).
Because ACE2 is mainly expressed in ECs and VSMCs of the vascular wall, ACE2 may display an antiatherosclerotic effect by targeting the vascular compartment. In this study, we provide experimental evidence to validate this hypothesis.
Results
ACE2 Expression and Activity.
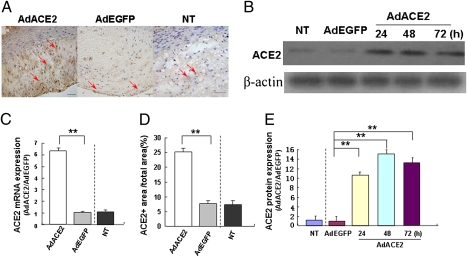
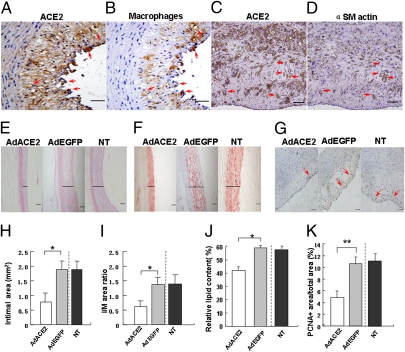
Immunohistochemistry and RT-PCR showed high levels of ACE2 protein and mRNA expression in atherosclerotic lesions in the Ad-ACE2 group relative to Ad-EGFP and nontransduced (NT) groups (Fig. 1 A, C, and D). Experiment in vitro demonstrated that ACE2 protein expression was increased in the Ad-ACE2 group of cultured human umbilical vein endothelial cells (HUVECs) 24 h after transfection in comparison with the nontransduced and Ad-EGFP groups (Fig. 1 B and E). Adjacent serial sections showed positive ACE2 protein expression in macrophages (Fig. 2 A and B) and VSMCs (Fig. 2 C and D). The ratio of peptide peaks [Ang(1-7):AngII] as an indicator of ACE2 activity in vascular tissues was significantly higher in the Ad-ACE2 group (1.20 ± 0.05) relative to the Ad-EGFP (0.32 ± 0.01) and nontransduced (0.31 ± 0.02) groups (Fig. S1A).
Fig. 1.
Expression of ACE2 in rabbit abdominal aortic segments and HUVECs. (A) ACE2 protein expression in atherosclerotic lesions by immunostaining (red arrows). (B) ACE2 protein expression in nontransduced (NT), Ad-EGFP, and Ad-ACE2 groups of HUVECs (24, 48, and 72 h after transfection) by Western blot analysis. (C) ACE2 mRNA expression in atherosclerotic lesions detected by real-time RT-PCR. **P < 0.01 vs. nontransduced or Ad-EGFP group. (D) Quantitative analysis of ACE2 protein expression by immunohistochemistry. **P < 0.01 vs. nontransduced or Ad-EGFP group. (E) Quantitative analysis of ACE2 protein expression (24, 48, and 72 h after transfection). **P < 0.01 vs. nontransduced or Ad-EGFP group. n = 12 in each group.
Fig. 2.
Pathology staining and quantitative analysis of rabbit atherosclerotic lesions. (A and B) Adjacent serial sections showing positive staining of ACE2 protein and positive staining of macrophages, respectively. Red arrows represented positive staining at similar locations. (C and D) Adjacent serial sections showing positive staining of ACE2 protein and positive staining of α-actin of SMCs, respectively. Red arrows represented positive staining at similar locations. (Scale bars: 50 μm.) (Magnification: 40×.) (E) Representative H&E staining of atherosclerotic lesions. (Magnification: 10×.) Solid lines indicated the thickness of the intima. (Scale bars: 200 μm.) (F) Lipid contents in atherosclerotic lesions by Oil-red O staining. Solid lines indicated the thickness of the intima. (G) Immunohistochemistry of PCNA protein expression (red arrows). (H) Measurements of the intimal area. *P < 0.05 vs. NT or Ad-EGFP group. (I) Measurements of the ratio of intimal-to-medial (I/M) area. *P < 0.05 vs. NT or Ad-EGFP group. (J) Quantification of the area positively stained by Oil-red O. *P < 0.05 vs. nontransduced or Ad-EGFP group. (K) Quantification of PCNA protein expression. **P < 0.01 vs. nontransduced or Ad-EGFP group.
ACE2 Attenuates Atherosclerotic Lesions.
Fatty streak formation in the abdominal aorta was significantly reduced in the Ad-ACE2 group relative to the Ad-EGFP and nontransduced groups (Fig. 2 E and F). Marked intimal thickening was present in the Ad-EGFP and nontransduced groups; the intimal area and the ratio of intimal to medial (I/M) area were significantly lower in the Ad-ACE2 group than the Ad-EGFP and nontransduced groups (Fig. 2 H and I), with no difference between the Ad-EGFP and nontransduced groups, although the serum lipid levels were similar among the three groups of rabbits (Tables S1 and S2). The relative lipid content in atherosclerotic lesions, depicted by Oil-red O staining, was significantly lower in the Ad-ACE2 group than the Ad-EGFP and nontransduced groups (Fig. 2 F and J).
Within the atherosclerotic lesions, the area that was positive for proliferating cell nuclear antigen (PCNA; Fig. 2 G and K) was significantly lower in the Ad-ACE2 group (4.87 ± 1.13%) than in the Ad-EGFP (10.63 ± 1.19%) and nontransduced groups (11.13 ± 1.25%). Double immunolabeling showed that the PCNA-positive staining was localized in VSMCs, macrophages, and ECs in atherosclerotic lesions (Fig. S2).
The percentage of macrophages in atherosclerotic lesions was lower in the Ad-ACE2 group (18.75 ± 4.00%) than in the Ad-EGFP (25.85 ± 4.72%) and nontransduced groups (23.73% ± 7.24%) (Fig. S3A). The percentage of apoptotic cells in the intima did not differ between the Ad-ACE2 (0.26 ± 0.03%), Ad-EGFP (0.25 ± 0.02%), and nontransduced (0.25 ± 0.03%) groups.
ACE2 Regulates MCP-1 and LOX-1 Expression.
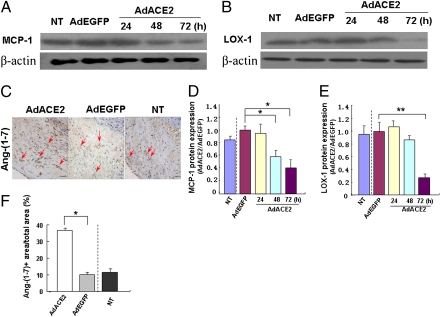
Immunohistochemistry was used to explore the changes in MCP-1 (Fig. S3B) and LOX-1 (Fig. S3C) protein expression induced by ACE2 gene transfection in the abdominal aorta. Staining was extensive in the Ad-EGFP and nontransduced groups (where it was found mainly in the endothelium and neointima) as compared with the Ad-ACE2 group. Quantitative analysis revealed that the relative MCP-1 protein expression was significantly lower in the Ad-ACE2 group (16.56 ± 2.34%) than in the Ad-EGFP (27.73 ± 5.82%) and nontransduced (26.97 ± 5.01%) groups. Similarly, the relative LOX-1 protein expression was significantly lower in the Ad-ACE2 group (16.64 ± 2.13%) than in the Ad-EGFP (25.03 ± 3.34%) and nontransduced (24.00 ± 3.64%) groups, with no difference in MCP-1 and LOX-1 expression between the Ad-EGFP and nontransduced groups.
Western blot analysis of HUVECs demonstrated a lower expression of MCP-1 in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups 48 h after Ad-ACE2 transfection (Fig. 3 A and D). Similarly, LOX-1 expression was significantly suppressed 72 h after Ad-ACE2 transfection (Fig. 3 B and E).
Fig. 3.
MCP-1 and LOX-1 protein expression in HUVECs and Ang(1-7) protein in atherosclerotic lesions. (A) MCP-1 protein expression in NT, Ad-EGFP, and Ad-ACE2 groups of HUVECs (24, 48, and 72 h after transfection). (B) LOX-1 protein expression in nontransduced, Ad-EGFP, and Ad-ACE2 groups (24, 48, and 72 h after transfection). (C) Immunohistochemical staining of Ang(1-7) protein expression in atherosclerotic lesions (red arrows). (Magnification: 40×.) (Scale bars: 50 μm.) (D) Quantitative analysis of A. *P < 0.05 vs. NT, Ad-EGFP and Ad-ACE2 groups (24 h after transfection). (E) Quantitative analysis of B. **P < 0.01 vs. NT, Ad-EGFP, and Ad-ACE2 groups (24 and 48 h after transfection). (F) Quantitative analysis of Ang(1-7) protein expression. *P < 0.05 vs. NT or Ad-EGFP group. n = 12 in each group.
In exploring the signaling pathways mediating the effects of ACE2 on MCP-1 and LOX-1 expression in HUVECs, PI3K (Fig. S4 A and B) and Akt (Fig. S4 C and D) protein expression, assessed by Western blot analysis, was significantly higher in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups at 24, 48, and 72 h after ACE2 transfection. MCP-1 (Fig. S4 E and F) and LOX-1 (Fig. S4 G and H) protein expression in the Ad-ACE2+Akt inhibitor group was higher than that in the Ad-ACE2 group but lower than that in the Akt-inhibitor, nontransduced and Ad-EGFP groups, with no difference in MCP-1 and LOX-1 expression between Akt-inhibitor, nontransduced and Ad-EGFP groups.
ACE2 Modulates AngII and Ang(1-7) Expression.
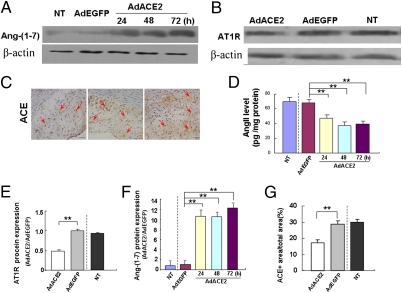
Ang(1-7) protein expression was higher in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups (Fig. 3 C and F), with no significant difference between the Ad-EGFP and nontransduced groups. AngII protein expression in aortic lesions, as measured by ELISA (ng/mg total protein), was markedly lower in the Ad-ACE2 group (2.53 ± 0.14) than the Ad-EGFP (3.56 ± 0.14) and nontransduced (3.59 ± 0.22) groups, with no difference between the Ad-EGFP and nontransduced groups.
Studies of HUVECs demonstrated a markedly lower AngII protein level in the Ad-ACE2 group than the Ad-EGFP and nontransduced groups (Fig. 4D). In contrast, Ang(1-7) protein expression was higher in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups (Fig. 4 A and F).
Fig. 4.
Ang(1-7) and AngII level in HUVECs and AT1R,ACE protein expression in atherosclerotic lesions. (A) Ang(1-7) protein expression in NT, Ad-EGFP, and Ad-ACE2 groups (24, 48, and 72 h after transfection) by Western blot analysis. (B) AT1R protein expression in atherosclerotic lesions of NT, Ad-EGFP, and Ad-ACE2 groups. (C) ACE1 protein expression in atherosclerotic lesions of NT, Ad-EGFP, and Ad-ACE2 groups (red arrows). (Magnification: 40×.) (Scale bars: 50 μm.) (D) Quantitative analysis of AngII protein levels in NT, Ad-EGFP, and Ad-ACE2 groups (24, 48, and 72 h after transfection) by ELISA. **P < 0.01 vs. NT or Ad-EGFP group. (E) Quantitative analysis of B. **P < 0.01 vs. NT or Ad-EGFP group. (F) Quantitative analysis of A. **P < 0.01 vs. NT or Ad-EGFP group. (G) Quantitative analysis of C. **P < 0.01 vs. NT or Ad-EGFP group.
ACE2 Regulates ACE and AT1R Expression.
ACE and AT1R expression levels were examined in vivo. ACE protein expression (as determined by immunohistochemistry; Fig. 4 C and G) and AT1R protein expression (as detected by Western blot analysis; Fig. 4 B and E) were significantly lower in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups.
Western blot analysis of HUVECs demonstrated that ACE protein expression (Fig. S5 A and B) was significantly lower in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups 24 h after gene transfection and that AT1R expression (Fig. S5 C and D) was lower beginning at 48 h.
AngI was converted to AngII in all three groups examined (Fig. S1B). The ratio of AngII to AngI was significantly lower in the Ad-ACE2 group (0.61 ± 0.12) than in the Ad-EGFP (1.11 ± 0.03) and nontransduced (1.15 ± 0.04) groups, with no difference between the Ad-EGFP and nontransduced groups.
ACE2 Inhibits VSMC Proliferation and Migration.
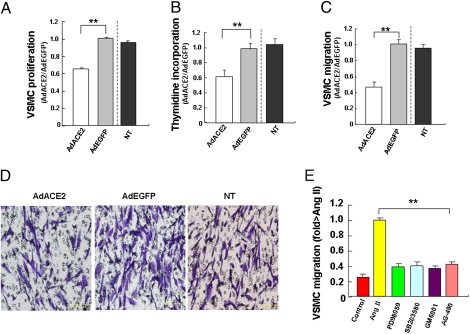
Ad-ACE2-transfected VSMCs showed a significant and time-dependent reduction in proliferation as compared with Ad-EGFP and nontransduced groups at 48 h after transfection (Fig. 5A). Thymidine incorporation assays showed that DNA replication of VSMCs was significantly lower in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups (Fig. 5B). VSMC migration was significantly inhibited in the Ad-ACE2 group as compared with the Ad-EGFP and nontransduced groups (Fig. 5 C and D). Thus, ACE2 markedly inhibited DNA replication and VSMC proliferation and migration.
Fig. 5.
VSMC proliferation and migration. (A) Quantitative analysis of VSMC proliferation as measured by BrdU incorporation (48 h after AngII stimulation). **P < 0.01 vs. NT or Ad-EGFP group. (B) Quantitative analysis of VSMC proliferation by thymidine incorporation assay. **P < 0.01 vs. NT or Ad-EGFP group. (C) Quantitative analysis of VSMC migration. **P < 0.01 vs. NT or Ad-EGFP group. (D) VSMC migration assay. (Magnification: 20×.) (Scale bars: 200 μm.) (E) Quantitative analysis of AngII-induced VSMC invasion into the ECM. Ang-II significantly promoted VSMC migration, which was substantially attenuated by treatment with GM6001, an MMP inhibitor, blockade of ERK1/2 MAPK, and p38 MAPK with SB203580 (10 μM) and PD98059 (20 μM), respectively, or administration of a specific JAK2 inhibitor (AG-490, 10 mM). **P < 0.01 vs. NT, PD98059, SB203580, GM6001, or AG-490 group.
Migration activity associated with up-regulation of matrix metalloproteinase (MMP) in VSMCs was examined by migration assay with the use of a modified Boyden chamber. The polycarbonate filter was coated with Matrigel. As shown in Fig. 5E, AngII significantly enhanced VSMC invasion into matrix barriers and promoted VSMC migration. Treatment with GM6001, an MMP inhibitor, inhibited VSMC migration induced by AngII. Furthermore, blockade of ERK1/2 mitogen-activated protein kinase (MAPK) and p38 MAPK with SB203580 and PD98059, respectively, or with a specific JAK2 inhibitor (AG-490) significantly attenuated VSMC migration induced by AngII.
ACE2 Improves Endothelial Function.
To characterize the role of ACE2 in regulating EC function, we evaluated proliferation, migration, and formation of tube-like structures in HUVECs. In comparison with the Ad-EGFP or nontransduced groups, Ad-ACE2-transfected HUVECs showed significantly inhibited EC proliferation (Fig. S6 A and B), enhanced EC migration (Fig. S6 C and D) and augmented tube-like structure formation, which is indicative of an increased number of infiltrating vessels, in the Ad-ACE2 group (Figs. S6E and S7A). In addition, ACE2 overexpression substantially reduced the protein expression of MCP-1 and intercellular adhesion molecule 1 (ICAM-1; Table S3), and the number of monocytes adhering to HUVECs (Fig. S7D), as compared with the Ad-EGFP and nontransduced groups. Endothelium-dependent relaxation induced by acetylcholine was significantly impaired in the aortic segments of the Ad-EGFP and nontransduced groups. In contrast, endothelium-dependent relaxation of the aortic segments was significantly improved in the Ad-ACE2 group (Table S4).
ACE2 Regulates Reactive Oxygen Species (ROS) and NF-κB Activity.
After AngII stimulation in HUVECs, levels of ROS were significantly lower in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups (Fig. S7B). Transcription factor assays were performed with nuclear protein extracted from HUVECs. NF-κB DNA-binding activity in HUVECs was significantly lower in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups after AngII stimulation (Fig. S7C).
Impact of ACE2 on MMP-2 and MMP-9 Activity.
To elucidate the association of AngII stimulation and MMP activation, we measured MMP-2 and MMP-9 activity in AngII-treated VSMCs by zymography. At the same dose that induced VSMC migration, AngII stimulation increased MMP-9 activity but did not affect MMP-2 activity. After ACE2 gene transfer, MMP-9 activity was markedly inhibited in the Ad-ACE2 group compared with the Ad-EGFP and nontransduced groups (Fig. S8 A and B). However, ACE2 had no effect on MMP-2 activity.
ACE2 Regulates ERK-p38 and JAK-STAT Signaling Pathways.
To clarify how ACE2 suppressed VSMC proliferation and migration, the ERK1/2 and p38 signaling pathways were explored. ERK1/2 (Fig. S8 C and D) and p38 (Fig. S8 E and F) phosphorylation in atherosclerotic lesions was significantly lower in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups.
Because JAK2 and STAT3 signaling pathways play an essential role in mediating VSMC proliferation, we explored the role of these two signaling pathways in the regulation of VSMC bioactivity after Ad-ACE2 transfection. Expression of both JAK2 (Fig. S9 A and B) and STAT3 (Fig. S9 C and D) protein in atherosclerotic lesions was significantly lower in the Ad-ACE2 group than in the Ad-EGFP and nontransduced groups.
Discussion
The key finding of this study is that local overexpression of ACE2 significantly inhibits the development of early atherosclerotic lesions in a rabbit atherosclerosis model. The antiatherosclerotic effect of ACE2 is associated with prohibited proliferation and migration of VSMCs and improved endothelial functions. The underlying mechanism involves down-regulation of the ERK-p38 and JAK-STAT pathways and up-regulation of the PI3K-Akt pathway.
The recent discovery of several new members in the RAS family, such as ACE2, Ang(1-7), and Ang(1–9), facilitates our understanding of the complex relationship between RAS and atherosclerosis (10–13). ACE2 catalyzes the formation of the vasoprotective peptide Ang(1-7) from AngII and is thus considered a natural antagonizing force for AngII. Ang(1-7) has both vasodilative and antiproliferative activities opposing those of AngII, and Mas, a proto-oncogene that is considered the receptor for Ang(1-7) is mainly expressed in the kidney, heart, and brain (14). It has been shown that high expression of Mas leads to decreased ROS. Moreover, inhibition of NF-κB activation by Ang(1-7) is partially reversed by the Mas receptor antagonist A779 (15). In this study, we show that ACE2 effectively attenuates atherosclerotic lesions by inducing a number of changes in the RAS-mediated signaling system. First, ACE2 overexpression led to greater conversion of AngII to Ang (1–7), with a reduction in the level of AngII, an effect similar to ACE inhibitors. In addition, Ang(1-7) has a potent and independent antiatherosclerotic effect, as revealed by our previous study (9). Thus, decreased AngII and increased Ang(1-7) levels are equally important in the therapeutic effects of ACE2 gene transfection in early atherosclerosis. Second, ACE2 overexpression suppressed ACE activity. Previous studies reported that AngII increased ACE expression but decreased ACE2 expression by activating the ERK-p38 pathway (16). We found that ACE2 attenuated ACE expression and activity by down-regulating the ERK-p38 pathway. Similarly, ACE inhibition may lead to enhanced expression and activity of ACE2 as reported in a rat model of myocardial infarction and Lewis normotensive rats (17, 18). Thus, ACE and ACE2 may regulate each other by feedback inhibition. Third, ACE2 overexpression affected AT1R activity. We found that the protein expression of AT1R was markedly attenuated after ACE2 gene transfection, a finding consistent with a previous study (19). The explanation for this finding may be the accelerated conversion of AngII to Ang(1-7) by overexpressed ACE2 combined with the decreased conversion of AngI to AngII due to suppressed ACE activity.
Neointima formation and vascular remodeling are characteristics of early atherosclerotic lesions, and these pathological processes may involve EC dysfunction, VSMC proliferation and migration, and ECM deposition (20, 21). Therefore, modulation of EC function and VSMC growth is of critical importance in the prevention and treatment of early atherosclerosis. In this study, we found that ACE2 overexpression led to a significant reduction of the intimal area and the ratio of intimal-to-medial area. Moreover, Ad-ACE2 transfection significantly inhibited DNA replication and VSMC proliferation and migration. These results suggest that improved VSMC bioactivity is one of the major mechanisms of the antiatherosclerotic effects of ACE2 overexpression, although the underlying signaling pathway remains unclear. Previous studies demonstrated that ERK-p38 and JAK-STAT signaling pathways activated by AngII and other cytokines had essential roles in VSMC proliferation and migration (22). In this study, we found that AngII-induced VSMC migration and MMP-9 activation were significantly attenuated by blocking the ERK-p38 and JAK-STAT signaling pathways in vitro and, more importantly, these pathways were significantly down-regulated in atherosclerotic lesions after Ad-ACE2 transfection. Together, these results suggest that ACE2 attenuated VSMC proliferation and migration in early atherosclerosis by inhibiting the ERK-p38 and JAK-STAT signaling pathways.
VSMC migration requires degradation of the basement membrane and ECM surrounding the cell. MMPs cleave components of the ECM and play an important role in inducing VSMC migration. Some studies have found that AngII stimulates MMP-9 secretion from VSMCs (23). In this study, ACE2 transfection significantly inhibited MMP-9 activity in AngII-stimulated VSMCs, which suggests that ACE2 may inhibit VSMC migration by suppressing MMP-9 activity. In contrast, Ad-ACE2 had no effect on MMP-2 activity in AngII-stimulated VSMCs, a result consistent with previous findings (23).
EC dysfunction in atherosclerosis involves vasoconstriction, leukocyte adherence, prooxidation, platelet activation, vascular inflammation, and thrombosis (24). In this study, we found that endothelium-dependent relaxation induced by acetylcholine was significantly impaired in the aortic segments of the Ad-EGFP and nontransduced groups but was significantly improved in the Ad-ACE2 group. We also found that ACE2 significantly inhibited HUVEC proliferation, promoted cell migration, and enhanced tube-like structure formation, which is regarded as an indicator of improved endothelial cell function and EC-mediated neovascularization (1). Because MCP-1 is a key chemokine that stimulates monocyte migration into the subendothelial space, we evaluated the effect of ACE2 on the adhesion of monocytes to HUVECs in vitro and on the extent of macrophage infiltration and MCP-1 and LOX-1 protein expression in vivo. ACE2 decreased the expression of MCP-1 and ICAM-1 in cultured HUVECs, decreased the number of monocytes adhering to HUVECs, inhibited MCP-1 and LOX-1 expression, and inhibited the extent of macrophage infiltration in atherosclerotic lesions. These results suggest that improved EC bioactivity and suppressed chemokine expression are an important mechanism of the antiatherosclerosis effects of ACE2 overexpression.
To further elucidate the signaling pathway responsible for the therapeutic effects of ACE2 on chemokine expression, we explored the association of the PI3K-Akt pathway and expression of MCP-1 and LOX-1. ACE2 gene transfection in HUVECs markedly activated the PI3K-Akt pathway, which led to attenuated expression of MCP-1 and LOX-1. In contrast, administration of the specific Akt inhibitor SH-6 with Ad-ACE2 transfection significantly counteracted the suppressive effect of ACE2 on MCP-1 and LOX-1 expression. Thus, ACE2 may exert its suppressive effects on LOX-1 and MCP-1 expression via the PI3K-Akt pathway.
Studies have found that AngII enhances endothelial NADPH oxidase activity that leads to increased ROS production and NF-κB activation (25–27) and initiates a number of pathological processes including endothelial dysfunction, vascular inflammation, and cell proliferation (28). In this study, we found that ACE2 gene transfection decreased ROS levels and inhibited NF-κB activity in HUVECs, suggesting that changes in the downstream inflammatory pathways may take part in the mechanisms of the antiatherosclerotic effects induced by ACE2 overexpression.
In summary, our study demonstrates that ACE2 overexpression significantly inhibited early atherosclerotic lesions by suppressing VSMC proliferation and migration and improving EC function. The antiatherosclerotic effects of ACE2 likely result from the interactions of various RAS components, such as decreased AngII levels, increased Ang(1-7) levels, reduced ACE activity, and reduced AT1R expression. The mechanisms underlying these therapeutic effects involved down-regulation of the ERK-p38, JAK-STAT, and AngII-ROS-NF-κB pathways and up-regulation of the PI3K-Akt pathway. Thus, ACE2 may provide a unique therapeutic target in the prevention and treatment of early atherosclerotic lesions.
Materials and Methods
Preparation of ACE2 Adenoviral Vector.
Murine ACE2 cDNA was amplified by RT-PCR from RNA of mouse kidney. Recombinant adenovirus carrying the murine ACE2 or a control transgene (enhanced green fluorescent protein, EGFP) were prepared as described with use of the AdMax system (29) (see SI Materials and Methods for details).
Animal Model and Gene Transfer.
Sixty-six male New Zealand White rabbits were fed an atherogenic chow, then arterial wall injury was induced by balloon injury after anesthesia by a described method (30) (see SI Materials and Methods for details). Rabbits were randomly divided into three groups (n = 22 each) for treatment with a suspension of Ad-ACE2, treatment with a suspension of Ad-EGFP, and no treatment.
HUVEC or VSMC Culture and Gene Transfer.
HUVECs or VSMCs incubated with AngII for 24 h were divided into three groups for treatment: Ad-ACE2, Ad-EGFP, and no treatment. Ad-ACE2 or Ad-EGFP was transfected into cells and harvested at 24, 48, and 72 h after gene transfection for Western blot analysis (see SI Materials and Methods for details).
Measurement of ACE2 and ACE Activity.
The enzymatic activities of ACE2 and ACE were evaluated by SELDI-TOF-MS (31) (see SI Materials and Methods for details).
Serum Lipid Measurement.
The serum concentrations of total cholesterol (TC) and triglycerides (TG) were determined by enzymatic assays.
Histopathological Analysis.
Serial sections were stained with hematoxylin and eosin and Oil-red O for histopathological analysis (see SI Materials and Methods for details).
Immunohistochemical Analysis.
Macrophages, LOX-1, MCP-1, Ang (1-7), AT1R, and PCNA were identified by using appropriate primary antibodies (see SI Materials and Methods for details). The identities of cells with positive PCNA staining were determined by double-labeled immunocytochemistry as reported (32).
ELISA.
Protein levels of MCP-1, ICAM-1, and Ang II were measured by ELISA (see SI Materials and Methods for details).
Real-Time RT-PCR.
The mRNA expression of ACE2 was quantitated by RT-PCR (see SI Materials and Methods for details).
Western Blot Analysis.
The protein expression of ACE2, ACE, AngII, AT1R, MCP-1, LOX-1, Ang (1-7), PI3K, AKT, JAK2, and STAT3 was assayed by Western blot analysis (see SI Materials and Methods for details).
Terminal dUTP Nick-End Labeling Staining.
The segments of the abdominal aorta were stained for apoptotic nuclei by using an in situ cell death detection kit as reported (ref. 33; see SI Materials and Methods for details).
Measurement of Endothelial Function.
Endothelium-dependent vasodilator activity was tested in the rabbit atherosclerosis model (see SI Materials and Methods for details).
Proliferation Assay of VSMCs and HUVECs.
Proliferation assay by BrdU incorporation was performed by ELISA (see SI Materials and Methods for details).
Thymidine Incorporation Assay of VSMCs and HUVECs.
Thymidine incorporation assay was conducted to quantitate cell proliferation (ref. 33; see SI Materials and Methods for details).
Migration Assay of VSMCs and HUVECs.
Cell migration was assayed by a modified Boyden's chamber method (see SI Materials and Methods for details).
Tube-like Structure Assay.
Tube-like structure assay was performed to evaluate endothelial cell function (ref. 34; see SI Materials and Methods for details).
Zymography.
The activity of MMP-2 and MMP-9 in VSMCs was evaluated by zymography (see SI Materials and Methods for details).
Quantification of Monocytes Adhering to ECs.
HUVECs were pretreated with Ad-ACE2 or Ad-EGFP at 100 multiplicities of infection and were activated with AngII for 16 h, then with PKH26-labeled THP-1 for 45 min. The percentage of monocytes adhering to HUVECs was correlated with the fluorescence, measured by use of a 544-nm/590-nm filter set.
Measurement of Reactive Oxygen Species (ROS) Levels.
ROS levels in HUVECs were assessed by measuring fluorescence at excitation and emission wavelengths of 490 and 530 nm, respectively (see SI Materials and Methods for details).
Detection of NF-κB p65 activity.
The NF-κB p65 activity in nuclear extracts was detected by using the TransAM-NF-κB p65 Transcription Factor Assay Kit (ref. 35; see SI Materials and Methods for details).
Statistical Analysis.
Data are expressed as mean ± SD. SAS Stat View-J v5.0 (SAS) was used for statistical analysis. One-way ANOVA and Student's t test were used to analyze differences among animal and cell groups. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Qing Tao Yu, Qiu li Dong, Ya Pei Yang, Jing Bo Feng, Xun Qu, Hong Jiang, Rong Wang, Mei Dong, and Xu Ping Wang for their excellent technical assistance. This work was supported by National 973 Basic Research Program of China Grant 2009CB521900, National High-tech Research and Development Program of China Grant 2006AA02A406, Program of Introducing Talents of Discipline to Universities Grant B07035, State Key Program of National Natural Science of China Grant 60831003, National Natural Science Foundation of China Grants 30900607 and 30772810, the Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China Grant 704030, and Shandong Natural Science Foundation of China Grants 03BS37 and Y2006C68. Y.C. is a Chang Jiang Scholar at Shandong University, China.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001253107/-/DCSupplemental.
References
- 1.Tian Y, Jain S, Kelemen SE, Autieri MV. AIF-1 expression regulates endothelial cell activation, signal transduction, and vasculogenesis. Am J Physiol Cell Physiol. 2009;296:C256–C266. doi: 10.1152/ajpcell.00325.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med. 2006;16:25–28. doi: 10.1016/j.tcm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki M, Takai S. [Involvement of angiotensin II in development of atherosclerosis] Nippon Rinsho. 2002;60:1904–1910. [PubMed] [Google Scholar]
- 4.Schieffer B, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: Potential implications for inflammation and plaque instability. Circulation. 2000;101:1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone MT, et al. Angiotensin receptor blockade with candesartan attenuates atherosclerosis, plaque disruption, and macrophage accumulation within the plaque in a rabbit model. Circulation. 2004;110:2060–2065. doi: 10.1161/01.CIR.0000143627.55926.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy JL, Lambert DW, Warner FJ, Hooper NM, Turner AJ. Membrane-associated zinc peptidase families: Comparing ACE and ACE2. Biochim Biophys Acta. 2005;1751:2–8. doi: 10.1016/j.bbapap.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danilczyk U, Eriksson U, Oudit GY, Penninger JM. Physiological roles of angiotensin-converting enzyme 2. Cell Mol Life Sci. 2004;61:2714–2719. doi: 10.1007/s00018-004-4241-6. [DOI] [PubMed] [Google Scholar]
- 8.Zulli A, et al. Immunolocalization of ACE2 and AT2 receptors in rabbit atherosclerotic plaques. J Histochem Cytochem. 2006;54:147–150. doi: 10.1369/jhc.5C6782.2005. [DOI] [PubMed] [Google Scholar]
- 9.Dong B, et al. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1270–1276. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- 10.Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: A novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol. 2006;91:163–198. doi: 10.1016/j.pbiomolbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM. Contribution of angiotensin-(1-7) to cardiovascular physiology and pathology. Curr Hypertens Rep. 2003;5:129–134. doi: 10.1007/s11906-003-0069-y. [DOI] [PubMed] [Google Scholar]
- 12.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira AJ, Santos RA, Almeida AP. Angiotensin-(1-7) improves the post-ischemic function in isolated perfused rat hearts. Braz J Med Biol Res. 2002;35:1083–1090. doi: 10.1590/s0100-879x2002000900009. [DOI] [PubMed] [Google Scholar]
- 14.Dias-Peixoto MF, et al. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension. 2008;52:542–548. doi: 10.1161/HYPERTENSIONAHA.108.114280. [DOI] [PubMed] [Google Scholar]
- 15.Al-Maghrebi M, Benter IF, Diz DI. Endogenous angiotensin-(1-7) reduces cardiac ischemia-induced dysfunction in diabetic hypertensive rats. Pharmacol Res. 2009;59:263–268. doi: 10.1016/j.phrs.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koka V, et al. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172:1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ocaranza MP, et al. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572–578. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- 18.Ferrario CM, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, et al. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu JR, et al. Lercanidipine inhibits vascular smooth muscle cell proliferation and neointimal formation via reducing intracellular reactive oxygen species and inactivating Ras-ERK1/2 signaling. Pharmacol Res. 2009;59:48–56. doi: 10.1016/j.phrs.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Hasenstab D, Lea H, Hart CE, Lok S, Clowes AW. Tissue factor overexpression in rat arterial neointima models thrombosis and progression of advanced atherosclerosis. Circulation. 2000;101:2651–2657. doi: 10.1161/01.cir.101.22.2651. [DOI] [PubMed] [Google Scholar]
- 22.Rauch BH, Müschenborn B, Braun M, Weber AA, Schrör K. ICAM-1 and p38 MAPK mediate fibrinogen-induced migration of human vascular smooth muscle cells. Eur J Pharmacol. 2007;577:54–57. doi: 10.1016/j.ejphar.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 23.Browatzki M, et al. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- 24.Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation. 2003;108:2054–2059. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 25.Bhandarkar SS, et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapidoth M, Ben-Amitai D, Bhandarkar S, Fried L, Arbiser JL. Efficacy of topical application of eosin for ulcerated hemangiomas. J Am Acad Dermatol. 2009;60:350–351. doi: 10.1016/j.jaad.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Perry BN, et al. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol. 2006;126:2316–2322. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- 28.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 29.Ng P, Parks RJ, Cummings DT, Evelegh CM, Graham FL. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Hum Gene Ther. 2000;11:693–699. doi: 10.1089/10430340050015590. [DOI] [PubMed] [Google Scholar]
- 30.Aikawa M, et al. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: A potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- 31.Elased KM, Cunha TS, Gurley SB, Coffman TM, Morris M. New mass spectrometric assay for angiotensin-converting enzyme 2 activity. Hypertension. 2006;47:1010–1017. doi: 10.1161/01.HYP.0000215588.38536.30. [DOI] [PubMed] [Google Scholar]
- 32.Rekhter M, Nicholls S, Ferguson M, Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993;13:609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- 33.Tulis DA, et al. Adenoviral gene transfer of fortilin attenuates neointima formation through suppression of vascular smooth muscle cell proliferation and migration. Circulation. 2003;107:98–105. doi: 10.1161/01.cir.0000047675.86603.eb. [DOI] [PubMed] [Google Scholar]
- 34.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 35.Sun HW, et al. Involvement of integrins, MAPK, and NF-kappaB in regulation of the shear stress-induced MMP-9 expression in endothelial cells. Biochem Biophys Res Commun. 2007;353:152–158. doi: 10.1016/j.bbrc.2006.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.