Abstract
Neutrophil extracellular traps (NETs) are part of the innate immune response to infections. NETs are a meshwork of DNA fibers comprising histones and antimicrobial proteins. Microbes are immobilized in NETs and encounter a locally high and lethal concentration of effector proteins. Recent studies show that NETs are formed inside the vasculature in infections and noninfectious diseases. Here we report that NETs provide a heretofore unrecognized scaffold and stimulus for thrombus formation. NETs perfused with blood caused platelet adhesion, activation, and aggregation. DNase or the anticoagulant heparin dismantled the NET scaffold and prevented thrombus formation. Stimulation of platelets with purified histones was sufficient for aggregation. NETs recruited red blood cells, promoted fibrin deposition, and induced a red thrombus, such as that found in veins. Markers of extracellular DNA traps were detected in a thrombus and plasma of baboons subjected to deep vein thrombosis, an example of inflammation-enhanced thrombosis. Our observations indicate that NETs are a previously unrecognized link between inflammation and thrombosis and may further explain the epidemiological association of infection with thrombosis.
Keywords: neutrophils, platelets, histones, red blood cells, chromatin
Thrombosis depends on the adhesion, activation, and aggregation of platelets (1). RBCs, whose function in thrombosis is not well defined, are especially abundant in venous thrombi. Final thrombus stability requires scaffolding provided by large polymers, such as fibrin and von Willebrand factor (VWF) (2, 3). Thrombus formation can be enhanced by inflammation and endothelial dysfunction (4). Deep vein thrombosis (DVT), which affects over 375,000 patients per year in the United States (5), is often linked to inflammation (6) and infections (7).
In sepsis, neutrophil extracellular traps (NETs) are formed within the vasculature (8). NETs are extracellular DNA fibers comprising histones and neutrophil antimicrobial proteins (9). They are known for their antimicrobial function and have been proven beneficial against infections (10). NETs are formed by a cell-death program, which proceeds from the dissolution of internal membranes followed by chromatin decondensation and cytolysis (11). In vitro, neutrophils, basophils, and mast cells release extracellular DNA traps (9, 12, 13) in response to microbial and inflammatory stimuli, like IL-8 and reactive oxygen species (12–14). Interestingly, extracellular DNA traps are also observed in inflammatory but noninfectious diseases, like pre-eclampsia (15) or small-vessel vasculitis (16).
Here, we show that extracellular DNA traps are a unique link between inflammation and thrombosis. Extracellular DNA traps provide a stimulus and scaffold for thrombus formation and markers of extracellular DNA traps are abundant in DVT.
Results
NETs Provide a Scaffold and Stimulus for Platelet Binding and Aggregation.
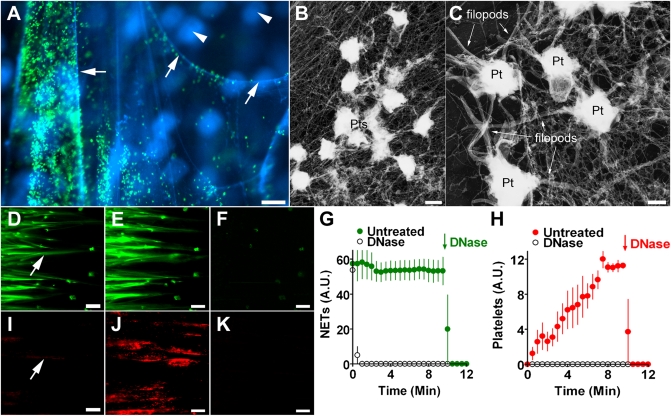
We used extracellular traps released from neutrophils as a model to study their interaction with blood. We perfused NETs with platelets suspended in plasma and observed 3D NETs with avidly adhering platelets (Fig. 1A). Electron micrographs showed platelet accumulation on a fibrous meshwork of NETs (Fig. 1B) and filopod formation indicated that platelets adherent on NETs were activated (Fig. 1C). Perfusion of NETs with anticoagulated blood at high shear rates (900/s) (Fig. 1 D–K and Movie S1) or low, typically venous shear rates (200/s) (Movie S2) resulted in time-dependent platelet aggregation. Strings of NETs aligned (Fig. 1 D and E) in the direction of flow and, importantly, NETs were not a static surface but moved in three dimensions (Movie S1). Within 1 min from onset of perfusion, small platelet aggregates appeared on NETs (Fig. 1 D and I, arrows). Platelet adhesion and aggregation on NETs increased over the next 9 min (Fig. 1 E and J). DNase treatment simultaneously removed NETs and platelets, indicating that platelets were indeed attached to NETs (Fig. 1 F and K, and Movie S1). Quantification showed that areas covered by NETs were constant (Fig. 1G), whereas platelets adhered and aggregated in a time-dependent manner (Fig. 1H). Both platelet aggregates and NETs were removed by DNase (Fig. 1 G and H). When blood was supplemented with DNase at the beginning of the perfusion, NETs were degraded rapidly (Fig. 1G) and platelet aggregates did not form (Fig. 1H). Thus, NETs were the only prothrombotic substrate in these experiments.
Fig. 1.
NETs provide a scaffold for platelet adhesion and aggregation. (A) Platelets (green) bound to NETs (blue, arrows). Neutrophils (blue, arrowheads) were out of focus and did not bind platelets. (Scale bar, 20 μm.) (B) Electron micrograph of platelets (Pts) attached to a fibrous meshwork of NETs. (Scale bar, 1 μm.) (C) Numerous filopods indicated that platelets (Pt) on NETs were activated. (Scale bar, 0.5 μm.) (D–K) Time-course of platelet adhesion and aggregation on NETs. (Scale bars, 100 μm.) NETs (D and E, green) were perfused with platelets (I and J, red) in whole blood. The flow direction was from left to right. Images showed NETs and platelets after 1 min (arrows in D and I) and 10 min (E, J) of perfusion. DNase added to blood after 10 min digested NETs (F) and removed platelets (K), indicating that platelets were attached to NETs. Quantification of NETs (G) or platelets (H) in the presence (open circles) or absence of DNase (closed circles). DNase was added to untreated samples after 10 min (arrow). DNase removes NETs and inhibits platelet aggregation. A.U., arbitrary units. Data presented are representative of at least three independent experiments and are shown as mean ± SEM, n = 3.
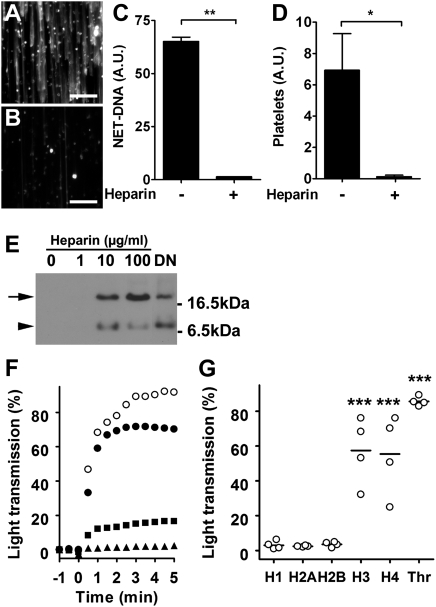
When we tested heparin, a common anticoagulant, on NET-induced platelet binding and aggregation, we observed that NETs were almost completely dismantled after perfusion with heparinized blood (Fig. 2 A–C). In addition, heparin removed platelet aggregates from NETs (Fig. 2D) as efficiently as DNase. The effect of heparin was also observed in medium, indicating a direct interaction of heparin with the NETs (Movie S3). Heparin has high affinity for histones (17) and releases histones from chromatin (18). Consequently, incubation of NETs under static conditions with heparin or DNase alone released histones to the culture supernatant (Fig. 2E). This result indicates that heparin removes histones from the chromatin fibers that built the backbone of NETs and this leads to the destabilization of NETs.
Fig. 2.
Heparin dismantles NETs and prevents histone induced platelet aggregation. SytoxGreen staining of NETs perfused for 10 min with blood in the absence (A) or presence (B) of heparin. (Scale bars, 100 μm.) (C) Quantification of NETs after 10 min perfusion with normal (-) or heparinized (+) blood. (D) Quantification of platelets on NETs perfused for 10 min with blood before (-) and after (+) treatment with heparin. Data presented as mean ± SEM, n = 3; (Student's t test; *P < 0.05; **P < 0.01). (E) Heparin and DNase released histones from NETs. Immunodetection of histone H2B (arrow) in the culture supernatants of NETs treated with heparin or DNase (DN). A second band (arrowhead) may represent cross reactivity of the antibody or a proteolytic product. Data presented are representative of three independent experiments. (F) Aggregometry of platelets stimulated with thrombin (open circles) or human recombinant histone H3 (solid circles). EDTA (solid squares) and heparin (solid triangles) inhibited platelet aggregation by histone H3. (G) Extent of platelet aggregation 3 min after addition of histones 1H, H2A, H2B, H3, or H4, or thrombin (Thr). Histones H3 and H4, and thrombin induced aggregation of platelets obtained from four different donors. (ANOVA; ***P < 0.001 compared with histone 1H).
We tested whether platelets could interact directly with histones. Interestingly, histones were sufficient to induce platelet aggregation. Incubation of platelets with histones H3 and H4 stimulated aggregation (Fig. 2 F and G), whereas histones 1H, H2A, and H2B had no such effect (Fig. 2G). Thrombin served as a positive control in these experiments (Fig. 2 F and G). Aggregation in response to histone H3 (Fig. 2F) and H4 (Fig. S1) was inhibited by EDTA, which excluded platelet agglutination caused by the positive charge of histones. Heparin completely abolished platelet response to these histones (Fig. 2F and Fig. S1). Dissociating NETs and inhibiting histones could add to the antithrombotic effects of heparin.
NETs Induce the Formation of a RBC-Rich Thrombus.
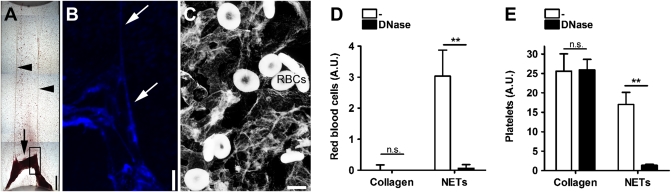
We washed NETs after perfusion with blood and observed macroscopically a red thrombus (Fig. 3A). DNA staining revealed a scaffold of DNA (Fig. 3B) and electron microscopy showed the presence of intact RBCs (Fig. 3C). Quantification of RBC hemoglobin in flow chambers coated with NETs or collagen showed that RBCs bound to NETs but not collagen (Fig. 3D), although platelets bound to both substrates (Fig. 3E). RBC adhesion to NETs was prevented when blood was supplemented with DNase, confirming that RBCs were attached to NETs. DNase had no effect on platelet adhesion to collagen-coated chambers, but prevented platelet adhesion to NETs (Fig. 3E). In summary, these data show that NETs provide a scaffold not only for platelets but also for RBC adhesion.
Fig. 3.
NETs provide a scaffold for RBC-rich thrombi. (A) Flow chamber coated with NETs after perfusion with blood. Light microscopy of a red thrombus (arrow) anchored on two strings (arrowheads). Figure is a composite of multiple photographs of the flow chamber. (Scale bar, 500 μm.) (B) DNA staining of thrombus (rectangle in A). Strings of DNA are seen in the thrombus (arrows). (Scale bar, 250 μm.) (C) Electron microscopy shows individual RBCs attached to NETs. (Scale bar, 5 μm.) Quantification of RBCs (D) or platelets (E) on collagen or NETs. Coated chambers were perfused with blood supplemented with DNase or not (-). (D) RBCs were detected in NETs- but not in collagen-coated flow chambers. Adhesion of RBCs to NETs could be prevented if blood was supplemented with DNase. (E) Addition of DNase had no effect on platelet adhesion to collagen but blocked platelet adhesion to NETs. A.U. arbitrary units; n.s. not significant. Data presented are representative of at least three independent experiments and presented as mean ± SEM, n = 3; (ANOVA; **P < 0.01).
NETs Bind Plasma Proteins Important for Thrombus Stability.
We questioned whether NETs can concentrate plasma proteins that promote and stabilize thrombi (19). Immunocytochemistry of NETs incubated with plasma showed that VWF and fibronectin, as well as fibrinogen, bound to NETs (Fig. S2). These findings are corroborated by previous reports that VWF and fibrinogen interact with histones (20, 21) and that fibronectin bears a DNA-binding domain (22). We then studied whether the interaction of fibrinogen with NETs could promote fibrin deposition. NETs were perfused with recalcified blood supplemented with fluorescent fibrinogen (Fig. S3A). We were able to detect fibrinogen along NET-DNA strings and the deposition drastically increased with perfusion time until the large fluorescent clot “embolized” together with the NETs. We inhibited thrombin in parallel samples to prevent fibrinogen conversion to fibrin and polymerization. Under these conditions, just traces of fibrinogen were found on NETs and NETs remained stable during the entire perfusion period (Fig. S3B). Taken together, these experiments show that NETs support platelet-adhesion molecule deposition and thrombin-dependent fibrin formation.
We compared the susceptibility of NETs and fibrin as scaffolds for blood clots to thrombolysis (Fig. S4). Therefore, we incubated neutrophils prestimulated with platelet-activating factor to release NETs with recalcified blood under stirring conditions (23). A single clot in which DNA intercalated with fibrin formed under these conditions (Fig. S4 D and G). Samples were treated with DNase to digest NETs or tissue plasminogen activator (tPA) for fibrin digestion. The tPA removed fibrin but did not prevent clot formation. In tPA-resistant clots, RBCs and platelets were held together by a DNA scaffold of NETs (Fig. S4F). Consequently, clot formation in the presence of activated neutrophils could be prevented only by simultaneous treatment with tPA and DNase. Thus, NETs may provide a clot scaffold independent from fibrin.
Extracellular DNA Traps Are Present in Baboon DVT.
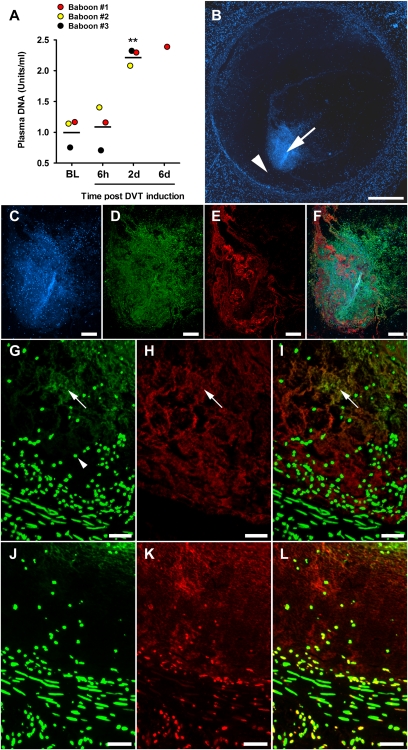
Red thrombi, as well as leukocyte recruitment, are characteristics of DVT (6). Thus, we investigated whether NETs are formed in experimental DVT in baboons. In brief, anesthetized juvenile male baboons underwent iliac vein thrombosis by temporary balloon catheter occlusion (6 h) as previously described (24). Plasma was collected before and during DVT and analyzed for circulating DNA (Fig. 4A), a marker of intravascular NET formation in sepsis (25). Plasma DNA levels were low before (Fig. 4A, BL) and after the 6 h-DVT induction (Fig. 4A, 6h); thus, the surgical procedure did not increase this marker. Elevated plasma DNA levels were detected 2 d after thrombus induction and remained increased at 6 d postinduction (Fig. 4A, 2d and 6d). It is interesting that the kinetics of the appearance of the fibrin degradation product D-dimer in plasma of baboons subjected to the same model is very similar (24).
Fig. 4.
Markers of NETs are abundant in baboon DVT. (A) Plasma DNA levels before (baseline: BL) or at indicated time points after initiation of DVT. Plasma DNA was elevated after 48 h (bar represents the mean value of groups; n = 3; Repeated measures ANOVA; **P < 0.01 compared with BL) and remained increased in baboon #1 at day 6. (B) Cross-section of thrombotic left iliac vein at day 6 stained for DNA. Figure is a composite of multiple micrographs of the thrombus. (Scale bar, = 500 μm.) (C–F) Immunostaining of the DNA core (arrow in B). (C) Staining for DNA shows a distinct dotted pattern indicating nuclei, as well as a diffuse pattern indicating extracellular DNA. (D) Positive staining for the DNA/H2A/H2B complex reveals that the extracellular DNA is of nuclear origin. (E) VWF strings are part of the DNA core. (F) Overlay of C, D, and E. (Scale bars C–F, 100 μm.) (G, H, and I) Immunostaining of area between DNA core and vessel wall (arrowhead in B). (G) DNA by SytoxGreen indicates nuclei (arrowhead) as well as extracellular DNA (arrow). (H) VWF in this area often colocalizes with extracellular DNA (arrow). (I) Overlay of G and H. (Scale bars G–I, 50 μm.) (J) Nuclei stained by SytoxGreen in an area free of extracellular DNA (arrowhead in B). (K) Extracellular histone H3 is abundantly present in this area although DNA is no longer detected. (L) Overlay of J and K. (Scale bars J–L, 20 μm.)
Six days postthrombosis, the affected iliac vein (including thrombus) and the contralateral unaffected iliac vein (control/without thrombus) were dissected. DNA staining of the thrombosed iliac vein showed the circular vessel wall and within the lumen a dispersed punctuate staining, indicating nuclei from leukocytes as well as a dense DNA core (Fig. 4B, arrow). This image comprised two distinct DNA patterns: the dotted staining of nuclei and a diffuse staining of extracellular DNA, reminiscent of extracellular traps (Fig. 4C). Positive staining using an antibody specific for DNA/histone complex showed that the DNA was of nuclear rather than mitochondrial origin (26) (Fig. 4D). Although we suspect that extracellular DNA came from neutrophils, we cannot exclude participation of other leukocytes as well (12, 13). Immunolocalization of VWF revealed abundant VWF strings within the DNA core (Fig. 4 E and F) and in the area between the DNA core and the vessel wall [Fig. 4 B (arrowhead) and H]. The DNA pattern often overlapped with that of VWF (Fig. 4 G and I). As a control, we analyzed the right iliac vein from the same baboon. No indications of extracellular DNA traps were observed in this tissue (Fig. S5). Areas within the thrombus lacking visible extracellular DNA (Fig. 4J) were abundant in histones (Fig. 4 K and L), indicating the degradation of extracellular DNA presumably by nucleases in plasma (27). In summary, markers of extracellular DNA traps are present in plasma and within the thrombus of baboons subjected to DVT.
Discussion
Inhibition of leukocyte infiltration in the baboon model of DVT produces unstable thrombi (24). One way leukocytes may promote thrombus stability is by producing NETs. Our results show that NETs colocalize with fibrin in vitro (Fig. S3 and Fig. S4), and it is conceivable that NETs interact closely with fibrin strands in the thrombus, thus potentially influencing thrombus organization and stability. Given the procoagulant activity of nucleic acids (28) and polyphosphates (29), future studies should address whether NETs promote coagulation, how NETs affect the mechanical properties of fibrin (2), and the susceptibility of clots to thrombolysis.
Histones in NETs or liberated after digestion of NET-DNA could also provide a stimulus for platelet aggregation. Our findings are corroborated by a recently published work showing microthrombi in lungs of mice infused with histones and a prominent role for histones in the pathology of sepsis (30). In humans, platelets and RBCs are the only blood cells lacking histones, which may help to prevent excessive thrombus propagation.
At present, the signaling mechanisms underlying induction of NETs in DVT are not understood. Ischemia results in the production of IL-8 (31) and reactive oxygen species (32). IL-8 is capable of inducing NETs (9) and is considered a risk factor for venous thrombosis (33). In vitro stimulation of neutrophils with exogenous reactive oxygen species is sufficient to induce NETs (11). Clinically, inflammation and infection are linked to thrombosis (7, 34) and it is conceivable that NETs contribute to this linkage. Mechanistically, NETs provide a scaffold for platelet and RBC adhesion and concentrate effector proteins involved in thrombosis. The interaction with NETs could be mediated in multiple ways. Platelet-binding to NETs could be accomplished via VWF, fibronectin, or fibrinogen immobilized on NETs. Alternatively, platelets could directly interact with DNA/histones in NETs. DNA has been detected on the cell surface of platelets from patients with systemic lupus erythematosus (35). Lupus patients are prone to develop venous thrombosis (6) and were recently described to have impaired NET degradation (36).
Adhesion of platelets and RBCs to NETs may be helped by electrostatic interaction of the negatively charged cells with positively charged histones in NETs (37). RBC adhesion to NETs could also play a role in sickle-cell disease, where a lethal crisis is often precipitated by infection (38).
In an infected wound, the synergy of antimicrobial and prothrombotic functions of NETs might be valuable to prevent sepsis and maintain hemostasis. In pathological leukocyte activation, targeting of the extracellular DNA and histones in NETs may prove beneficial to prevent thrombosis.
Materials and Methods
Isolation of Platelets and Neutrophils from Human Blood.
The investigation conforms to the principles outlined in the Declaration of Helsinki and received approval from the Immune Disease Institute Institutional Review Board. After explaining the nature and possible consequences of the study, we obtained informed consent from all donors. Blood donors were healthy and had not taken any medication for at least 10 d. Platelets and neutrophils were prepared from ACD-blood (39) or EDTA-blood (11), respectively.
Perfusions of NETs.
Neutrophils were seeded into flow chambers (μ-Slide IV, IBIDI) at 0.5 to 1 × 107 cells/mL. NET formation by the majority of cells was induced by phorbol 12-myristate 13-acetate (PMA, 50 nM, 4 h; Sigma Aldrich) or glucose oxidase (GO, 1 U/mL, 4 h; Worthington Biochem), as previously described (11). PMA-induced NETs were used for initial observations (Fig. 1 A–C). Other experiments were done with GO-induced NETs. NETs were washed and blocked with 1% BSA (Calbiochem). NET-DNA was stained with Hoechst 33342 (1 μg/mL; Invitrogen) or SytoxGreen (1 μM, Invitrogen). Washed platelets were loaded with fluorescent Calcein-AM (2.5 μg/mL, 10 min, 37 °C; Invitrogen) or platelets in whole blood were labeled with Rhodamine 6G (5 μg/mL, 10 min, 37 °C; Sigma). NETs were perfused with ACD-anticoagulated blood supplemented with the irreversible thrombin inhibitor PPACK-Dihydrochloride (100 μM; Calbiochem) and recalcified by addition of 2 mM CaCl2. Perfusions were carried out at a shear rate of 200/s or 900/s and 37 °C using a peristaltic pump. If indicated, blood was supplemented with DNase1 (100 U/mL; Worthington Biochem) or unfractionated heparin (100 μg/mL; Sigma). To analyze fibrin deposition, we supplemented blood with fluorescent fibrinogen (100 μg/mL; Invitrogen) and 20 mM CaCl2 at the beginning of the perfusions.
Immunoblotting.
For immunoblotting, 1 × 106 neutrophils/mL were stimulated with GO to release NETs, washed, and incubated with heparin (Sigma) or DNase1 (1 U/mL; Worthington Biochem) for 30 min. Culture supernatants were collected and centrifuged for 5 min at 10,000 × g. Aliquots were mixed with SDS reducing sample buffer. After 3 min at 95 °C, samples were subjected to 15% (wt/vol) SDS/PAGE, followed by an immunoblotting procedure as described (18). We used a polyclonal rabbit antihistone H2B (Abcam) as primary antibody and goat-anti-rabbit-IgG conjugated to horseradish peroxidase (Biorad) as secondary antibody. Detection was carried out with a Pierce ECL Western blotting substrate (Thermo Scientific).
Aggregometry.
Platelet aggregation was determined by an optical aggregation system (Chrono-Log). Washed platelets were resuspended in Tyrode's-hepes buffer containing 1 mM CaCl2 to a concentration of 1.5 × 108 platelets per milliliter. Recombinant human histones (New England Biolabs) or thrombin were added at a final concentration of 5 μg/mL and 0.5 U/mL, respectively. If indicated, 10 μg/mL heparin (Sigma) or 5 mM EDTA was added.
Fluorescence Microscopy.
Fluorescent images were acquired by a Zeiss Axiovert 200 inverted fluorescence microscope in conjunction with a monocrom camera (AxioCam MRm). Colors for fluorescence channels were assigned using Axiovision software. Fluorescent areas in images were quantified using ImageJ software.
Electron Microscopy.
We induced NETs on glass coverslips and perfusion was performed using a parallel-plate flow chamber system (Glycotech). NETs were washed and fixed with 2.5% glutaraldehyde and electron microscopy was performed as previously described (40).
Quantification of RBCs and Platelets.
Cells firmly attached to collagen or NETs were lysed with 100 μL of 0.5% Triton ×100 in water. Hemoglobin content was measured using the method of Drabkin (41). To quantify platelets, Rhodamine-6G fluorescence of the sample was analyzed using a fluorometer.
Plasma Coating of NETs.
Human NETs were induced on glass coverslips or in tissue-culture plates by stimulating cells with PMA (50 nM, 4 h). After the activation, NETs were incubated with 1% BSA for 1 h at 37 °C. NETs were washed and incubated with 50% plasma in PBS for 30 min at 37 °C. Next, NETs were washed and treated with DNase1 (100 U/mL, 10 min). After another wash, cells were fixed with paraformaldehyde (2%, 1 h at 37 °C) and unspecific binding sites were blocked with BSA (3%, 1 h at 37 °C). To visualize and quantify binding of VWF, fibrinogen, or fibronectin to NETs, samples were subjected to immunocytochemistry. Primary antibodies were used at 1 μg/mL in PBS supplemented with 1% BSA and 0.1% Triton ×100 [mouse-antifibrinogen, rabbit-antifibronectin (both Sigma); rabbit-anti-VWF, (Chemicon)]. After incubation at 37 °C for 1 h, samples were washed with PBS and fluorescently conjugated secondary antibodies (Invitrogen) were applied at 10 μg/mL for 30 min at 37 °C. NET-DNA was stained with 1 μg/mL Hoechst 33258 (Invitrogen) for 15 min at 37 °C. Coverslips were mounted on slides and analyzed by fluorescence microscopy. DNA and Alexa 488 fluorescence was quantified for samples in tissue culture using a fluorescence microplate reader.
Clot Lysis Studies.
Two-hundred-fifty microliters of 5 × 106 neutrophils per milliliter in RPMI medium were activated by platelet activating factor (50 μM, Calbiochem) at 37 °C under static conditions to induce NET formation. Control samples were 250 μL medium alone, 250 μL of unstimulated neutrophils, or 250 μL of 50 μM PAF in medium. After 10 min, 250 μL of recalcified (40 mM CaCl2) ACD anticoagulated blood was added. The mixture was incubated under stirring conditions (1,000 rpm) at 37 °C using an Eppendorf Thermomixer. Indicated samples were supplemented with tPA (25 μg/mL, Baxter) or DNase1 (100 U/mL, Worthington Biochem). After 20 min, the blood was passed through a 100-μm cell strainer to isolate the clot. Images were acquired and the clot was determined. Thereafter, 10-μm frozen sections of the clot were prepared and stained for fibrinogen (mouse-antihuman-fibrinogen, Sigma) and VWF (rabbit-antihuman-VWF, Chemicon). Isotype control antibodies were used to determine background staining and DNA was labeled fluorescently by Hoechst 33258 (Invitrogen).
Baboon DVT model.
Juvenile male baboons were anesthetized and underwent iliac vein thrombosis by temporary balloon catheter occlusion (6 h) (24). Six days postthrombosis, the animal was humanely killed and both the thrombosed and nonthrombosed iliac veins were harvested. The iliac vein samples were then fixed and paraffin-embedded for immunohistochemical analysis. The animal research protocol was approved by the University of Michigan and its Committee on Use and Care of Animals.
Baboon Plasma Analysis.
Blood was drawn from the left iliac vein using a 22-G vacutainer needle with a 4.5-mL sodium citrate vacutainer. After centrifugation at 3,350 × g for 15 min at room temperature, aliquots were flash-frozen in liquid nitrogen and stored in −80 °C. Time-points included: before (baseline), 6 h, 2 d, and 6 d postthrombus induction. We diluted plasma 10-fold in PBS and mixed diluted plasma with an equal volume of 1 μM of the fluorescent DNA dye SytoxGreen (Invitrogen) in PBS. Fluorescence was determined by a fluorescence microplate reader (Fluoroskan, Thermo Scientific). Samples were normalized to the mean of values obtained from plasma collected before induction of DVT (baseline).
Immunohistochemistry.
We analyzed baboon DVT using a blood vessel staining kit (Millipore). We followed the manufacturer's instructions, but replaced the antibodies. We used the following primary antibodies: mouse-antihistone H2A/H2B/DNA complex (42), rabbit-anti-VWF (Chemicon), and rabbit-antihistone H3 (Abcam). Primary and isotype control antibodies were employed at 1 μg/mL; fluorescently conjugated secondary antibodies (Invitrogen) at 10 μg/mL DNA was labeled with Hoechst 33342 (1 μg/mL, Invitrogen), or SytoxGreen (1 μM, Invitrogen).
Statistical Evaluation.
Statistical analysis included mean ± SEM, ANOVA, Student's t test, and repeated measures ANOVA. Results were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Drs. Richard Hynes and Bernhard Lämmle for critical reading of the manuscript and helpful discussions, and Lesley Cowan for help preparing the manuscript. This research was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health Grants P01 HL056949 (to D.D.W. and J.H.H.), R01 HL095091 (to T.W.W. and D.D.W.), and R01 HL070766 (to T.W.W.), and a fellowship from the Deutsche Forschungsgemeinschaft, Germany (FU 742/1-1) (to T.A.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005743107/-/DCSupplemental.
References
- 1.Denis CV, Wagner DD. Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:728–739. doi: 10.1161/01.ATV.0000259359.52265.62. [DOI] [PubMed] [Google Scholar]
- 2.Lord ST. Fibrinogen and fibrin: Scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14:236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120(Suppl 1):S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heit JA. Venous thromboembolism: Disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–1617. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 6.Esmon CT. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. 2009;23:225–229. doi: 10.1016/j.blre.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smeeth L, et al. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 8.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan JT, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Köckritz-Blickwede M, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 13.Yousefi S, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann V, Zychlinsky A. Beneficial suicide: Why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal PK, Starr T, Gertler MM. Neutralization of heparin by histone and its subfractions. Thromb Res. 1983;31(1):69–79. doi: 10.1016/0049-3848(83)90008-7. [DOI] [PubMed] [Google Scholar]
- 18.Napirei M, Ludwig S, Mezrhab J, Klöckl T, Mannherz HG. Murine serum nucleases—contrasting effects of plasmin and heparin on the activities of DNase1 and DNase1-like 3 (DNase1l3) FEBS J. 2009;276:1059–1073. doi: 10.1111/j.1742-4658.2008.06849.x. [DOI] [PubMed] [Google Scholar]
- 19.Frenette PS, Wagner DD. Adhesion molecules—Part II: Blood vessels and blood cells. N Engl J Med. 1996;335(1):43–45. doi: 10.1056/NEJM199607043350108. [DOI] [PubMed] [Google Scholar]
- 20.Ward CM, Tetaz TJ, Andrews RK, Berndt MC. Binding of the von Willebrand factor A1 domain to histone. Thromb Res. 1997;86:469–477. doi: 10.1016/s0049-3848(97)00096-0. [DOI] [PubMed] [Google Scholar]
- 21.Gonias SL, Pasqua JJ, Greenberg C, Pizzo SV. Precipitation of fibrinogen, fibrinogen degradation products and fibrin monomer by histone H3. Thromb Res. 1985;39(1):97–116. doi: 10.1016/0049-3848(85)90125-2. [DOI] [PubMed] [Google Scholar]
- 22.Pande H, Calaycay J, Hawke D, Ben-Avram CM, Shively JE. Primary structure of a glycosylated DNA-binding domain in human plasma fibronectin. J Biol Chem. 1985;260:2301–2306. [PubMed] [Google Scholar]
- 23.Yost CC, et al. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neonates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier TR, et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99:343–351. doi: 10.1160/TH07-10-0608. [DOI] [PubMed] [Google Scholar]
- 25.Margraf S, et al. Neutrophil-derived circulating free DNA (cf-DNA/NETs): A potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30:352–358. doi: 10.1097/SHK.0b013e31816a6bb1. [DOI] [PubMed] [Google Scholar]
- 26.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 27.von Köckritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114:5245–5246. doi: 10.1182/blood-2009-08-240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannemeier C, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci USA. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller F, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakurum M, et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994;93:1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 33.van Aken BE, Reitsma PH, Rosendaal FR. Interleukin 8 and venous thrombosis: Evidence for a role of inflammation in thrombosis. Br J Haematol. 2002;116(1):173–177. doi: 10.1046/j.1365-2141.2002.03245.x. [DOI] [PubMed] [Google Scholar]
- 34.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25:1321–1324. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 35.Frampton G, Perl S, Bennett A, Cameron JS. Platelet-associated DNA and anti-DNA antibody in systemic lupus erythematosus with nephritis. Clin Exp Immunol. 1986;63:621–628. [PMC free article] [PubMed] [Google Scholar]
- 36.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson K, Gooderham NJ, Davies DS, Edwards RJ. Nucleosomes bind to cell surface proteoglycans. J Biol Chem. 1999;274:21707–21713. doi: 10.1074/jbc.274.31.21707. [DOI] [PubMed] [Google Scholar]
- 38.Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: A review. Int J Infect Dis. 2010;14(1):e2–e12. doi: 10.1016/j.ijid.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Brill A, et al. Oxidative stress activates ADAM17/TACE and induces its target receptor shedding in platelets in a p38-dependent fashion. Cardiovasc Res. 2009;84(1):137–144. doi: 10.1093/cvr/cvp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drabkin DL. Heme binding and transport—a spectrophotometric study of plasma glycoglobulin hemochromogens. Proc Natl Acad Sci USA. 1971;68:609–613. doi: 10.1073/pnas.68.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(-)+/+ mouse. Oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.