Abstract
Homozygous mutations in SLC4A4, encoding the electrogenic Na+-HCO3− cotransporter NBCe1, have been known to cause proximal renal tubular acidosis (pRTA) and ocular abnormalities. In this study, we report two sisters with pRTA, ocular abnormalities, and hemiplegic migraine. Genetic analysis ruled out pathological mutations in the known genes for familial hemiplegic migraine, but identified a homozygous 65-bp deletion (Δ65bp) in the C terminus of NBCe1, corresponding to the codon change S982NfsX4. Several heterozygous members of this family also presented glaucoma and migraine with or without aura. Despite the normal electrogenic activity in Xenopus oocytes, the Δ65bp mutant showed almost no transport activity due to a predominant cytosolic retention in mammalian cells. Furthermore, coexpression experiments uncovered a dominant negative effect of the mutant through hetero-oligomer formation with wild-type NBCe1. Among other pRTA pedigrees with different NBCe1 mutations, we identified four additional homozygous patients with migraine. The immunohistological and functional analyses of these mutants demonstrate that the near total loss of NBCe1 activity in astrocytes can cause migraine potentially through dysregulation of synaptic pH.
Keywords: SLC4A4, glaucoma, epilepsy, proximal renal tubular acidosis, dominant negative effect
Migraine is a common, disabling, multifactorial disorder, affecting more than 10% of the population (1). Although genetic factors certainly play a role, a Mendelian type of inheritance has been established only in familial hemiplegic migraine (FHM). This rare autosomal dominant subtype of migraine with aura is genetically heterogeneous. Thus far, mutations have been found in three different genes: CACNA1A encoding the α1 subunit of voltage-gated neuronal Cav2.1 calcium channels (2), ATP1A2 encoding the α2 subunit of the Na+/K+ pump (3), and SCN1A encoding the neuronal voltage-gated sodium channel Nav1.1 (4). Functional alterations of these cation transporters are thought to cause migraine by enhancing neuronal excitability (5, 6).
Several acid/base transporters may also affect neuronal excitability by regulating the local pH in the brain (7). Among them, the electrogenic Na+-HCO3− cotransporter NBCe1, encoded by SLC4A4, has two N-terminally spliced variants, NBCe1A and NBCe1B, and one C-terminally spliced variant, NBCe1C (8–10). Whereas NBCe1A is predominantly expressed in the kidney, the expression of NBCe1C is almost limited to the brain (8, 10). On the other hand, NBCe1B is expressed in several tissues including pancreas, eye, and brain, and its transport activity in astrocytes is thought to modulate neuronal excitability by regulating local pH (7, 11, 12). Homozygous inactivating mutations in SLC4A4 cause proximal renal tubular acidosis (pRTA) and ocular abnormalities, invariably associated with short stature (13–18). In this study, we found a previously undescribed homozygous C-terminal mutation in SLC4A4 in two sisters who presented normal stature, relatively mild pRTA, and severe ocular abnormalities. Both of them had hemiplegic migraine, but had no pathological mutations in CACNA1A, ATP1A2, or SCN1A genes. In addition, several heterozygous members of this family presented with glaucoma and migraine with or without aura. This mutant showed a predominant cytosolic retention and failed to induce a functional activity in mammalian cells, supporting the pathogenicity of this mutation. Furthermore, coexpression experiments identified a dominant negative effect of the mutant protein through hetero-oligomer formation with the wild-type protein, which could explain the occurrence of migraine and glaucoma in heterozygous members of the pedigree. Among the other pRTA pedigrees previously reported (13–18), we identified four additional homozygous SLC4A4 mutations associated with migraine: hemiplegic migraine with episodic ataxia in L522P, migraine with aura in Δ2311A, and migraine without aura in R510H and R881C. From the immunohistological and functional analyses of the corresponding NBCe1B mutants, we propose that the near total loss of NBCe1B activity in astrocytes can cause migraine through dysregulation of the synaptic pH.
Results
Clinical Phenotypes Caused by a Δ65bp NBCe1 Mutation.
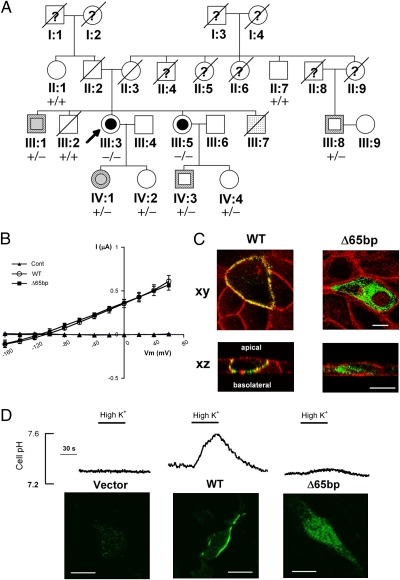
The proband (III: 3) was 50 y old and of normal intelligence and stature (Fig. 1A). She had bilateral high-tension glaucoma, band keratopathy, cataract, and mild pRTA (blood HCO3− 17.3 mmol/L). She had five episodes of hemiplegic migraine, which typically started with a transient left sensorimotor deficit lasting around 20 min, followed by a severe hemicranial headache lasting hours to days, accompanied by photo-, sono-, and kinesiophobia, nausea, and vomiting. At the age of 46, she had a first episode of complex partial status epilepticus, characterized by unresponsiveness, left hemiparesis, and continuous ictal epileptic activity over the right posterotemporal and occipital regions. With antiepileptic drugs she made a full recovery over 10 d. MRI of the brain was normal. She developed a difficult-to-treat migraleptic (migraine-associated epileptic seizures) condition. Sequence analysis of her NBCe1 cDNA and genomic DNA revealed a unique, homozygous 65-bp deletion (Δ65bp) in the C-terminal region, which was not found in 160 control subjects (Fig. S1). The amino acids substitutions due to a frameshift in exon 23 introduce a premature stop codon for both NBCe1A (S982NfsX4) and NBCe1B (S1026NfsX4), yielding the mutant proteins with 51 fewer amino acids than the wild-type proteins. On the other hand, this mutation abolishes the translation of NBCe1C, the C-terminal variant skipping exon 24 (10). For simplicity, we hereafter designate other SLC4A4 mutations according to the corresponding codon numbers for NBCe1A throughout the text. Analysis of her genomic DNA ruled out pathological mutations in the three genes (CACNA1A, ATP1A2, and SCN1A) linked to FHM (2–4). The younger sister of the proband (III: 5), who was 42 y old and of normal intelligence and stature, was also homozygous for this mutation. She had bilateral high-tension glaucoma, cataracts, and pRTA (blood HCO3− 14.8 mmol/L). Since the age of 15 she had had episodes of hemiplegic migraine, two of which had evolved into migraine stupor lasting 2–3 days. MRI of the brain was normal. pRTA was not found in other members of this pedigree. Among six heterozygous members, one had migraine with visual aura, one had migraine without aura, two had normal-tension glaucoma in advanced stages, and two had normal-tension glaucoma in early stages. Members without the Δ65bp mutation had none of these phenotypes.
Fig. 1.
Identification and characterization of the NBCe1 Δ65bp mutation. (A) The pedigree. Arrow: proband; squares: men; circles: women; slashes: deceased. Inner symbols: black—pRTA with hemiplegic migraine; gray—migraine with or without aura; dotted—convulsions; white—normal. Symbol borders: white—high-tension glaucoma; hatched—normal-tension glaucoma. −/−: homozygous mutation; +/−: heterozygous mutation; +/+: no mutation; question marks: no history available. (B) Current (I)/voltage (Vm) relationship of the NBCe1A constructs expressed in Xenopus oocytes. The data represent the mean ± SEM (n = 8 for each construct) obtained for wild-type NBCe1A (WT), the Δ65bp mutant (Δ65bp), or the H2O injected Control (Cont). (C) Confocal images of GFP-tagged wild-type NBCe1A (WT) or the Δ65bp mutant in polarized MDCK cells. Green: GFP fluorescence; red: actin cytoskeletons; xy: front view; xz: side view. Scale bars, 10 μm. (D) The NBCe1B activity in C6 cells, analyzed by cell pH responses to high K+ (20 mM) solution. Confocal images of NBCe1B localization are at the bottom. (Scale bars, 10 μm.)
Δ65bp Mutation Causes Cytosolic Retention of NBCe1.
We first performed functional analysis in Xenopus oocytes, which are suitable for the evaluation of the electrophysiologic properties of NBCe1 (8, 16). We found that the Δ65bp mutation did not change the NBCe1A activity (Fig. 1B). In confluent Madin-Darby canine kidney (MDCK) cells, which are suitable for the evaluation of NBCe1 trafficking in the polarized epithelia (18), the wild-type NBCe1A was predominantly expressed in the basolateral membrane as reported (18). By contrast, the Δ65bp NBCe1A mutant was predominantly retained in the cytoplasmic region (Fig. 1C), supporting the pathogenicity of this mutation.
NBCe1B-mediated HCO3− uptake into astrocytes may play a key role in preventing neuronal hyperexcitability (7, 19), suggesting a causal relationship between NBCe1B inactivation and migraine. Accordingly, we performed functional analysis in C6 glioma cells, which retain several properties of astrocytes but lack the endogenous NBCe1 activity (19). In stably expressed cells, wild-type NBCe1B showed a definite membrane localization and induced a prompt pHi increase in response to a high K+ solution (Fig. 1D), reproducing the phenomenon known as depolarization-induced alkalinization (DIA) in astrocytes (7, 19). By contrast, the Δ65bp NBCe1B mutant showed a predominant cytosolic retention and induced virtually no pHi response to high K+. The average rate of cell pH increase following high K+ for the wild type was 0.35 ± 0.02 pH unit/min (n = 24). Among three selected clones displaying the abundant cytoplasmic expression of the Δ65bp mutant protein, two showed no response to high K+ (n = 12 for each), whereas the third showed only a trace pHi increase (0.04 ± 0.01 pH unit/min, n = 25). These results strongly suggest that defective membrane expression in mammalian cells is responsible for the unique clinical manifestations of the Δ65bp mutation.
Δ65bp NBCe1 Mutation Can Exert a Dominant Negative Effect.
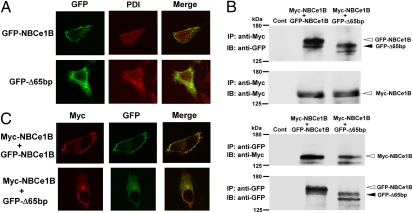
Several mutant proteins that are retained in the endoplasmic reticulum (ER) are known to exert a dominant negative effect through the formation of hetero-oligomer complexes with wild-type proteins (20, 21). The occurrence of migraine and glaucoma in several heterozygous members of this pedigree suggests that the Δ65bp mutant may have a similar dominant negative effect. To explore this possibility, we first examined the intracellular localization of GFP-tagged wild-type NBCe1B (GFP-NBCe1B) and GFP-tagged Δ65bp mutant (GFP-Δ65bp) transiently expressed in C6 cells. Although GFP-NBCe1B was predominantly expressed at the plasma membrane, GFP-Δ65bp expression largely overlapped with the expression of the ER marker protein disulfide isomerase (PDI), indicating that the mutant was mostly retained in the ER (Fig. 2A). We next tested the interaction between the wild-type and the Δ65bp mutant by a coimmunoprecipitation assay. We transiently expressed Myc-tagged wild-type NBCe1B (Myc-NBCe1B) together with GFP-NBCe1B or GFP-Δ65bp in C6 cells and subjected cell lysates to immunoprecipitation with an anti-Myc or an anti-GFP antibody. Consistent with the oligomer formation of NBCe1 (22), we found not only the interaction between Myc-NBCe1B and GFP-NBCe1B but also the interaction between Myc-NBCe1B and GFP-Δ65bp (Fig. 2B). Analysis by confocal microscopy showed that the Myc-NBCe1B and the GFP-NBCe1B signals predominantly colocalized at the plasma membrane. By contrast, the Myc-NBCe1B and the GFP-Δ65bp signals predominantly colocalized in the cytoplasmic region (Fig. 2C), supporting the dominant negative effect of the Δ65bp mutant through the formation of hetero-oligomer complexes with wild-type NBCe1.
Fig. 2.
Association of wild-type NBCe1B and the Δ65bp mutant in C6 cells. (A) Localization of GFP-NBCe1B and GFP-Δ65bp. Green: GFP; red: PDI. (B) Immunoprecipitation with anti-Myc (Upper) or anti-GFP antibody (Lower). Control (Cont): cells transfected with vector alone. (C) Coexpression of Myc-NBCe1B together with GFP-NBCe1B or GFP-Δ65bp. Red: Myc; green: GFP.
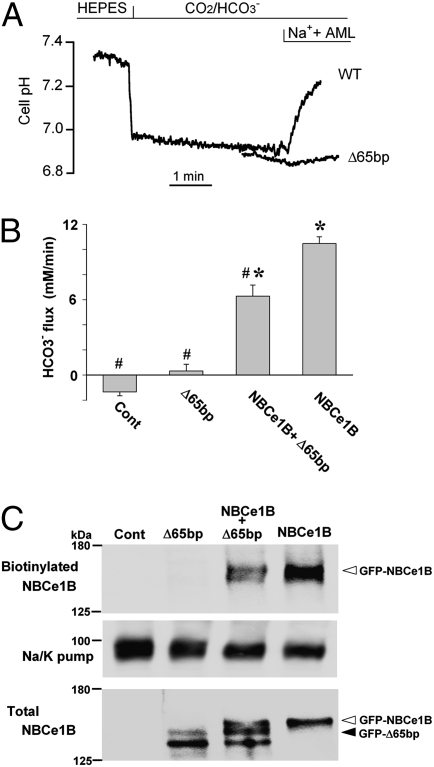
To examine the dominant negative effect of the Δ65bp mutant in more detail, we transiently expressed wild-type NBCe1B and/or the Δ65bp mutant in HEK293 cells, which are suitable for the evaluation of the transport activities of NBCe1 (22). Although the expression of the wild-type alone induced a robust NBCe1 activity, the expression of the Δ65bp mutant alone induced virtually no NBCe1 activity (Fig. 3A). More importantly, the coexpression of the wild-type and the Δ65bp mutant significantly reduced the net NBCe1 activity (P < 0.01 vs. wild type alone), which corresponded to 60.0% ± 8.5% of that by the wild-type alone (Fig. 3B). Analysis by confocal microscopy in three independent coexpression studies using Myc-NBCe1B and GFP-Δ65bp showed that 88.1% of the NBCe1-expressing cells also expressed the Δ65bp mutant. Biotinylation Western blotting revealed that the coexpression of GFP-NBCe1B and GFP-Δ65bp resulted in the reduction in surface expression of NBCe1 (Fig. 3C). Densitometric analysis of four independent experiments confirmed that the coexpression of GFP-Δ65bp reduced the surface expression of NBCe1, which corresponded to 57.9% ± 5.4% of that by GFP-NBCe1B alone (P < 0.05). To exclude the possibility that the observed net reduction of transport activity was due to any nonspecific effects potentially originating from coexpression procedures, we also tested an artificial mutant, 667KK-667QQ, which has no transport activity (23) but is properly expressed at the plasma membrane of mammalian cells (Fig. S2). The coexpression of wild-type NBCe1B with the 667KK-667QQ mutant did not change the net NBCe1 activity, which corresponded to 98.5% ± 9.1% of that by the wild-type alone. Because haploinsufficiency of the NBCe1 gene alone has not been linked to any phenotypes (13–18), we conclude that the dominant negative effect of the Δ65bp mutant is responsible for the occurrence of migraine and glaucoma in the heterozygous family members.
Fig. 3.
Dominant negative effect of the Δ65bp mutant in HEK293 cells. (A) Original traces showing the Na+-dependent cell pH recovery for wild-type NBCe1B (WT) and the Δ65bp mutant. AML: amiloride (1 mM). (B) Summary data for the functional analyses. Control (Cont): cells transfected with vector alone.*P < 0.01 vs. Cont. #P < 0.01 vs. NBCe1B. Numbers observed were 11 (Control), 12 (Δ65bp), 16 (Δ65bp + NBCe1B), and 19 (NBCe1B). (C) Cell surface expression of NBCe1B. Blots with the anti-GFP antibody in biotinylated (2 μL per lane) and total fractions (15 μL per lane) were shown. Equal protein loading in the biotinylated fraction was confirmed by blots with the anti-Na/K pump antibody. Control (Cont): cells transfected with vector alone.
Other NBCe1 Mutations with Similar Cellular Effects Also Cause Migraine.
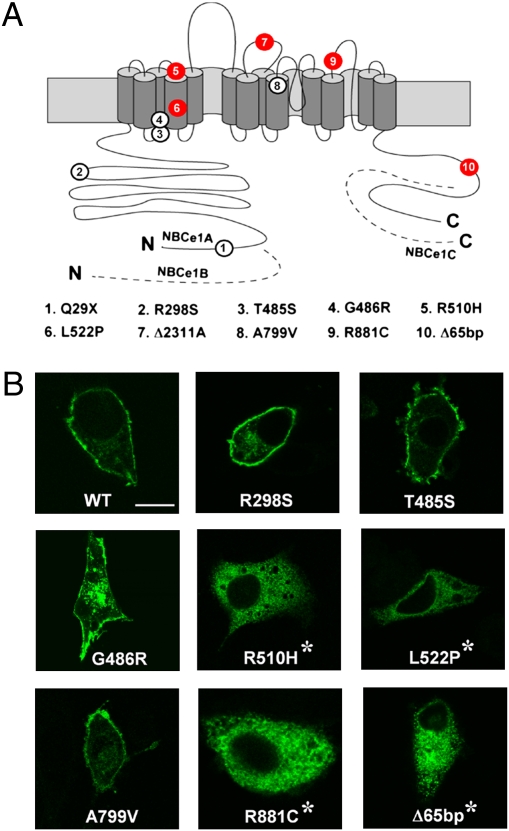
To further substantiate NBCe1 mutations as a cause of migraine, we investigated the other pRTA pedigrees with distinct NBCe1 mutations and found four additional homozygous patients with migraine (Fig. 4A): hemiplegic migraine with episodic ataxia in L522P (17), migraine with aura in Δ2311A (15), and migraine without aura in R510H and R881C (13, 16). By contrast, migraine subtypes meeting the International Headache Society criteria (24) were not found in another five patients homozygous for Q29X, R298S, T485S, G486R, and A799V (13, 14, 16). Among their heterozygous parents, only the mother of the R510H patient (13) had migraine without aura. Transient expression of GFP-tagged NBCe1B constructs carrying these mutations in C6 cells revealed a remarkable coincidence between the apparent lack of membrane expression and the occurrence of migraine (Fig. 4B). Notably, Δ2311A is a premature-stop mutation yielding no functional NBCe1 proteins (15), whereas Q29X is a NBCe1A-specific mutation leaving NBCe1B intact (14). Thus, all of the NBCe1B mutants showing a defective membrane expression in C6 cells were associated with migraine and vice versa (Table S1). Statistical analysis based on the migraine occurrence rate of 13% in the general population (1) indicates that the association in six of six individuals between migraine and homozygous NBCe1B mutations leading to defective membrane expression cannot be attributed to a chance coincidence (P = 4.8 × 10−6, binominal distribution). Functional analysis in HEK293 cells showed that the NBCe1B mutants reaching the plasma membrane (R298S, T485S, G486R, and A799V) had reduced transport activities corresponding to 30–40% of the wild-type activity, whereas the mutants defective in membrane expression (R510H, L522P, and R881C) had almost no transport activities (Fig. S3). These results further support a view that the near total loss of NBCe1B activity in astrocytes is associated with migraine in the pRTA patients with homozygous NBCe1 mutations.
Fig. 4.
Association of NBCe1 mutations and migraine. (A) Topology of NBCe1 and mutations identified in pRTA patients. Red circles denote mutations associated with migraine and white circles mutations not associated with migraine. The dashed lines represent the specific regions of NBCe1B or NBCe1C, and the N and C denote the N and C terminus, respectively. (B) Confocal images of GFP-tagged wild-type NBCe1B (WT) and the mutants transiently expressed in C6 cells. Asterisks indicate mutations associated with migraine. (Scale bar, 10 μm.)
Discussion
The present study identified the homozygous NBCe1 mutation Δ65bp associated with pRTA, ocular abnormalities, and hemiplegic migraine. Although the Δ65bp mutant had normal activity in Xenopus oocytes, it had almost no activity due to ER retention in mammalian cells. Such a discrepancy in trafficking behaviors in different expression systems was also noted for other NBCe1 mutants (16, 18). Moreover, the C-terminal region of NBCe1 was shown to be essential for the proper trafficking in MDCK cells (25). Investigation of other pRTA pedigrees identified four additional homozygous NBCe1 mutations associated with migraine. On the basis of the remarkable coincidence between the lack of plasma membrane expression of these mutants in C6 glioma cells and the occurrence of migraine, we conclude that homozygous NBCe1 mutations leading to defective membrane expression in astrocytes cause migraine. Although some of these patients with homozygous NBCe1 mutations had only ordinary migraine without hemiplegia, similar phenotypic variations were also reported for mutations in other FHM genes (26, 27), supporting the view that hemiplegic migraine and ordinary migraine represent a phenotypic spectrum caused by the same disease mechanism (28).
Cerebral cortical hyperexcitability causing cortical spreading depression (CSD) could be the pathophysiological mechanism underlying migraine aura (6). Although neuronal firing might lead to a rise in extracellular K+ concentration and further depolarization, uptake of K+ into astrocytes can counteract this process. Thus, enhanced neurotransmitter release by CACNA1A mutations, excessive neuronal firing by SCN1A mutations, or impaired clearance of K+ and/or glutamate by ATP1A2 mutations can all induce CSD (6). Neuronal excitation also leads to a transient extracellular alkalosis, possibly mediated by Ca2+/H+ exchange (7, 29). However, glial cell depolarization causes glial cell acid secretion via inward electrogenic Na+-HCO3− cotransport, i.e., DIA (7, 19), overwhelming the initial extracellular alkalosis. Under normal conditions, the net extracellular acidosis due to DIA makes the surrounding neuronal cells less excitable because excitatory NMDA receptors are blocked by protons, with a steep sensitivity in the physiological range of extracellular pH (7). Indeed, extracellular acidosis is known to suppress neuronal excitability (7), indicating that pRTA itself is unlikely to be a risk factor for migraine. Instead, the absence of DIA due to defective NBCe1 expression in the plasma membrane may cause a positive feedback loop of increased neuronal activity leading to further NMDA-mediated neuronal hyperactivity and causing complete depolarization of a sizable population of brain cells, i.e., CSD. We therefore propose that migraine associated with NBCe1 mutations represents a primary headache most likely caused by dysfunctional local pH regulation in the brain, and not a secondary headache associated with disorders of systemic homeostasis (24). Interestingly, topiramate, a widely used medication in migraine prophylaxis, has a weak inhibitory action on carbonic anhydrase. The resultant mixed RTA, although not observed in every case (30), could be at least partially involved in migraine prophylaxis. The enhancement of the DIA-induced extracellular acidosis by carbonic anhydrase inhibition (7) could also have a favorable effect.
Heterozygous mutations in the Cl−/HCO3− exchanger AE1 cause autosomal dominant distal renal tubular acidosis through either the mistargeting of mutant AE1 to the apical membrane or ER retention of hetero-oligomer complexes consisting of wild-type and mutant proteins (20, 21). By contrast, all of the mutations in NBCe1 identified so far cause pRTA in an autosomal recessive manner. Nevertheless, we found that several heterozygous members in the pedigree with the Δ65bp mutation had glaucoma and migraine. A series of coexpression analyses mimicking the heterozygous status revealed that the Δ65bp mutant forms hetero-oligomer complexes with the wild-type NBCe1, resulting in the net reduction of transport activity. This kind of dominant negative effect of the Δ65bp mutation can be responsible for the neurological and ocular phenotypes in the heterozygous members. The heterozygous members without acidemia may have higher basal activities of NMDA receptors than the homozygous patients suffering from acidemia. Therefore, a modest reduction in NBCe1B activity in astrocytes may induce migraine in some of the heterozygous, but not in the homozygous, members. NBCe1 variants show the distinct patterns of tissue expression and mediate different physiological roles (8). Among the phenotypes caused by NBCe1 mutations, we can primarily attribute pRTA to NBCe1A inactivation and the ocular and neurological phenotypes to NBCe1B inactivation. Because NBCe1C was recently reported to be expressed in rat astrocytes (29), however, we cannot exclude a possibility that NBCe1C inactivation is also involved in the occurrence of migraine.
The expression of NBCe1B in trabecular meshwork cells (11) suggests that a disturbance in aqueous humor outflow may be involved in the occurrence of high-tension glaucoma in pRTA patients with the homozygous Δ65bp and other NBCe1 mutations. On the other hand, dysfunctional local pH regulation in the retina may be involved especially in the occurrence of normal-tension glaucoma in the heterozygous members. Indeed, retinal excitation by light is known to induce an increase in extracellular pH resulting in a gain of synaptic activity (31, 32), and NBCe1B in retinal Müller cells counteracts this light-induced extracellular alkalosis (33, 34).
The reason why the heterozygous Δ65bp mutation does not cause pRTA through its dominant negative effect remains speculative. It is possible that the mechanism of trafficking control may be slightly different in in vitro and in vivo situations. If a small amount of Δ65bp mutant can escape from ER retention and reach the plasma membrane in vivo, its transport activity will at least partially counteract the dominant negative effect. In this scenario, the acid secretory ability of renal distal tubules may be sufficient to compensate for a substantial reduction in bicarbonate absorption from the proximal tubules. However, such a compensatory mechanism would be absent or insufficient in the brain and in ocular tissues.
Finally, the demonstration of the dominant negative effect of the Δ65bp mutation raises an important question as to whether heterozygous NBCe1 mutations without pRTA represent a risk factor for common forms of migraine. This possibility should be tested in future studies, especially in view of the known association between normal-tension glaucoma and migraine (35).
Methods
Mutation Analysis of the Family with Δ65bp Mutation.
All of the subjects provided written informed consent, and the institutional review boards at each institute approved this study. RNA was extracted using acid guanidinium thiocyanate–phenol–chloroform from Epstein-Barr virus–transformed cells of the proband's peripheral lymphocytes. NBCe1 cDNA was constructed using a first-strand cDNA synthesis kit (Life Science). PCR was performed to amplify each coding exon of SLC4A4 using the specific primers as described (14). Genomic DNA was extracted from the peripheral leukocytes of the family members, and exon 23 and intron 23 of SLC4A4 were amplified. The PCR products were sequenced on a model 373A automated DNA sequencer (Perkin-Elmer). Genomic analysis of FHM genes was performed as described (2–4, 27).
Diagnosis of Migraine.
The diagnostic criteria of the International Headache Society (24) were used to define migraine subtypes. A direct interview of the family members or a literature search was performed for the homozygous NBCe1 mutations Q29X, R298S, T485S, G486R, R510H, L522P, Δ2311A, A799V, R881C, and Δ65bp.
Construction of NBCe1-Expressing Constructs.
cDNAs encoding human NBCe1A or NBCe1B were subcloned into pcDNA3.1 as described (16, 36). The QuikChange Site-Directed Mutagenesis kit (Stratagene) was used to introduce mutations. For GFP- and Myc-tagged constructs, coding sequences of NBCe1 constructs were subcloned into pcDNA3.1/NH2-terminal GFP-TOPO or pcDNA3.1/myc-His (C-terminal), respectively (both from Invitrogen).
Expression in Xenopus laevis Oocytes.
cRNAs were transcribed from the appropriately linearized templates with the mMESSAGE mMACHINE high-yield Capped RNA Transcription kit (Ambion). Oocytes were removed from Xenopus laevis, dissociated with collagenase and were injected with 50 nl of a cRNA solution containing 5 ng of cRNA expressing each NBCe1 construct. Two-electrode voltage clamp experiments were performed 3–5 d after injection as described (16). In brief, oocytes were perfused with a HCO3−-containing solution in the presence of 5% CO2, and the current (I)/voltage (Vm) relationship was determined by applying pulse trains between Vm = −160 and +60 mV. The currents sensitive to 0.4 mM 4, 4′-diisothiocyanatostilbene-2, 2′-disulphonic acid (Sigma) were shown to represent the NBCe1 currents (16, 18, 36).
Expression in Cultured Cells.
MDCK, C6 glioma, and HEK293 cells were grown on culture dishes in Dulbecco's modified Eagles's medium supplemented with 10% FCS. These cells were transfected with plasmid expressing each NBCe1 construct with LipofectAMINE 2000 (Invitrogen). For stable expression, C6 cells were selected with G-418 (Sigma). For transient expression, the same amounts of DNA (4 μg per well of six-well dishes) were transfected for the wild-type NBCe1 and the mutants. For coexpression, the total amounts of DNA were kept constant (2 or 12 μg for each construct per well of six-well dishes or per dish of 10-cm dishes, respectively) by adding empty vector if necessary.
Cell pH Measurements.
C6 or HEK293 cells, grown on fibronectin-coated coverslips, were incubated with the pH dye acetoxymethylester of bis(carboxyethyl)carboxyfluorescein (Molecular Probes). Cell pH measurements were performed with a microscopic fluorescence photometry system (OSP-10; Olympus) as described (16, 18). C6 cells were perfused with HCO3−-Ringer solution containing (in mM) the following: NaCl 115, KCl 5, CaCl2 1.5, MgCl2 1, NaH2PO4 2, Na2SO4 1, NaHCO3 25, glucose 5.5; pH 7.4 equilibrated with 5% CO2/95% O2 gas. The K+ concentration in the perfusate was increased to 20 mM (high K+) by replacing 15 mM NaCl by equimolar KCl. NBCe1 activity in HEK 293 cells was analyzed by the standard method (22, 23). In brief, after perfusion with a Hepes-buffered solution, solution was exchanged to a Na+-free HCO3−-solution (replacement of Na+ by N-methyl-D-glucamine), which induced a marked intracellular acidification due to CO2 entry. Thereafter, solution was exchanged to a HCO3−-Ringer solution containing 1 mM amiloride, which blocks the endogenous Na+/H+ exchange activity. The resultant Na+-dependent cell pH recovery (dpHi/dt) represents the NBCe1 activity (22, 23). The intrinsic cell buffer capacity (βi) was measured as described (16). Total cell buffer capacity (βT) in the HCO3−-containing solution was equal to the sum of βi and the HCO3− buffer capacity, and the HCO3−-flux through NBCe1 was calculated as dpHi/dt × βT.
Immunoprecipitation and Cell Surface Biotinylation.
For immunoprecipitation, the C6 cells, grown on a 10-cm culture dish, were lysed with a lysis buffer containing 50 mM Tris/pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF. The cell lysate was precleaned by incubation with Protein G Sepharose 4 Fast Flow (GE Healthcare Bio-Science) and incubated with an anti-Myc (Bethyl Laboratories) or an anti-GFP antibody (Clontech) coupled to Protein G Sepharose 4 Fast Flow. The immunoprecipitates were washed three to five times with lysis buffer and subjected to Western blotting. Cell surface biotinylation in HEK293 cells was performed with the Pierce Cell Surface Protein Isolation kit (Thermo Scientific) according to the manufacturer's protocol. In brief, cells grown on four dishes of a 10-cm culture dish were washed with PBS and incubated with EZ-LINK Sulfo-NHS-SS-biotin for 30 min at 4 °C followed by the addition of a quenching solution. Cells were lysed with lysis buffer (500 μL) containing the Halt protease inhibitor mixture kit. An aliquot (100 μL) of the lysate (total) was saved for Western blotting. The biotinylated NBCe1 was isolated with NeutrAvidin agarose gel, eluted by the sample buffer (400 μL) containing DTT, and subjected to Western blotting with the anti-GFP or anti-Na/K pump α1 antibody (Upstate Biotech).
Immunofluorescence.
Confluent MDCK cells grown on fibronectin-coated coverslips were fixed and stained with the F-actin dye tetramethylrhodamine isothiocyanate-phalloidin (Sigma) and observed by a TCS SL laser-scanning confocal microscope (Leica) as described (18). To visualize untagged NBCe1B in C6 cells, an affinity-purified rabbit polyclonal antibody against the N-terminal specific region of NBCe1B (Kumamoto Immunochemical) was used as the primary antibody (11), and Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes) was used as the secondary antibody. To visualize the ER, a rabbit polyclonal antibody against PDI (Stressgen Bioreagents) was used as the primary antibody. Alexa Fluor 546 goat anti-rabbit IgG (Molecular Probes) was used as the secondary antibody for the detection of ER protein and Myc-NBCe1B. We performed at least three independent transfection studies examining at least 100 NBCe1-expressing cells in each case.
Statistical Analysis.
The data were represented as mean ± SEM. Significant differences were determined by applying Student's t test or ANOVA with Bonferroni's correction, as appropriate. Statistical significance was set at P < 0.05. To examine whether the chance coincidence can explain the occurrence of migraine in six patients with homozygous NBCe1 mutations leading to a defective membrane expression, we compared the migraine occurrence rate (100%) in the patients with that (13%) in the general population using the bimodal distribution (1). The null hypothesis of equal occurrence was tested at the significance level of 0.05 with two-tailed analysis.
Supplementary Material
Acknowledgments
We thank the patients and their families for participating in this study and Kumamoto Immunochemical for providing the anti-NBCe1B antibody. This work was in part supported by grants from the Ministry of Education, Science and Culture of Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008705107/-/DCSupplemental.
References
- 1.Lipton RB, et al. Migraine in the United States: Epidemiology and patterns of health care use. Neurology. 2002;58:885–894. doi: 10.1212/wnl.58.6.885. [DOI] [PubMed] [Google Scholar]
- 2.Ophoff RA, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 3.De Fusco M, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33:192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 4.Dichgans M, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366:371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 5.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 6.Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39–44. doi: 10.1016/j.molmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 8.Romero MF, Boron WF. Electrogenic Na+/HCO3− cotransporters: Cloning and physiology. Annu Rev Physiol. 1999;61:699–723. doi: 10.1146/annurev.physiol.61.1.699. [DOI] [PubMed] [Google Scholar]
- 9.Abuladze N, et al. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene. 2000;251:109–122. doi: 10.1016/s0378-1119(00)00204-3. [DOI] [PubMed] [Google Scholar]
- 10.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278:C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- 11.Usui T, et al. Molecular basis of ocular abnormalities associated with proximal renal tubular acidosis. J Clin Invest. 2001;108:107–115. doi: 10.1172/JCI11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satoh H, et al. Localization of Na+-HCO3− cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284:C729–C737. doi: 10.1152/ajpcell.00166.2002. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi T, et al. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi T, et al. Novel nonsense mutation in the Na+/HCO3− cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol. 2001;12:713–718. doi: 10.1681/ASN.V124713. [DOI] [PubMed] [Google Scholar]
- 15.Inatomi J, et al. Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch. 2004;448:438–444. doi: 10.1007/s00424-004-1278-1. [DOI] [PubMed] [Google Scholar]
- 16.Horita S, et al. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol. 2005;16:2270–2278. doi: 10.1681/ASN.2004080667. [DOI] [PubMed] [Google Scholar]
- 17.Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: A novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1) Mol Vis. 2006;12:324–330. [PubMed] [Google Scholar]
- 18.Suzuki M, et al. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflugers Arch. 2008;455:583–593. doi: 10.1007/s00424-007-0319-y. [DOI] [PubMed] [Google Scholar]
- 19.Shrode LD, Putnam RW. Intracellular pH regulation in primary rat astrocytes and C6 glioma cells. Glia. 1994;12:196–210. doi: 10.1002/glia.440120305. [DOI] [PubMed] [Google Scholar]
- 20.Quilty JA, Cordat E, Reithmeier RA. Impaired trafficking of human kidney anion exchanger (kAE1) caused by hetero-oligomer formation with a truncated mutant associated with distal renal tubular acidosis. Biochem J. 2002;368:895–903. doi: 10.1042/BJ20020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alper SL. Genetic diseases of acid-base transporters. Annu Rev Physiol. 2002;64:899–923. doi: 10.1146/annurev.physiol.64.092801.141759. [DOI] [PubMed] [Google Scholar]
- 22.Kao L, et al. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. J Biol Chem. 2008;283:26782–26794. doi: 10.1074/jbc.M804006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abuladze N, et al. Critical amino acid residues involved in the electrogenic sodium-bicarbonate cotransporter kNBC1-mediated transport. J Physiol. 2005;565:717–730. doi: 10.1113/jphysiol.2005.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 25.Li HC, et al. Identification of a carboxyl-terminal motif essential for the targeting of Na+-HCO3− cotransporter NBC1 to the basolateral membrane. J Biol Chem. 2004;279:43190–43197. doi: 10.1074/jbc.M405780200. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen LL, et al. The genetic spectrum of a population-based sample of familial hemiplegic migraine. Brain. 2007;130:346–356. doi: 10.1093/brain/awl334. [DOI] [PubMed] [Google Scholar]
- 27.Ducros A, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med. 2001;345:17–24. doi: 10.1056/NEJM200107053450103. [DOI] [PubMed] [Google Scholar]
- 28.Barrett CF, van den Maagdenberg AM, Frants RR, Ferrari MD. Familial hemiplegic migraine. Adv Genet. 2008;63:57–83. doi: 10.1016/S0065-2660(08)01003-1. [DOI] [PubMed] [Google Scholar]
- 29.Majumdar D, et al. Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience. 2008;155:818–832. doi: 10.1016/j.neuroscience.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirza N, Marson AG, Pirmohamed M. Effect of topiramate on acid-base balance: Extent, mechanism and effects. Br J Clin Pharmacol. 2009;68:655–661. doi: 10.1111/j.1365-2125.2009.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borgula GA, Karwoski CJ, Steinberg RH. Light-evoked changes in extracellular pH in frog retina. Vision Res. 1989;29:1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- 32.Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bok D, et al. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Am J Physiol Renal Physiol. 2001;281:F920–F935. doi: 10.1152/ajprenal.2001.281.5.F920. [DOI] [PubMed] [Google Scholar]
- 34.Newman EA. Sodium-bicarbonate cotransport in retinal astrocytes and Müller cells of the rat. Glia. 1999;26:302–308. doi: 10.1002/(sici)1098-1136(199906)26:4<302::aid-glia4>3.0.co;2-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cursiefen C, et al. Migraine and tension headache in high-pressure and normal-pressure glaucoma. Am J Ophthalmol. 2000;129:102–104. doi: 10.1016/s0002-9394(99)00289-5. [DOI] [PubMed] [Google Scholar]
- 36.Shirakabe K, et al. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1) Proc Natl Acad Sci USA. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.