Abstract
Motivated by the potential high-temperature superconductivity in hydrogen-rich materials, the high-pressure structures of SiH4(H2)2 in the pressure range 50–300 GPa were extensively explored by using a genetic algorithm. An intriguing layered orthorhombic (Ccca) structure was revealed to be energetically stable above 248 GPa with the inclusion of zero-point energy. The Ccca structure is metallic and composed of hydrogen shared SiH8 dodecahedra layers intercalated by orientationally ordered molecular H2. Application of the Allen-Dynes modified McMillan equation yields remarkably high superconducting temperatures of 98–107 K at 250 GPa, among the highest values reported so far for phonon-mediated superconductors. Analysis reveals a unique superconducting mechanism that the direct interactions between H2 and SiH4 molecules at high pressure play the major role in the high superconductivity, while the contribution from H2 vibrons is minor.
Keywords: H2-containing compounds, high-pressure, high-temperature superconductors, metallization
One of the major targets in high-pressure research is to metalize solid hydrogen on the search for a high-temperature superconductor. However, the experimental metallization is yet achieved even at the pressures up to ∼300 GPa (1, 2), above which experiment remains a great difficulty. As an alternative, hydrogen-rich group IV hydrides (i.e., CH4, SiH4, GeH4, and SnH4) are extensively explored since their metallization can happen at much lower pressures (3–9) accessible to current experiments. Electric resistivity measurement on SiH4 (6) has revealed a relatively low-temperature superconductivity with a transition temperature (Tc) of 17 K at 96 GPa and 120 GPa, though debates remain (10). The low-temperature superconductivity in SiH4 has then been confirmed by theory (8), where SiH4 is found to basically remain at its molecular form at 220 GPa with the shortest H-H distance of 1.35 Å (8). However, subsequent theoretical researches on GeH4 and SnH4 have predicted much higher superconductivities with maximal Tc reaching 64 K at 220 GPa for GeH4 (7) and 62 K at 200 GPa for SnH4 (9), respectively. A common feature on the high-pressure structures of GeH4 and SnH4 is the formation of intriguing semimolecular “H2” units with H-H lengths of 0.87 Å (220 GPa) and 0.79 Å (120 GPa), respectively. These H2 units have been found to contribute mostly to the high superconductivities. This fact illustrates the importance of H2 units in the superconductivity and will inevitably motivate further search of high-temperature superconductors on those H2 units containing materials.
Remarkably, recent experiments have demonstrated that these hydrogen-rich full-shell group IV hydrides (CH4 and SiH4) can adsorb additional H2 molecules at high pressures forming a series of new H2-containing compounds, e.g., CH4(H2)2, (CH4)2H2, CH4(H2)4, CH4H2 (11), and SiH4(H2)2 (12, 13). In a further survey of literatures, H2 was even found to interact with H2O (14, 15), NH3BH3 (16), Ar (17), and Xe (18) at readily accessible pressures stabilizing at stoichiometric compounds. The exploration of high-pressure metallization of these compounds is greatly desirable in search of high-temperature superconductors since here the demanding H2 units automatically apply.
In view of the low metallization pressure observed in SiH4 (5, 6), we have then targeted on the metallization and superconductivity of SiH4(H2)2 by first-principles calculations via genetic algorithm on crystal structure prediction and linear response theory on electron-phonon coupling (EPC) calculations. We focus on the high-pressure regime of 50–300 GPa, below which SiH4(H2)2 is clearly insulating (13). Our calculations have revealed the metallization and a remarkable high superconductivity of SiH4(H2)2 at high pressures. The current results have unraveled a superconducting mechanism which could have important implication on other high-pressure H2-containing compounds, e.g., H2O-H2, CH4-H2, NH3BH3-H2, Ar-H2, and Xe-H2.
Results and Discussion
Evolutionary variable-cell structure predictions were performed at 10, 50, 80, 120, 200, 250, and 300 GPa with one to four SiH4(H2)2 formula units (f.u.) per cell. At 10 GPa, our calculations predict the correct crystal structure of the synthesized SiH4(H2)2 (12, 13), where SiH4 molecules form a peculiar tetragonal or a distorted face-centered cubic lattice with the orientationally disordered H2 molecules occupying the interstitial sites. The validity of our structural model has been supported by the excellent mutual agreement between theoretical and experimental equation of states, Raman, and X-ray diffraction data (Fig. S1). Above 50 GPa, analysis of the predicted results gives three best structures at certain pressure regions, triclinic P-1 (2 f.u./cell) at 50 and 80 GPa, monoclinic Cc (4 f.u./cell) at 120 GPa, and orthorhombic Ccca (4 f.u./cell) at 200, 250, and 300 GPa, as depicted in Fig. 1 and Table S1. All three structures contain SiHx (x = 6, 8) layers intercalated by molecular H2 layers. The enthalpy curves of the predicted structures together with the low pressure tetragonal (tI18) phase are plotted as a function of pressure in Fig. 2A. The P-1 structure (Fig. 1A) is energetically stable between 24 and 92 GPa and still an insulator (Fig. S2). Different from the tetrahedral Si-H bonding in SiH4 molecules of tI18 structure at low pressure, sixfold-coordinated SiH6 octahedra was formed in P-1 structure. This new bonding feature involves three d orbitals of Si due to the pressure-induced p-d charge transfers. The H2 molecules sit in between SiH6 layers with H-H bond lengths of 0.747 and 0.748 Å, slightly longer than that (0.737 Å) in pure solid H2 (19). Total charge density (Fig. 1D) analysis evidences the domination of van der Waals interactions between SiH4 and H2 molecules.
Fig. 1.
High-pressure crystal structures of SiH4(H2)2: (A) P-1, (B) Cc and (C) Ccca. Large spheres represent Si and small spheres labeled by H1 and H2 denote H atoms in SiH4 and H2 molecules, respectively. (D–F) The total charge density contours in planes containing Si-H2 and H1-H2 bonds in the P-1 (50 GPa), Cc (120 GPa) and Ccca (200 GPa) structures, respectively.
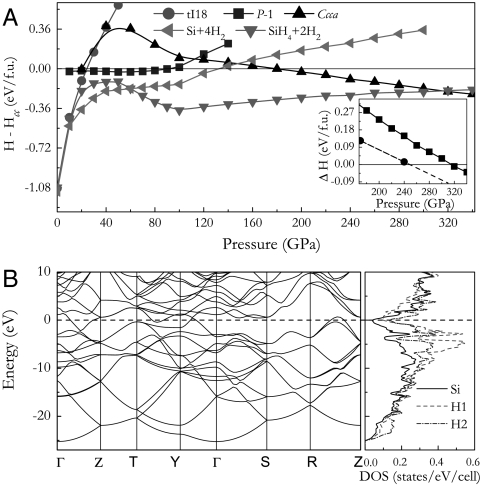
Fig. 2.
Calculated enthalpy curves for various structures relative to our predicted Cc structure as a function of pressure. The decomposition enthalpies into Si + 4H2 and SiH4 + 2H2 were also plotted. Inset in (A) is the enthalpies of Ccca structure relative to the SiH4 + 2H2 without (solid) and with (dashed) zero-point energy corrections. (B) The electronic band structure and projected DOS of the Ccca structure at 250 GPa. In the decomposition calculations, structures of Fd-3m (40) at 0–20 GPa, P6/mmm (41) at 20–40 GPa, P63/mmc (41) at 40–80 GPa and Fm-3m (42) above 80 GPa for Si, P21c (43) at 0–40 GPa, Fdd2 (8) at 40–60 GPa, I41a (44) at 40–220 GPa and Pbcn (8) at 220–250 GPa and C2/c (45) above 250 GPa for SiH4, P63m at 0–100 GPa and C2/c at 100–250 GPa and Cmca (19) above 250 GPa for H2 (19) are adopted.
On compression, the P-1 structure transforms to Cc structure (Fig. 1B) at 92 GPa. The P-1 → Cc transition is accompanied by an increased Si coordination number from six to eight with the formation of hydrogen shared SiH8 dodecahedra. Note also that the puckered Si layers in P-1 structure become planar in Cc structure. The H2 molecules distribute in between SiH8 layers in various orientations with H-H bond lengths of 0.742 and 0.754 Å at 120 GPa. Weakly covalent H1-H2 bonds have been found in the Cc structure (Fig. 1E), indicating the increased SiH4-H2 interactions under pressure. The Cc structure is a very weak metal with an extremely low density of states (DOS) at Fermi level (0.17 states/eV/unit cell) (Fig. S2) and stable up to 180 GPa, above which the Ccca structure (Fig. 1C) takes over. At the Cc → Ccca transition, the H2 orientations become clearly ordered in two directions without the major modification of SiH8 dodecahedra, but the intramolecular H-H bond was significantly stretched to be 0.843 Å at 200 GPa, much longer than that in pure solid H2 (19). The elongation of H-H bonds might be mainly resulted from the continuously increased SiH4-H2 interactions under pressure. Indeed, our total charge density (Fig. 1F) analysis at 200 GPa has revealed considerably strong covalent bonding characteristics between SiH4 and H2 molecules. This fact further implies that SiH4(H2)2 is no longer a van der Waals compound. We then devised the total energy calculations within the Ccca structure by gradually rotating H2 molecules in b-c plane while remaining the H2 centers unchanged. A pronounced energy variation of 0.17 eV/f.u. is found with a 10° rotation of H2. Such a large energy barrier naturally prevents H2 from the rotation, resulting in an orientationally ordered H2 in SiH4(H2)2.
The decomposition enthalpies into Si + 4H2 and SiH4 + 2H2 are examined to check the phase stability of SiH4(H2)2 under pressure (Fig. 2A). It is surprisingly found that the P-1 and Cc structures are metastable relative to the decomposition and the Ccca structure only becomes stable above ∼315 GPa. One keeps in mind that quantum effects related to hydrogen atoms are very important. Particularly, the hydrogen zero-point (ZP) energy is expected to be large enough to significantly revise the structural stability as in the cases of solid hydrogen (19) and hydrides (7, 9). We therefore have estimated the ZP energies of SiH4(H2)2, SiH4, and H2 at 170 and 240 GPa using the quasi-harmonic approximation (20). It turns out that the inclusion of ZP effects does not change the topology of the phase diagram but lowers the decomposition pressure of the Ccca structure into ∼248 GPa (inset of Fig. 2A). Therefore, the subsequent electronic, phonon, and EPC calculations are focused on the Ccca structure at 250 GPa.
We have explored the electronic band structure and projected DOS for the Ccca structure and found that Ccca structure is a good metal with a large DOS at Fermi level (0.41 states/eV/unit cell) at 250 GPa (Fig. 2B). Flat bands along T-Y and R-Z directions and steep bands along Γ-Z and Y-Γ directions are evidenced and apparently satisfied with the “Flat-Deep” band scenario for a good superconductor (21). The projected DOS clearly indicates strong hybridizations of Si, H1 (H atom in SiH4) and H2 (H atom in H2) orbitals. This hybridization results in a 25 eV broad valence band favorable for a good superconductor, in accordance with the previous predictions for dense hydrogen alloys (3). The phonon band structure and projected phonon DOS of the Ccca structure at 250 GPa are shown in Fig. 3. The absence of any imaginary frequency establishes the dynamical stability of the Ccca structure. Here, the low frequency bands below 13 THz and high energy bands above 81 THz are dominated by Si vibrations and H2 vibrons, respectively. It is noteworthy that strong coupling of Si-H2 (13–31 THz) and H1-H2 (31–81 THz) vibrations is revealed and largely contrasted to the clear separation of frequency regions found in SiH4, GeH4, and SnH4 (7–9). The strong covalent interactions between SiH4 and H2 molecules are responsible for this phenomenon.
Fig. 3.
Calculated phonon dispersions (A), phonon density of states (PHDOS) (B), and the Eliashberg phonon spectral function α2F(ω)/ω and electron-phonon integral λ(ω) (C) for the Ccca structure at 250 GPa.
The Eliashberg spectral function α2F(ω)/ω and the EPC parameter λ of the Ccca structure (Fig. 3C) as a function of frequency at 250 GPa are calculated to explore the possible superconductivity of SiH4(H2)2. The resulting total λ is 1.625 indicating that the EPC is extremely strong. By applying the calculated logarithmic average frequency ωlog (871 K) into the Allen and Dynes modified McMillan equation (22) with a typical choice of Coulomb pseudopotential μ∗ as 0.1–0.13 (3), a remarkable large Tc in the range of 98–107 K was derived. The current results support the earlier theoretical proposal that hydrogen dominant metallic alloys are high-temperature superconductors (3). However, the Tc magnitude of ∼100 K strikingly achieved for SiH4(H2)2 is among the highest values for all previously reported hydrides (6, 7, 9). This result invites us to probe the underlying superconducting mechanism originated from the additional H2 molecules. We then decompose the total λ into several major individual contributions. The low frequency Si translational vibrations (below 13 THz) were found to contribute 33% in total. It is regretful to find that H2 vibrons (> 81 THz) contribute only 1% of λ. Instead, a remarkable feature prompts to us that the H2-SiH4 coupling (13–81 THz) constitutes ∼66% of the total λ. This result highlights the significant role played by H2 molecules on the superconductivity through the strong interactions with SiH4 molecules enhancing the attractive EPC over the repulsive Coulomb interaction between electrons. This physical mechanism is apparently contrasted to those in high-pressure GeH4 (7) and SnH4 (9), where the semimolecular H-H vibrations dominate the superconductivity.
Remarks on the enthalpy results (Fig. 2A) are necessary before we turn to the summary. We find that SiH4(H2)2 decomposes into Si + 4H2 at 10–60 GPa, and then SiH4 + 2H2 at 60–248 GPa. However, it is noteworthy that at relatively low temperatures, a large kinetic barrier might exist and prevent SiH4(H2)2 from decomposition. Indeed, experiments (12, 13) observe the stability of SiH4(H2)2 at least up to 35 GPa. Previous studies on several hydrogen containing systems have revealed fairly large activation energies on decomposition, e.g., 79.3–102.2 KJ/mol for Al-H (23), 71–74 KJ/mol for Mg-H (24), and 78 KJ/mol for Pd-Ni-H (25). An accurate evaluation of decomposition kinetic barrier is needed to give a precise conclusion, but remains a major challenge and beyond the scope of this study. Nevertheless, if the large kinetic barrier could efficiently prevent SiH4(H2)2 from decomposition, the compound can follow the transition paths P-1 → Cc → Ccca and the experimental observation of high superconductivity can be significantly lowered to 180 GPa where much less experimental effort is requested. By all appearance, the current prediction of extremely high superconductivity in SiH4(H2)2 might be observable in the range of 180–248 GPa accessible to the current high-pressure experiments.
In summary, a metallic Ccca phase has been predicted for SiH4(H2)2 most stable above 248 GPa by using first-principle calculations. EPC calculations and application of the Allen-Dynes modified McMillan equation at 250 GPa yield remarkably high critical temperatures of 98–107 K for the Ccca phase, among the highest values reported for hydrogen-rich compounds to date. The current results have unraveled a unique superconducting mechanism where the strong interactions between H2 and SiH4 molecules rather than H2 vibrons dominate the superconductivity of SiH4(H2)2. The stability fields of the Ccca structure are within the reach of current experimental techniques. Therefore, the predicted high Tc for SiH4(H2)2 will inevitably stimulate extensive experiments. Note that other such high-pressure compounds, e.g., H2O-H2, CH4-H2, NH3BH3-H2, Ar-H2, and Xe-H2, all contain rich H2 molecules. Future theoretical and experimental exploration on the metallization and superconductivity of these compounds at high pressures are highly demanded.
Materials and Methods
Evolutionary search for the high-pressure structures of SiH4(H2)2 was performed by a genetic algorithm (26–28), which has been applied successfully to a number of systems (7, 9, 29–33). In the evolutionary structural predictions, the first generation was created randomly. The subsequent generation is produced from 60% of the lowest-enthalpy structures of the preceding generation. In addition, the lowest-enthalpy structures always survived into the next generation. The variation operators used for producing offspring included heredity (60%), lattice mutation (20%), and atomic permutation (20%). The underlying ab initio structure relaxation was performed using density-functional theory (34) within projector augmented wave method (35) as implemented in the Vienna Ab-inito Simulation Package code (VASP) (36). The projector augmented wave potentials used here were derived using the generalized gradient approximation (37) functional with valence electrons of 3s23p2 and 1s1 and cutoff radii of 1.9 and 0.8 a.u. for Si and H, respectively, suitable for the high-pressure study. A plane-wave kinetic energy cutoff of 1,000 eV and the use of Monkhorst-Pack (MP) k-points meshes of 10 × 10 × 8 for the Ccca structure were shown to given excellent convergence of the total energy. In the geometrical optimization, all forces on atoms were converged to less than 0.001 eV/Å, and the total stress tensor was reduced to the order of 0.01 GPa. Lattice dynamic and EPC were calculated using the plane-wave pseudopotential method and density-functional perturbation theory, through the Quantum-opEn Source Package for Research in Electronic Structure, Simulation, and Optimization (ESPRESSO) package (38). Forces and stresses for the converged structures are optimized and checked to be within the error between the VASP and ESPRESSO code. Troullier-Martins norm-conserving scheme (39) was used to generate the pseudopotentials for Si and H. A 6 × 6 × 6 q-point mesh in the first Brillouin zone was used in the EPC calculation. A MP grid of 16 × 16 × 14 was used to ensure k-point sampling convergence with Gaussians of width 0.03 Ry, which approximates the zero-width limits in the calculations of EPC parameter λ.
Supplementary Material
Acknowledgments.
We thank the following financial support: the National Natural Science Foundation of China under Grant No. 10874054, the China 973 Program under Grant No. 2005CB724400; and the 2007 Cheung Kong Scholars Programme of China. G.G. is also grateful to the Project 20092004 supported by Graduate Innovation Fund of Jilin University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007354107/-/DCSupplemental.
References
- 1.Loubeyre P, Occelli F, LeToullec R. Optical studies of solid hydrogen to 320 GPa and evidence for black hydrogen. Nature. 2002;416:613–617. doi: 10.1038/416613a. [DOI] [PubMed] [Google Scholar]
- 2.Goncharov AF, Gregoryanz E, Hemley RJ, Mao H. Spectroscopic studies of the vibrational and electronic properties of solid hydrogen to 285 GPa. Proc Nat Acad Sci USA. 2001;98:14234–14237. doi: 10.1073/pnas.201528198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft NW. Hydrogen dominant metallic alloys: high temperature superconductors? Phys Rev Lett. 2004;92:187002. doi: 10.1103/PhysRevLett.92.187002. [DOI] [PubMed] [Google Scholar]
- 4.Tse JS, Yao Y, Tanaka K. Novel superconductivity in metallic SnH4 under high pressure. Phys Rev Lett. 2007;98:117004. doi: 10.1103/PhysRevLett.98.117004. [DOI] [PubMed] [Google Scholar]
- 5.Chen XJ, et al. Pressure-induced metallization of silane. Proc Nat Acad Sci USA. 2008;105:20–23. doi: 10.1073/pnas.0710473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eremets MI, et al. Superconductivity in hydrogen dominant materials: silane. Science. 2008;319:1506–1509. doi: 10.1126/science.1153282. [DOI] [PubMed] [Google Scholar]
- 7.Gao G, et al. Superconducting high pressure phase of germane. Phys Rev Lett. 2008;101:107002. doi: 10.1103/PhysRevLett.101.107002. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Canales M, et al. Novel structures and superconductivity of silane under pressure. Phys Rev Lett. 2009;102:87005. doi: 10.1103/PhysRevLett.102.087005. [DOI] [PubMed] [Google Scholar]
- 9.Gao G, et al. High-pressure crystal structures and superconductivity of Stannane (SnH4) Proc Nat Acad Sci USA. 2010;107:1317–1320. doi: 10.1073/pnas.0908342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degtyareva O, et al. Formation of transition metal hydrides at high pressures. Solid State Commun. 2009;149:1583–1586. [Google Scholar]
- 11.Somayazulu MS, Finger LW, Hemley RJ, Mao HK. High-pressure compounds in methane-hydrogen mixtures. Science. 1996;271:1400–1402. [Google Scholar]
- 12.Wang S, Mao H, Chen XJ, Mao WL. High pressure chemistry in the H2-SiH4 system. Proc Nat Acad Sci USA. 2009;106:14763–14767. doi: 10.1073/pnas.0907729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strobel TA, Somayazulu M, Hemley RJ. Novel pressure-induced interactions in silane-hydrogen. Phys Rev Lett. 2009;103:65701. doi: 10.1103/PhysRevLett.103.065701. [DOI] [PubMed] [Google Scholar]
- 14.Mao WL, et al. Hydrogen clusters in clathrate hydrate. Science. 2002;297:2247–2249. doi: 10.1126/science.1075394. [DOI] [PubMed] [Google Scholar]
- 15.Mao WL, Mao H. Hydrogen storage in molecular compounds. Proc Nat Acad Sci USA. 2004;101:708–710. doi: 10.1073/pnas.0307449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Mao WL, Mao H. Storage of molecular hydrogen in an ammonia borane compound at high pressure. Proc Nat Acad Sci USA. 2009;106:8113–8116. doi: 10.1073/pnas.0903511106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loubeyre P, Letoullec R, Pinceaux JP. Compression of Ar (H2)2 up to 175 GPa: a new path for the dissociation of molecular hydrogen? Phys Rev Lett. 1994;72:1360–1363. doi: 10.1103/PhysRevLett.72.1360. [DOI] [PubMed] [Google Scholar]
- 18.Somayazulu M, et al. Pressure-induced bonding and compound formation in xenon-hydrogen solids. Nat Chem. 2010;2:50–53. doi: 10.1038/nchem.445. [DOI] [PubMed] [Google Scholar]
- 19.Pickard CJ, Needs RJ. Structure of phase III of solid hydrogen. Nat Phys. 2007;3:473–476. [Google Scholar]
- 20.Ma Y, Tse JS. Ab initio determination of crystal lattice constants and thermal expansion for germanium isotopes. Solid State Commun. 2007;143:161–165. [Google Scholar]
- 21.Simon A. Superconductivity and chemistry. Angew Chem Int Engl. 1997;36:1788–1806. [Google Scholar]
- 22.Allen PB, Dynes RC. Transition temperature of strong-coupled superconductors reanalyzed. Phys Rev B. 1975;12:905–922. [Google Scholar]
- 23.Graetz J, Reilly JJ. Decomposition kinetics of the AlH3 polymorphs. J Phys Chem B. 2005;109:22181–22185. doi: 10.1021/jp0546960. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen P, Ledovskikh A, Danilov D, Notten PHL. Thermodynamics and kinetics of the thin film magnesium-hydrogen system. Acta Mater. 2009;57:4967–4973. [Google Scholar]
- 25.Gavra Z, Johnson JR, Reilly JJ. Decomposition kinetics of palladium nickel hydride. J Less-Common Met. 1991;172:107–115. [Google Scholar]
- 26.Oganov AR, Glass CW, Ono S. High-pressure phases of CaCO3: Crystal structure prediction and experiment. Earth Planet Sci Lett. 2006;241:95–103. [Google Scholar]
- 27.Oganov AR, Glass CW. Crystal structure prediction using ab initio evolutionary techniques: Principles and applications. J Chem Phys. 2006;124:244704. doi: 10.1063/1.2210932. [DOI] [PubMed] [Google Scholar]
- 28.Glass CW, Oganov AR, Hansen N. USPEX: Evolutionary crystal structure prediction. Comput Phys Commun. 2006;175:713–720. [Google Scholar]
- 29.Oganov AR, et al. Ionic high-pressure form of elemental boron. Nature. 2009;457:863–867. doi: 10.1038/nature07736. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, et al. Novel high pressure structures of polymeric nitrogen. Phys Rev Lett. 2009;102:065501. doi: 10.1103/PhysRevLett.102.065501. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, et al. Transparent dense sodium. Nature. 2009;458:182–185. doi: 10.1038/nature07786. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, et al. Twofold coordinated ground-state and eightfold high-pressure phases of heavy transition metal nitrides MN2 (M = Os, Ir, Ru, and Rh) Inorg Chem. 2009;48:9904–9909. doi: 10.1021/ic9014702. [DOI] [PubMed] [Google Scholar]
- 33.Li Q, et al. Superhard monoclinic polymorph of carbon. Phys Rev Lett. 2009;102:175506. doi: 10.1103/PhysRevLett.102.175506. [DOI] [PubMed] [Google Scholar]
- 34.Baroni S, Giannozzi P, Testa A. Green's-function approach to linear response in solids. Phys Rev Lett. 1987;58:1861–1864. doi: 10.1103/PhysRevLett.58.1861. [DOI] [PubMed] [Google Scholar]
- 35.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B. 1999;59:1758–1775. [Google Scholar]
- 36.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B. 1996;54:11169–11186. doi: 10.1103/physrevb.54.11169. [DOI] [PubMed] [Google Scholar]
- 37.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 38.Scandolo S, et al. First-principles codes for computational crystallography in the Quantum-ESPRESSO package. Z Kristallogr. 2005;220:574–579. [Google Scholar]
- 39.Troullier N, Martins JL. Efficient pseudopotentials for plane-wave calculations. Phys Rev B. 1991;43:1993–2006. doi: 10.1103/physrevb.43.1993. [DOI] [PubMed] [Google Scholar]
- 40.Dutta BN. Lattice constants and thermal expansion of silicon up to 900 °C by X-ray method. Physica Status Solidi B. 2006;2:984–987. [Google Scholar]
- 41.Olijnyk H, Sikka SK, Holzapfel WB. Structural phase transitions in Si and Ge under pressures up to 50 GPa. Phys Lett A. 1984;103:137–140. [Google Scholar]
- 42.Duclos SJ, Vohra YK, Ruoff AL. hcp to fcc transition in silicon at 78 GPa and studies to 100 GPa. Phys Rev Lett. 1987;58:775–777. doi: 10.1103/PhysRevLett.58.775. [DOI] [PubMed] [Google Scholar]
- 43.Degtyareva O, et al. Crystal structure of SiH4 at high pressure. Phys Rev B. 2007;76:064123. [Google Scholar]
- 44.Pickard CJ, Needs RJ. High-pressure phases of silane. Phys Rev Lett. 2006;97:045504. doi: 10.1103/PhysRevLett.97.045504. [DOI] [PubMed] [Google Scholar]
- 45.Chen X-J, et al. Superconducting behavior in compressed solid SiH4 with a layered structure. Phys Rev Lett. 2008;101:077002. doi: 10.1103/PhysRevLett.101.077002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.