Abstract
Ghrelin, an octanoylated peptide hormone produced in the stomach, rises dramatically in mouse plasma during chronic severe calorie deprivation, an event that is essential to maintain life. The mechanism for this increase is not understood. Here, we study the control of ghrelin secretion in tissue culture cells derived from mice bearing ghrelinomas induced by a tissue-specific SV40 T-antigen transgene. We found that the ghrelin-secreting cells express high levels of mRNA encoding β1-adrenergic receptors. Addition of norepinephrine or epinephrine to the culture medium stimulated ghrelin secretion, and this effect was blocked by atenolol, a selective β1-adrenergic antagonist. When WT mice were treated with reserpine to deplete adrenergic neurotransmitters from sympathetic neurons, the fasting-induced increase in plasma ghrelin was blocked. Inhibition was also seen following atenolol administration. We conclude that ghrelin secretion during fasting is induced by adrenergic agents released by sympathetic neurons and acting directly on β1 receptors on the ghrelin-secreting cells of the stomach.
Keywords: endocrine tumors, PG-1 cells, atenolol, octanoate, SV40-T antigen
Ghrelin, an octanoylated peptide hormone, is secreted by a highly specialized population of cells in stomach epithelium (1). Secretion is tightly regulated in response to circadian rhythms, food ingestion, stress, and other factors. Plasma ghrelin concentrations rise dramatically before meals and decline immediately after eating commences (2–4). Once released, ghrelin reaches the pituitary, where it binds to its receptor and stimulates the secretion of growth hormone. Ghrelin receptors are also located in several other central nervous system sites, including many known to be involved with body weight determination (1, 5). Administration of excess ghrelin leads to increased food intake in rodents and humans. Therefore, ghrelin has been considered to be an important factor in regulating food intake (1, 6).
In contrast to the unequivocal appetite-stimulating effect of excess ghrelin, deficiency of the hormone has little or no effect on food intake. Genetically engineered mice lacking ghrelin or its receptor gain nearly the same amount of weight as normal mice, except when the animals are placed on high-calorie diets early in life (7–10). Although ghrelin is not essential for food intake in mice, it is required for certain food reward behaviors that occur in the setting of chronic calorie restriction (11). Ghrelin is also essential in mice to prevent hypoglycemia and death when the animals are subjected to severe calorie restriction. The latter function of ghrelin became apparent in studies of genetically engineered mice that lack the gene encoding ghrelin O-acyltransferase (GOAT), the enzyme that attaches octanoate to ghrelin, a reaction that is essential for the biological activity of the hormone (12).
GOAT-deficient mice (Goat−/−) were unable to produce any active ghrelin, although they did secrete normal or even excess amounts of des-acyl ghrelin (12). When subjected to severe calorie restriction (daily intake of 40% of normal calories), WT and Goat−/− mice lost about 30% of body weight and 60% of adipose tissue mass over 3 d. Fasting blood glucose levels dropped to ≈60 mg/dL in both lines. Thereafter, in WT mice, blood glucose stabilized in this range and the mice exhibited normal behavior for the next 4 d. In Goat−/− mice, the blood sugar never stabilized. It reached levels of 12–36 mg/dL after 7 d, and the mice became moribund. In WT mice, glucose stabilization was associated with a progressive rise in plasma ghrelin levels and a consequent rise in plasma growth hormone. In Goat−/− mice, ghrelin remained undetectable and a consequent rise in growth hormone was blunted. Infusion of ghrelin or growth hormone into the calorie-restricted Goat−/− mice restored blood glucose to the same levels seen in WT mice and prevented death (12).
The studies in the Goat−/− mice highlighted the protective role of ghrelin in preventing hypoglycemia and death during periods of famine. The question arises as to the mechanism by which ghrelin secretion is regulated. Previous studies have shown that ghrelin secretion increases when sympathetic nerves are stimulated artificially (13) or when adrenergic hormones are infused locally into the stomach lining (14). The mechanism by which adrenergic stimulation enhances ghrelin secretion is not known.
Direct study of ghrelin secretion in isolated cells is difficult because these cells account for less than 1% of cells in the gastric epithelium (1, 15). To circumvent this problem, in the current studies, we produced ghrelin-secreting gastric and pancreatic tumors in mice through the use of a transgene encoding SV40 T-antigen under control of the preproghrelin promoter. From the tumors, we established permanent tissue culture cell lines that secrete ghrelin constitutively. In these cells, ghrelin secretion was stimulated by the adrenergic agents norepinephrine and epinephrine and inhibited by atenolol, a selective antagonist of the β1 class of adrenergic receptors (16). The mRNA for the β1 receptor was increased several hundred fold in the tumor cells as compared with the level in mRNA prepared from total stomach. Administration of reserpine or atenolol to mice prevented the fasting-induced increase in plasma ghrelin levels. We conclude that fasting-induced ghrelin secretion is mediated via the sympathetic nervous system acting through the β1-adrenergic receptor.
Results
Generation of Mice with Ghrelinomas in Stomach and Pancreas.
Fig. S1A shows the schematic of the construct used to generate transgenic mice that express SV40 large T-antigen in ghrelin cells (hereafter referred to as TgGhrelin-SV40-T). This construct was generated by BAC engineering, such that the SV40 T-antigen coding region was inserted immediately downstream of the start codon of preproghrelin, thereby allowing both the transcription and translation of SV40 T-antigen to be controlled by preproghrelin regulatory elements. The modified BAC was used to generate four independent lines of TgGhrelin-SV40-T mice. At 13–14 wk of age, in all transgenic mice, expression of SV40 T-antigen was highest in the stomach, where levels were more than 500-fold higher than those in the intestine and pancreas. All studies in this report were carried out in line H7. Identical findings were obtained in studies of line H2.
The levels of plasma ghrelin and des-acyl ghrelin rose progressively with age in TgGhrelin-SV40-T mice (Fig. S1 B and C). When measured at 20 wk of age in ad libitum-fed animals during the light cycle, the plasma ghrelin level in TgGhrelin-SV40-T mice (6.1 ± 0.3 ng/mL) was 25-fold higher than that in WT littermates (0.24 ± 0.02 ng/mL) (Fig. S1D). Similarly, the plasma des-acyl ghrelin level (16.3 ± 0.5 ng/mL) was 37-fold higher than that of WT littermates (0.44 ± 0.01 ng/mL) (Fig. S1D). Despite the sustained increase in plasma ghrelin levels, body weights in the TgGhrelin-SV40-T mice were indistinguishable from those in WT animals up to 20 wk of age (Fig. S1E). Moreover, we observed no differences in food intake between the two groups of mice studied in metabolic cages at 16 wk of age.
By 24 wk of age, all 22 of the autopsied TgGhrelin-SV40-T mice exhibited visible gastric tumors (Fig. S1F) and thickened gastric epithelium (Fig. S1G). Eleven of the mice also had tumors in the pancreas, 10 had tumors in the kidney, and 7 had tumors in the liver. All 7 of the mice with liver tumors had a tumor in the stomach plus a tumor in either the pancreas or the kidney. The liver tumors were multifocal, suggesting a metastatic origin.
Histologically, the gastric ghrelinomas consisted of sheets of neoplastic cells divided into smaller nests by a fibrovascular stroma (Fig. S2 A–F). The majority of the cells within the tumors were labeled with a riboprobe specific for ghrelin mRNA (Fig. S2G) but not with the corresponding sense riboprobe (Fig. S2H). Fig. S3 shows the histology of the pancreatic ghrelinomas. Many of the small- to medium-sized tumors contained surviving pancreatic islet cells, suggesting an islet origin (Fig. S3 A and B). The largest of the pancreatic tumors contained areas of hemorrhage and necrosis (Fig. S3A). Occasional subcapsular infiltrates of neoplastic cells were found in the peripancreatic lymph nodes (Fig. S3C).
Establishment of Ghrelinoma Cell Lines.
We next set out to establish ghrelinoma cell lines from the pancreatic and stomach tumors. To minimize contamination by fibroblasts and other cell types, the tumors were not treated with trypsin. Instead, after the tumor capsule was removed, the tumor cells were obtained by mechanical dispersion and incubated at a high density (3 × 106 cells per 35-mm well). The ghrelinoma cells did not attach to the plastic. They were collected by centrifugation and resuspended in fresh medium every week. The medium was assayed weekly for des-acyl ghrelin levels. The adaptation process required about 3 mo, after which the level of des-acyl ghrelin in the medium was >20 ng/mL. The resulting pancreatic and stomach ghrelinoma cell lines are designated as PG-1 and SG-1, respectively.
As a first step in characterizing the newly derived ghrelinoma cell lines, we treated the PG-1 cells with octanoate and assessed the effects on the synthesis and secretion of ghrelin, as measured quantitatively with a double-antibody sandwich technique. In the absence of octanoate, little ghrelin was found in the cells (Fig. 1A) or medium (Fig. 1B). After incubation with octanoate for 24 h, the cellular content of ghrelin was increased by 5-fold (from 11 to 54 pg/μg of cell protein), and the amount of ghrelin in the medium rose by more than 39-fold (from 6.7 to 264 pg/μg of cell protein). In contrast, octanoate did not alter the levels of des-acyl ghrelin in the cells (Fig. 1C) or the medium (Fig. 1D). SDS/PAGE and immunoblotting analysis of cells and medium with antibodies specific for the two forms of ghrelin (17) showed bands consistent with the known molecular weights of the two peptides.
Fig. 1.
Time-dependent stimulation of synthesis and secretion of ghrelin by octanoate in PG-1 cells. On day 0, PG-1 cells were set up in medium A with 10% delipidated FBS as described in Materials and Methods. On day 3, cells were centrifuged, resuspended in serum-free medium B, aliquoted into a 24-well plate (5 × 104 cells per well), and treated in the absence (△) or presence (▲) of 50 μM sodium octanoate-albumin. After incubation at 37 °C for the indicated time, the medium and cells from each well were harvested for measurement of ghrelin (A and B) and des-acyl ghrelin (C and D) levels. Each value is the average of duplicate incubations. Numbers in B denote the percentage of ghrelin relative to the total amount of ghrelin plus des-acyl ghrelin secreted into the medium.
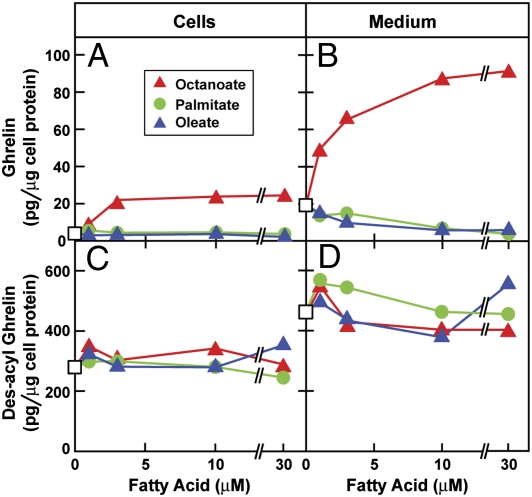
Whereas octanoate stimulated ghrelin synthesis (Fig. 2A) and secretion (Fig. 2B), neither palmitate nor oleate had any effect. Des-acyl ghrelin levels in the cells (Fig. 2C) or medium (Fig. 2D) were not affected by treatment with any of the fatty acids. In all subsequent experiments, we included 50 μM octanoate in the culture medium to provide substrate for ghrelin synthesis.
Fig. 2.
Dose-dependent stimulation of synthesis and secretion of ghrelin by octanoate in PG-1 cells. On day 0, PG-1 cells were set up in medium A with 10% delipidated FBS as described in Materials and Methods. On day 3, cells were centrifuged, resuspended in serum-free medium B, aliquoted into 24-well plates (5 × 104 cells per well), and treated with varying concentrations of the indicated fatty acid. After incubation at 37 °C for 24 h, the medium and cells from each well were harvested for measurement of ghrelin (A and B) and des-acyl ghrelin (C and D). Each value is the average of duplicate incubations. □, none.
Stimulation of Ghrelin Secretion in PG-1 and SG-1 Cells by β-Adrenergic Agonists but Not by Peptide Hormones.
Previous studies in rats showed that plasma ghrelin levels are increased by stimulation of the sympathetic nervous system (13, 14). To determine whether this response persists in vitro, we treated the PG-1 cells with two adrenergic agonists, norepinephrine and epinephrine. Both agonists stimulated secretion of ghrelin (Fig. 3B) and des-acyl ghrelin (Fig. 3D) in a dose-dependent manner. At 1 μM, both agents produced a 2.5-fold increase in ghrelin secretion. At lower concentrations, norepinephrine was 6-fold more potent than epinephrine with one-half maximal stimulation occurring at 0.1 vs. 0.6 μM (Fig. S4). The levels of ghrelin (Fig. 3A) and des-acyl ghrelin (Fig. 3C) in the cells were decreased by each of these treatments, suggesting that adrenergic agents primarily stimulate ghrelin secretion without immediately increasing synthesis.
Fig. 3.
Dose-dependent stimulation of ghrelin secretion by adrenergic but not cholinergic or GABA agonists in PG-1 cells. On day 0, PG-1 cells were set up in medium A with 10% FBS. On day 2, octanoate was added to the medium at a final concentration of 50 μM. On day 3, cells were centrifuged, resuspended in serum-free medium B with 50 μM octanoate, aliquoted into 24-well plates (5 × 104 cells per well), and treated with varying concentrations of the indicated compound. After incubation at 37 °C for 6 h, the medium and cells from each well were harvested for measurement of ghrelin (A and B) and des-acyl ghrelin (C and D). Each value is the average of duplicate incubations. □, none.
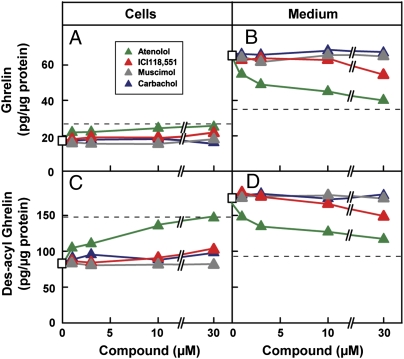
Inasmuch as epinephrine and norepinephrine stimulate the production of cAMP, we incubated the PG-1 cells with forskolin, a compound that directly activates adenylyl cyclase (18). Forskolin stimulated ghrelin secretion in a fashion that mimicked the two adrenergic agonists (Fig. 3). In contrast, neither muscimol (GABAA receptor agonist) nor carbachol (cholinergic receptor agonist) had any effect on the secretion of ghrelin in PG-1 cells (Fig. 3B).
To determine the time course of stimulation, we treated the PG-1 cells with various agonists for 1, 3, or 6 h. Treatment with epinephrine, norepinephrine, or forskolin increased secretion of ghrelin and des-acyl ghrelin in a fashion that was linear with time. The levels of ghrelin (Fig. 4A) and des-acyl ghrelin (Fig. 4C) in the cells were initially decreased by the adrenergic agents, but the levels then stabilized, suggesting that these agents initially stimulate the secretion of ghrelin and des-acyl ghrelin and that this is followed by increased synthesis. Again, neither muscimol nor carbachol had any effect on ghrelin production.
Fig. 4.
Time-dependent stimulation of ghrelin secretion by epinephrine and norepinephrine in PG-1 cells. On day 0, PG-1 cells were set up in medium A with 10% FBS. On day 2, octanoate was added to the medium at a final concentration of 50 μM. On day 3, cells were centrifuged, resuspended in serum-free medium B with 50 μM octanoate, aliquoted into 24-well plates (5 × 104 cells per well), and treated with 10 μM of the indicated compound. After incubation at 37 °C for the indicated time, the medium and cells from each well were harvested for measurement of ghrelin (A and B) and des-acyl ghrelin (C and D). Each value is the average of duplicate incubations.
Epinephrine and norepinephrine increase intracellular cAMP levels by binding to β-adrenergic receptors (19). To confirm the role of these receptors, we treated PG-1 cells with norepinephrine in the presence of various receptor agonists and antagonists. In the presence of norepinephrine, atenolol (a selective β1-adrenergic receptor antagonist) lowered the secretion of ghrelin (Fig. 5B) and des-acyl ghrelin (Fig. 5D) to basal levels, as indicated by the dashed lines. Simultaneously, atenolol increased the cellular content of ghrelin and des-acyl ghrelin (Fig. 5 A and C), consistent with a primary inhibition of secretion. In contrast, treatment with ICI118,551 (a selective β2-adrenergic receptor antagonist) had no effect on norepinephrine-stimulated secretion of ghrelin and des-acyl ghrelin. As expected, the norepinephrine-mediated increase of ghrelin secretion was not affected by treatment with muscimol, a GABAA receptor agonist, or carbachol, a cholinergic receptor agonist.
Fig. 5.
Effect of various receptor agonists and antagonists on norepinephrine-mediated stimulation of ghrelin secretion in PG-1 cells. On day 0, PG-1 cells were set up in medium A with 10% FBS. On day 2, octanoate was added to a final concentration of 50 μM. On day 3, cells were centrifuged, resuspended in serum-free medium B with 50 μM octanoate, aliquoted into 24-well plates (5 × 104 cells per well), and treated with 1 μM norepinephrine and different concentrations of the indicated compounds. After incubation at 37 °C for 6 h, the medium and cells from each well were harvested for measurement of ghrelin (A and B) and des-acyl ghrelin (C and D) levels. The dashed lines indicate the basal levels of ghrelin or des-acyl ghrelin without any treatment. Each value is the average of duplicate incubations.
Fig. 6 shows an experiment in which we treated PG-1 cells with 10 peptide hormones that regulate fuel metabolism or hormone secretion. At a concentration of 100 nM and an incubation time of 6 h, none of them affected the levels of ghrelin and des-acyl ghrelin in cells or in medium. Norepinephrine was included in the same experiment as a positive control.
Fig. 6.
Lack of effect of peptide hormones on secretion of ghrelin in PG-1 cells. On day 0, PG-1 cells were set up in medium A with 10% FBS. On day 2, octanoate was added to the medium at a final concentration of 50 μM. On day 3, cells were centrifuged, resuspended in serum-free medium B with 50 μM octanoate, aliquoted into 24-well plates (5 × 104 cells per well), and treated with 100 nM of the indicated peptide hormone. Norepinephrine (Norepi., added at 10 μM) was included as a positive control. After incubation at 37 °C for 6 h, the medium and cells from each well were harvested for measurement of ghrelin (A and B) and des-acyl ghrelin (C and D). Each bar represents the mean ± SEM of three incubations. GRP, gastrin-releasing peptide.
All the studies thus far were carried out in the pancreatic PG-1 ghrelinoma cells. We repeated these studies with stomach SG-1 ghrelinoma cells and obtained similar results. Fig. S5 shows a representative experiment. As in PG-1 cells (Figs. 2 and 3), secretion of ghrelin (Fig. S5A) and des-acyl ghrelin (Fig. S5B) in SG-1 cells was stimulated by epinephrine, norepinephrine, and forskolin but not by muscimol or carbachol. Likewise, insulin, insulin-like growth factor 1 (IGF-1), leptin, and glucagon did not have a discernible effect on the secretion of either ghrelin or des-acyl ghrelin in SG-1 cells (Fig. S5 C and D).
mRNA Expression Profiles in PG-1 Cells, SG-1 Cells, and Ghrelin Cells Isolated from the Stomach.
Table S1 shows an experiment in which we used quantitative real-time PCR to measure the expression levels of various hormones, hormone receptors, adrenergic receptors, and neuroendocrine markers in PG-1 and SG-1 ghrelinoma cells. For controls, we also measured the mRNA levels of the same genes in stomachs from WT and TgGhrelin-SV40-T mice. Inasmuch as ghrelin-secreting cells represent only a small proportion of stomach cells, we would expect that any mRNA that is selectively expressed in ghrelin-secreting cells should show a relative increase in the tumor cells as compared with whole stomach. Compared with stomach from WT mice, the PG-1 cells and SG-1 cells showed a 5.5- to 7.7-fold and 13.4- to 13.8-fold increase in the mRNA levels of preproghrelin and GOAT, respectively (Table S1, experiment A). These increases were similar to the corresponding 7.1- and 8.3-fold increases seen in the stomach ghrelinoma from a TgGhrelin-SV40-T mouse. Compared with stomach from WT mice, the PG-1 and SG-1 cells expressed very high levels of β1-adrenergic receptor (138- to 416-fold increases, respectively) but low to undetectable levels of β2 and β3 subtypes, further supporting the notion that norepinephrine stimulates ghrelin secretion in PG-1 and SG-1 cells via the β1-adrenergic receptor (Figs. 2 and 3 and Fig. S5). The PG-1 and SG-1 cells also expressed high levels of mRNA-encoding neuroendocrine proteins, including prohormone convertases PC1/3 and PC2; chromogranin A; and secretogranin II, III, and V. Neither PG-1 nor SG-1 cells showed detectable expression of genes encoding pancreatic islet hormones, including insulin, preproglucagon, pancreatic polypeptide, and somatostatin (Table S1, experiment B).
Table S2 shows an experiment in which we used quantitative real-time PCR to measure expression of various adrenergic receptors in ghrelin-enriched (GFP+) and nonenriched (GFP−) gastric mucosal cells. Gastric mucosal cells from mice harboring a transgene encoding GFP driven by the mouse preproghrelin promoter (15) were separated by FACS as previously described (20). Compared with nonenriched gastric mucosal cells, the ghrelin-enriched cells showed increases in the mRNA levels of preproghrelin (8,870-fold) and GOAT (90-fold), as expected. The ghrelin cell-enriched population also expressed very high levels of β1-adrenergic receptors (59-fold increase). Of the eight other adrenergic receptors examined in PG-1 and SG-1 cells, the only one that showed enrichment was the mRNA for the α1B receptor (37- to 65-fold increase). This increase did not appear to be functionally significant in that a α1-selective agonist (methoxamine) and a α1-selective antagonist (prazosin) (16) did not affect secretion of ghrelin or des-acyl ghrelin in PG-1 cells (Table S3). Moreover, the mRNA for the α1B receptor was not detected in the FACS-separated ghrelin cells from the transgenic mice (Table S2).
In Vivo Role of Sympathetic Nervous System in Fasting-Induced Ghrelin Secretion.
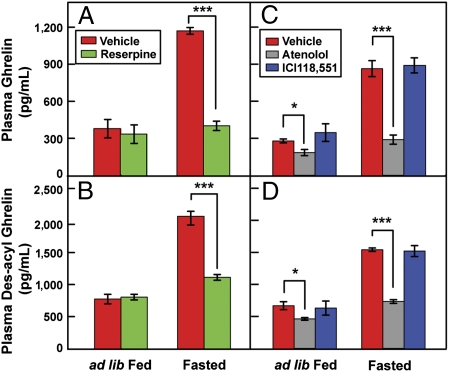
We next sought to determine whether adrenergic activity is essential for the rise in plasma ghrelin induced by fasting. In initial experiments, we depleted the sympathetic nervous system of catecholamines by treating mice with reserpine. This agent did not affect plasma ghrelin levels in fed animals (Fig. 7A). However, reserpine completely blocked the increase in plasma ghrelin (Fig. 7A) and des-acyl ghrelin (Fig. 7B) that was induced by a 24-h fast. To determine whether the adrenergic response is mediated by β1-adrenergic receptors, we treated mice with atenolol, the selective β1-antagonist. As a control in the same experiment, we treated mice with ICI118,551, a selective antagonist of β2-adrenergic receptors. Even in the fed state, atenolol lowered plasma levels of ghrelin (Fig. 7C) and des-acyl ghrelin (Fig. 7D). Moreover, atenolol abolished the increase in both peptides that was induced by fasting (Fig. 7 C and D). ICI118,551 had none of these effects.
Fig. 7.
Suppression of fasting-induced ghrelin secretion by reserpine (A and B) and atenolol (C and D). Male C57BL/6J mice (8 wk of age) were injected i.p. with either reserpine (0.5 mg/kg of body weight) or its vehicle (0.02% acetic acid) once daily (8:30 AM) for 3 d (A and B). A separate group of mice was injected i.p. with atenolol (10 mg/kg), ICI118,551 (1.0 mg/kg), or their vehicles (2 mM HCl) twice daily (8:30 AM and 8:30 PM) for 3 d (C and D). [A 10-fold lower dose of ICI118,551 relative to atenolol was used because of ICI118,511’s 550-fold higher affinity for β2 than β1 receptor (31)]. On day 2, blood samples were obtained at 10:00 AM from all mice (ad lib-Fed), after which the same mice were fasted for 24 h. On day 3, mice were euthanized at 10:00 AM, and blood was collected (Fasted). The blood samples were used for measurement of levels of plasma ghrelin (A and C) and des-acyl ghrelin (B and D). Each value represents the mean ± SEM of data from four mice. Asterisks denote the level of statistical significance (Student's t test). *P < 0.05; ***P < 0.001.
Discussion
The current studies were designed to further our understanding of the mechanisms underlying the large changes in plasma ghrelin levels that are associated with circadian rhythms and fasting. Understanding of the molecular mechanisms that control ghrelin secretion has been hampered because ghrelin-secreting cells make up only a tiny fraction of cells in the gastric mucosa. Accordingly, our first step was to prepare a transgene that employs the preproghrelin promoter to drive expression of the SV40 T-antigen. The resulting mice developed gastric and pancreatic tumors that were composed of ghrelin-secreting cells. These experiments followed the precedent set by Hanahan and colleagues (21–24), who used a similar strategy to produce insulinomas, glucagonomas, and carcinoid tumors as well as cell lines derived from these tumors. The ghrelinomas that we produced were used as a source from which to prepare lines of immortalized tissue culture cells that secrete ghrelin.
When studied in tissue culture, both the pancreatic and gastric ghrelin-secreting tumor cells exhibited the features expected of neuroendocrine cells. As shown in Table S1, their mRNAs were enriched for components of the secretory pathway, including chromogranins, secretogranins, and prohormone convertases. There was little difference between SG-1 cells obtained from stomach tumors and PG-1 cells obtained from pancreatic tumors (Table S1, experiment A). The cells were enriched in the mRNAs encoding preproghrelin and GOAT, but there was no enrichment for a variety of peptide hormone receptors (i.e., receptors for growth hormone, glucagon, leptin, IGF-1, insulin) (Table S1, experiment A). There was also no enrichment in the mRNAs for the classic pancreatic islet hormones (i.e., insulin, preproglucagon, somatostatin) (Table S1, experiment B). Notably, cells from stomach and pancreatic tumors were highly enriched in the mRNA encoding the β1-adrenergic receptor (138- to 416-fold increase), as were FACS-separated gastric ghrelin cells isolated from transgenic preproghrelin-GFP mice (Table S2).
The cultured ghrelinoma cells required exogenous octanoate to secrete appreciable amounts of ghrelin (Fig. 1). Long-chain fatty acids such as oleate and palmitate failed to substitute for octanoate (Fig. 2). These findings are consistent with previous whole-animal data indicating that ghrelin secretion is dependent on an exogenous source of octanoate (25). It appears that the ghrelin-secreting cells cannot synthesize octanoate de novo, nor can they shorten long-chain fatty acids.
The most important finding in the current study was the specific stimulation of ghrelin secretion by the adrenergic hormones norepinephrine and epinephrine (Figs. 3 and 4). Previous studies have shown that ghrelin secretion rose in vivo in response to stimulation of sympathetic nerves (13, 26) or by local infusion of adrenergic hormones into the stomach lining (14). Epinephrine i.v. infusion did not increase plasma ghrelin, suggesting that local release is important (13). The current findings are consistent with these results and demonstrate that the adrenergic agents act directly on β1 receptors in ghrelin-secreting cells.
Activation of the β1 receptor triggers an increase in cAMP levels. Indeed, the adrenergic effect on the ghrelinoma cells was mimicked by forskolin, an activator of adenylyl cyclase (18, 19). The effect was antagonized by atenolol, a selective β1 blocker. These findings are consistent with the demonstration that the ghrelin-secreting cells are markedly enriched in the mRNA encoding the β1 receptor. Additional evidence for β1 involvement was the observation that norepinephrine was 6-fold more potent on a molar basis than epinephrine in stimulating ghrelin secretion (Fig. S4), a finding consistent with the differential affinities of the β1 receptor for these agents (27).
We confirmed the role of β1 receptors in vivo by administering atenolol to mice (Fig. 7 C and D). This agent prevented the increase in plasma ghrelin that normally follows a 24-h fast. Depletion of neuronal catecholamines with reserpine also prevented the increase of ghrelin on fasting (Fig. 7 A and B). In as much as reserpine does not affect adrenal catecholamines (16), the reserpine blockade is consistent with the notion that fasting acts through the sympathetic nervous system to release catecholamines in proximity to the ghrelin-secreting cells. In vivo, the sympathetic neurotransmitter is primarily norepinephrine rather than epinephrine (16), consistent with the role of β1 receptors as the relevant adrenergic receptor subtype in ghrelin-secreting cells.
The aforementioned data provide strong support to the idea that the fasting-induced elevation in plasma ghrelin is produced by locally released norepinephrine acting directly on ghrelin-secreting cells through the β1 receptor. The sympathetic nervous system is also likely to mediate the ghrelin elevation observed in several models of acute or chronic stress (reviewed in ref. 28). Inasmuch as atenolol is a commonly used β-adrenergic agent in humans, it should be possible to determine whether this agent blocks the rise in plasma ghrelin that is known to be induced by fasting or in various settings of stress. If so, atenolol might prove useful in probing the physiologic function of ghrelin in humans.
In the current studies, we did not seek to characterize the effects of chronic ghrelin elevations on metabolic parameters in the tumor-bearing mice. We did note that the body weights of these mice were the same as those in control mice despite the massive elevation in plasma ghrelin (Fig. S1). This result differs from that reported by Iwakura et al. (29), who also used the SV40 T-antigen to create mice bearing ghrelinomas. These researchers found that the body weights in their mice with ghrelinomas were slightly reduced in association with a slight reduction in food intake. We failed to see this difference even though the plasma levels of ghrelin in our mice were somewhat higher than those studied by Iwakura et al. (29) (6.1 ng/mL in the Dallas mice as compared with 1 ng/mL in the Kyoto/Osaka mice at 16–18 wk of age). Body weight changes are difficult to interpret in ghrelinoma-bearing mice because body weight likely reflects a balance between cancer-related cachexia and ghrelin-stimulated appetite. In preliminary studies, we did find elevated fasting plasma glucose levels in our mice, which agrees with the finding reported by Iwakura et al. (29).
Recently, Iwakura et al. (30) also reported the establishment of ghrelin-secreting cell lines from their ghrelinoma-bearing mice. There are two main differences between our results and theirs. First, Iwakura et al. (30) did not study the effects of adrenergic agents on ghrelin secretion. Second, our ghrelinoma cell lines are grown under different conditions than the MGN3-1 cells of Iwakura et al. (30). Our PG-1 and SG-1 cells are grown in suspension culture in the absence of a feeder layer, whereas the MGN3-1 cells are cultured on a feeder layer of mitomycin C-treated embryonic fibroblasts. The PG-1 cell line has been grown continuously for more than 100 generations, and aliquots have been frozen in liquid nitrogen for more than 8 mo without a change in either growth properties or β-adrenergic stimulation of ghrelin secretion. PG-1 cells may prove especially useful in screening for inhibitors of GOAT or other molecules essential for ghrelin secretion.
Materials and Methods
Establishment of Ghrelinoma Cell Lines from Pancreas and Stomach.
TgGhrelin-SV40-T mice (6 mo of age) were anesthetized by i.p. injection of chloral hydrate (500 mg/kg). The tumor-containing pancreas and stomach were dissected, and the capsules were removed, washed with PBS, and then placed in 10 mL of medium A [DMEM/F-12 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 1% (vol/vol) insulin-transferrin-selenium-X (Invitrogen)] containing 10% (vol/vol) FBS. The tissues were cut into 1- to 2-mm2 pieces, which were then mechanically dispersed by pipetting up and down 20–30 times.
A pancreatic ghrelinoma cell line was established as follows. The dispersed cells were passed through a 100-μm filter and then centrifuged at 500 × g for 5 min at room temperature. The cell pellet containing 9 × 106 cells was resuspended in 9 mL of medium A with 10% (vol/vol) FBS. Aliquots of the suspension were seeded into a six-well Costar plate (catalog no. 3516; Corning) at a density of ∼3 × 106 cells per 35-mm well. The cells were cultured in a 37 °C incubator with 5% (vol/vol) CO2 and 5% (vol/vol) O2. Every week, the unattached cells in the medium from each well were collected by centrifugation at 1,000 × g for 5 min and resuspended in 3 mL of fresh medium A with 10% FBS. The centrifuged medium was assayed for its concentration of des-acyl ghrelin. After 3 mo of repeated weekly centrifugations and resuspensions, the level of des-acyl ghrelin in the medium reached a concentration of ≥20 ng/mL Thereafter, the cells were split at a ratio of 1:1.5, passaged every 3 or 4 d, and frozen in multiple aliquots in liquid nitrogen. The resulting pancreatic cell line, designated PG-1, was derived from TgGhrelin-SV40-T mouse 8175.
A stomach ghrelinoma cell line was derived essentially by the same procedure as described above. This cell line, designated SG-1, was derived from TgGhrelin-SV40-T mouse 1672.
Cell Culture.
Cells were maintained in medium A with 10% FBS in a 37 °C incubator with 5% CO2 and 5% O2. For experiments, cells were plated (day 0) onto 100-mm dishes at a density of 5 × 104 cells/mL. On day 2, sodium octanoate-albumin was added to the medium at a final concentration of 50 μM. On day 3, cells were centrifuged, resuspended in serum-free medium B (DMEM low-glucose containing 100 U/mL penicillin and 100 μg/mL streptomycin sulfate), aliquoted into Costar 24-well plates (1 mL per 18-mm well), and treated with different compounds as described in the figure legends. After incubation at 37 °C for various times, the medium and cells from each well were harvested for measurement of ghrelin and des-acyl ghrelin levels.
Other Methods.
Additional information, including primers for real-time PCR (Table S4), can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Doug Hanahan (University of California at San Francisco and Swiss Institute for Experimental Cancer Research) for his generosity in providing the RIP1-tag plasmid and for helpful discussions; Lisa Beatty for invaluable help in establishing the PG-1 and SG-1 cell lines; and Sherri Osborne-Lawrence for excellent technical assistance in generating transgenic mice. This work was supported by the National Institutes of Health (Grants HL20948, K08DK068069, and R01DA024680) and grants from the Perot Family Foundation. J.M.Z. is the recipient of a Disease-Oriented Clinical Scholars Award from the University of Texas Southwestern Medical Center. R.L.L. was supported by Medical Scientist Training Grant ST32GM08014.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011116107/-/DCSupplemental.
References
- 1.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 2.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 3.Small CJ, Bloom SR. Gut hormones and the control of appetite. Trends Endocrinol Metab. 2004;15:259–263. doi: 10.1016/j.tem.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Nass R, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wortley KE, et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest. 2005;115:3573–3578. doi: 10.1172/JCI26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zigman JM, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149:843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perello M, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao TJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mundinger TO, Cummings DE, Taborsky GJ., Jr Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- 14.de la Cour CD, Norlén P, Håkanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: A microdialysis study. Regul Pept. 2007;143:118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Sakata I, et al. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155:91–98. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westfall TC, Westfall DP. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. Brunton LL, Lazo JS, Parker KL, editors. New York: McGraw–Hill; 2006. pp. 237–295. [Google Scholar]
- 17.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol. 2003;23:305–314. doi: 10.1023/A:1023684503883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibley DR, Lefkowitz RJ. Beta-adrenergic receptor-coupled adenylate cyclase. Biochemical mechanisms of regulation. Mol Neurobiol. 1987;1:121–154. doi: 10.1007/BF02935266. [DOI] [PubMed] [Google Scholar]
- 20.Sakata I, et al. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab. 2009;297:E134–E141. doi: 10.1152/ajpendo.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D. Heritable formation of pancreatic β-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 22.Efrat S, et al. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci USA. 1988;85:9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powers AC, et al. Proglucagon processing similar to normal islets in pancreatic alpha-like cell line derived from transgenic mouse tumor. Diabetes. 1990;39:406–414. doi: 10.2337/diab.39.4.406. [DOI] [PubMed] [Google Scholar]
- 24.Grant SGN, Seidman I, Hanahan D, Bautch VL. Early invasiveness characterizes metastatic carcinoid tumors in transgenic mice. Cancer Res. 1991;51:4917–4923. [PubMed] [Google Scholar]
- 25.Nishi Y, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146:2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 26.Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul Pept. 2008;146:12–18. doi: 10.1016/j.regpep.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Frielle T, et al. Cloning of the cDNA for the human β1-adrenergic receptor. Proc Natl Acad Sci USA. 1987;84:7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang J-C, Zigman JM. Ghrelin's roles in stress, mood, and anxiety regulation. Int J Peptides. 2010 doi: 10.1155/2010/460549. Article ID 460549:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwakura H, et al. A mouse model of ghrelinoma exhibited activated growth hormone-insulin-like growth factor I axis and glucose intolerance. Am J Physiol Endocrinol Metab. 2009;297:E802–E811. doi: 10.1152/ajpendo.00205.2009. [DOI] [PubMed] [Google Scholar]
- 30.Iwakura H, et al. Establishment of a novel ghrelin-producing cell line. Endocrinology. 2010;151:2940–2945. doi: 10.1210/en.2010-0090. [DOI] [PubMed] [Google Scholar]
- 31.Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.