Abstract
Protein tyrosine phosphatases (PTPs) are regulated through reversible oxidation of the active-site cysteine. Previous studies have implied soluble reactive oxygen species (ROS), like H2O2, as the mediators of PTP oxidation. The potential role(s) of peroxidized lipids in PTP oxidation have not been described. This study demonstrates that increases in cellular lipid peroxides, induced by disruption of glutathione peroxidase 4, induce cellular PTP oxidation and reduce the activity of PDGF receptor targeting PTPs. These effects were accompanied by site-selective increased PDGF β-receptor phosphorylation, sensitive to 12/15-lipoxygenase (12/15-LOX) inhibitors, and increased PDGF-induced cytoskeletal rearrangements. Importantly, the 12/15-LOX–derived 15-OOH-eicosatetraenoic acid lipid peroxide was much more effective than H2O2 in induction of in vitro PTP oxidation. Our study thus establishes that lipid peroxides are previously unrecognized inducers of oxidation of PTPs. This identifies a pathway for control of receptor tyrosine kinase signaling, which might also be involved in the etiology of diseases associated with increased lipid peroxidation.
Keywords: phospholipid hydroperoxide glutathione peroxidase, PDGF, redox regulation, reversible oxidation
Signaling through receptor tyrosine kinases (RTKs), such as the PDGF β-receptor, is subjected to negative control by protein tyrosine phosphatases (PTPs) (1–3). Consequently, alterations in expression levels or the specific activity of PTPs will affect the cellular response to ligands of RTKs. With regard to the PDGF β-receptor, a series of PTPs, including T cell protein tyrosine phosphatase (TC-PTP), density enhanced phosphatase-1 (DEP-1), SHP-2, and PTP-1B, have been found to modulate receptor signaling (4–7). Detailed analyses of the consequences of deletion of individual PTPs have shown that individual PDGF receptor-antagonizing PTPs preferentially dephosphorylate particular phospho-tyrosine residues of the receptor and thereby are able to modulate the signaling output (4, 8–10).
Inhibitory and reversible oxidation of the active-site cysteine has emerged as a general mechanism for PTP regulation (11, 12). As a consequence of the microenvironment of the conserved active site of PTPs, the catalytic cysteine of PTPs usually exists as thiolate anion, which is highly susceptible to oxidation. Studies in cellular models, as well as in vitro studies, indicate that PTPs display intrinsic differences in oxidation susceptibility (13–15). PTP oxidation has been shown after activation of reactive oxygen species (ROS)-inducing cell-surface receptors, such as RTKs, G protein-coupled receptors (GPCRs), integrins, B cell receptors, and T cell receptors (6, 16–20). Manipulation of the expression levels of reducing enzymes such as peroxiredoxin II, cytosolic glutaredoxin, and glutathione peroxidase 1 has also been shown to affect RTK signaling and PTP oxidation in vitro and in vivo (21–23). In most of these cases, the PTP oxidation could be reverted by addition of the soluble antioxidant N-acetyl-cysteine (NAC) or DTT, and soluble ROS, such as H2O2, have been implied as the mediator of PTP oxidation. The importance of spatial control of ROS production was recently emphasized by demonstration of the site-restricted inactivation of peroxiredoxins after RTK activation (24).
Glutathione peroxidases (GPxs) play key functions in the control of cyclooxygenase and lipoxygenase (LOX) activities (25). GPxs, a family of eight enzymes, are required for the scavenging of H2O2 and (phospho-)lipid hydroperoxides. Glutathione peroxidase 4 (Gpx4; also named phospholipid hydroperoxide glutathione peroxidase) was initially discovered as an enzyme efficiently protecting liposomes and biomembranes from peroxidative degradation, preferentially acting at the membrane, where it reduces complex phospholipid hydroperoxides and oxidized cholesterylester in lipoproteins (26). Unlike other glutathione peroxidases, Gpx4 is not restricted to glutathione (GSH) as electron source but is also able to use protein thiols as reducing substrates when GSH becomes limiting. Under these conditions Gpx4 converts into a protein thiol peroxidase (27–29). To date, Gpx4 has been shown to be the sole Gpx being essential for early embryonic development (30). Using mice and cells with inducible disruption of Gpx4 (31), it was recently shown that Gpx4 along with GSH is a sensor of oxidative stress and a specific regulator of a 12/15-LOX–dependent and apoptosis-inducing factor–mediated cell death pathway (31, 32).
In this study we took advantage of the recently described inducible Gpx4 disruption system (31) to investigate the effects of peroxidized lipids on PTP oxidation.
Results
Gpx4 Deletion Leads to an Increase in Cellular PTP Oxidation.
As previously reported, Gpx4 disruption, involving 4-hydroxytamoxifen (Tam)-inducible disruption of Gpx4 in mouse embryonic fibroblasts, caused an NAC-insensitive substantial lipid peroxidation 30 h after Tam treatment (Fig. S1 A and B). Tam treatment of MERCreMER;Gpx4+/fl control cells did not induce lipid peroxidation (Fig. S1C).
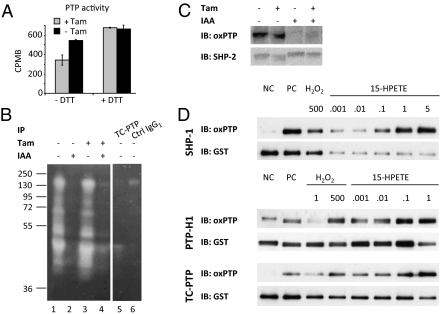
The effects of increased lipid peroxidation on PTP oxidation were analyzed in Gpx4-deleted cells. The lysates from the Gpx4−/− cells displayed a much lower PTP activity, as compared with control lysates, when assays were performed in the absence of DTT (Fig. 1A). In contrast, both lysates displayed equal activity when DTT was included in the in vitro dephosphorylation experiment. The “reverse in-gel assay” for PTP oxidation (6) was also used to monitor the effects of Gpx4 deletion on PTP oxidation. As shown in Fig. 1B, Gpx4−/− cells were characterized by an increase in PTP oxidation, as revealed by an increased PTP activity in lane 4 as compared with lane 2. Immunoprecipitated TC-PTP (lane 5) comigrated with the most prominent oxidized PTP in Gpx4−/− cells (lane 4), suggesting that this phosphatase might be one of the PTPs affected in Gpx4−/− cells.
Fig. 1.
Peroxidized lipids induce inhibitory oxidation of PTP activity. (A) Measurement of total PTP activity in the absence of exogenous reducing agent revealed that Gpx4 knockout cells (+ Tam, 60 h) had significantly less PTP activity compared with control cells (- Tam). No significant difference in PTP activity was observed when assays were performed in the presence of 10 mM DTT. (B) “Reverse in-gel assay” of PTP oxidation revealed increased PTP oxidation in Gpx4−/− cells (lane 4) compared with wild-type cells (lane 2). Immunoprecipitated TC-PTP from nonalkylated lystates of control cells (lanes 5 and 6) comigrates with the major oxidized PTP in the Gpx4−/− cells. (C) Analyses of SHP-2 oxidation using the oxPTP antibody demonstrated increased oxidation of SHP-2 in Gpx4−/− cells. (D) In vitro treatment of purified GST-tagged PTPs (SHP-1, PTP-H1, TC-PTP) induced PTP oxidation in a dose-dependent manner. 15-HPETE–induced PTP oxidation was compared with H2O2 treatment. Concentrations are given in micromolars [NC (negative control) represents samples that have not been treated with pervanadate, whereas PC (positive control) indicates samples that have been pervanadate treated in the absence of alkylation].
An increase in SHP-2 oxidation was also detected (Fig. 1C) using the oxPTP antibody-based method for PTP oxidation (14). Independent evidence for increased PTP oxidation in Gpx4−/− cells was also provided by analyses of LAR oxidation (Fig. S2) using an alternative assay for PTP oxidation, which relies on differences in alkylation-sensitivity of reduced and oxidized PTPs (33).
Together these analyses thus demonstrate that increased lipid peroxidation in Gpx4 null cells is associated with an increase in PTP oxidation.
Purified Peroxidized Lipids Induce PTP Oxidation in Vitro.
To substantiate the findings from the Gpx4-deleted cells, in vitro experiments were performed to analyze whether peroxidized lipids were able to induce PTP oxidation.
For this purpose an antibody-based PTP oxidation assay, previously used to monitor PTP oxidation in vitro, was applied (13). Fig. 1D shows 15-hydroperoxy-eicosatetraenoic acid (15-HPETE)–induced prominent oxidation of GST-tagged SHP-1, PTP-H1, and TC-PTP in a dose-dependent manner. Most interestingly, these 15-HPETE–mediated effects occurred at concentrations in the nanomolar range, whereas micromolar concentrations of H2O2 were required to obtain similar oxidizing effects. Importantly, the 15-HPETE effects could be reverted almost to background levels when Trolox, a water-soluble vitamin E derivative, was included in the 15-HPETE–treated samples (Fig. S3).
This experiment thus demonstrates a previously unrecognized ability of peroxidized arachidonic acid-derived lipids to induce oxidation of PTPs.
Gpx4−/− Cells Display Augmented PDGF β-Receptor Phosphorylation and Reduced PDGF Receptor Dephosphorylating Activity.
PDGF β-receptor phosphorylation and signaling is strongly influenced by multiple PTPs, including TC-PTP, PTP-1B, and DEP-1 (4, 34–36). We therefore investigated whether the increase in PTP oxidation in the Gpx4−/− cells was associated with changes in PDGF β-receptor phosphorylation.
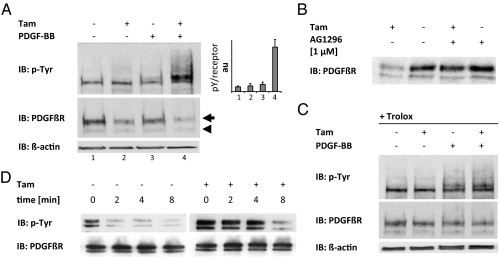
Tam-induced Gpx4 depletion in MERCreMER;Gpx4fl/fl cells resulted in an increased PDGF β-receptor phosphorylation after stimulation with 10 ng/mL PDGF-BB (lane 4, Fig. 2A), as compared with cells not treated with Tam (lane 3, Fig. 2A). When normalized for the amount of receptor protein, the ligand-stimulated Gpx4−/− cells displayed an ≈10-fold higher phosphorylation/receptor level, as compared with Gpx4-expressing cells.
Fig. 2.
Gpx4 null cells show reduced levels of mature PDGF β-receptor, increased receptor phosphorylation, and reduced activity of receptor-targeting PTPs. (A) Gpx4−/− cells (+ Tam) showed increased receptor phosphorylation levels in response to PDGF-BB stimulation for 3 min (lane 4) as compared with control cells (lane 3). In addition, knockout cells (lanes 2 and 4) displayed reduced levels of mature PDGF β-receptor (arrow), whereas the levels of immature PDGF β-receptor remained constant (arrowhead). (B) Treatment with the PDGF β-receptor–inhibitor AG1296 increased receptor levels in Tam-treated cells. (C) Trolox blocked the PDGF β-receptor down-regulation and the increased β-receptor phosphorylation observed in Gpx4−/− cells. (D) Monitoring of in vivo dephosphorylation of the PDGF β-receptor revealed a reduced rate of dephosphorylation in knockout cells.
Gpx4 depletion was also associated with a reduction in the steady-state levels of PDGF β-receptor (lanes 1 and 3 vs. lanes 2 and 4, Fig. 2A). By contrast, none of these changes could be observed in Tam-treated MERCreMER;Gpx4+/fl control cells (Fig. S4A) or in Gpx4−/− cells in which Gpx4 expression was reconstituted (Fig. S4B).
PDGF receptor phosphorylation leads to internalization and degradation. It was therefore hypothesized that the reduced expression levels of PDGF receptors after Gpx4 deletion in unstimulated cells was caused by increased ligand-independent receptor phosphorylation, internalization, and degradation. To test this, cells were treated with the PDGF β-receptor inhibitor AG1296 (37), and the effects on the levels of receptor expression were analyzed. As shown in Fig. 2B, treatment with AG1296 led to a clear increase in the levels of receptor protein in Tam-treated cells, whereas the inhibitor had only minor effects on receptor levels in noninduced cells.
Treatment with Trolox has previously been shown to reduce lipid peroxidation, caused by Gpx4 deletion, to background levels (31). As shown in Fig. 2C, Trolox fully blocked the effects on PDGF β-receptor phosphorylation caused by Gpx4 deletion. Furthermore, NAC treatment, which did not change the levels of peroxidized lipids (Fig. S1B), failed to revert the Gpx4-dependent alterations in PDGF β-receptor expression and phosphorylation (Fig. S5A).
To further demonstrate that the increased PDGF β-receptor phosphorylation in Gpx4-deleted cells was caused by reduced activity of PDGF β-receptor–targeting PTPs, we analyzed the rate of in vivo receptor dephosphorylation by monitoring PDGF receptor phosphorylation at different time points after addition of the PDGF receptor inhibitor AG1296. As depicted in Fig. 2D, p-Tyr of the PDGF β-receptor remained high for a longer time in Gpx4-deleted cells, in accordance with decreased PDGF-receptor–targeting PTP activity.
We thus conclude from these experiments that the lipid peroxidation caused by Gpx4 deletion leads to a reduction in the activity of PDGF β-receptor–targeting PTPs and an increased PDGF β-receptor phosphorylation.
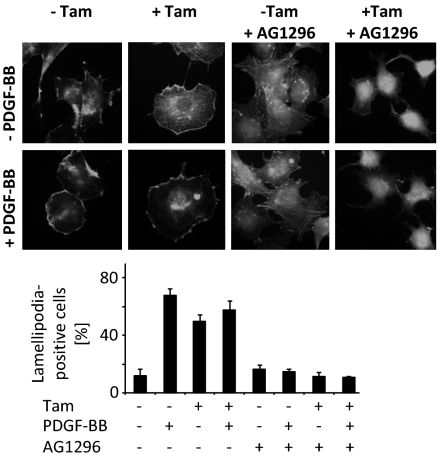
Increased PDGF Receptor-Dependent Lamellipodia Formation in Gpx4−/− Cells.
We next asked whether the augmented PDGF β-receptor phosphorylation in Gpx4-deleted cells also increased cellular responses induced by PDGF β-receptor activation. For this purpose we analyzed the formation of lamellipodia, which are formed after PDGF β-receptor activation (38). As expected from previous studies, control cells showed a PDGF-dependent increase in lamellipodia (Fig. 3). In contrast, Gpx4-deleted cells displayed high constitutive levels of lamellipodia. Pretreatment with the PDGF receptor inhibitor blocked the lamellipodia formation induced by Gpx4 deletion, as well as the PDGF-induced response (Fig. 3). The prominent effect of AG1296 on lamellipodia formation in unstimulated cells is compatible with an enhanced ligand-independent PDGF receptor activation suggested by earlier experiments (Fig. 2).
Fig. 3.
Gpx4-deficient cells display a PDGF receptor-dependent increase in lamellipodia formation. Filamentous actin was visualized by FITC-labeled phalloidin. For determination of the cell morphology phenotypes, cells were scored according to whether the treatment had induced the formation of lamellipodia at the cell margin. At least 100 cells were scored in triplicates for each condition. Data represent mean ± SD.
Site-Specific Alterations in PDGF β-Receptor Phosphorylation Pattern, and Selective Activation of PLC-γ1, After Gpx4 Deletion.
The PTPs involved in PDGF β-receptor dephosphorylation exert their activity in a site-specific manner (4, 8, 10). TC-PTP depletion leads to a preferential increase in phosphorylation of Y1021, whereas Y579 is particularly increased after PTP-1B deletion (4). As shown in Fig. S6, the effects of Tam-induced Gpx4 deletion varied between the different autophosphorylation sites, such that the increase in phosphorylation of Y1009 and Y1021 was much more prominent than the increase in phosphorylation of Y751 and Y771.
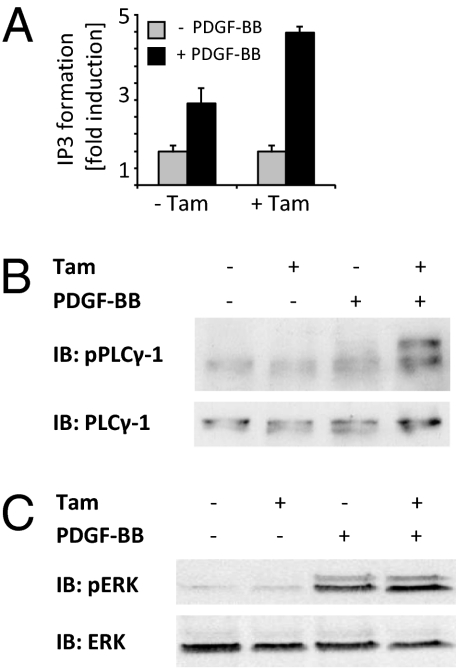
Consistent with the site-specific changes in phosphorylation pattern, differential effects on downstream effector molecules were also observed (Fig. 4 A–C). Phospholipase Cγ-1 (PLCγ-1) activation, dependent on phosphorylation of Y1021, was clearly augmented in Gpx4−/− cells as measured by ligand-induced inositol 1,4,5-trisphosphate (IP3) formation (Fig. 4A) and PLCγ-1 phosphorylation (Fig. 4B). In contrast, ERK phosphorylation was not affected by Gpx4 depletion (Fig. 4C).
Fig. 4.
Gpx4 deletion leads to pathway-specific alterations in PDGF β-receptor signaling. (A and B) Analysis of PLCγ-1 activity by monitoring IP3 formation revealed an increased response to PDGF-BB stimulation in knockout cells (A), as also confirmed by immunoblotting against tyrosine-phosphorylated PLCγ-1 (B). (C) No differences in Erk phosphorylation were detectable between control cells and knockout cells.
We thus conclude that the lipid peroxidation caused by Gpx4 deletion affects different site-specific PTPs to a variable extent.
12/15-LOX Dependence of Lipid Peroxidation and PDGF β-Receptor Phosphorylation in Gpx4−/− Cells.
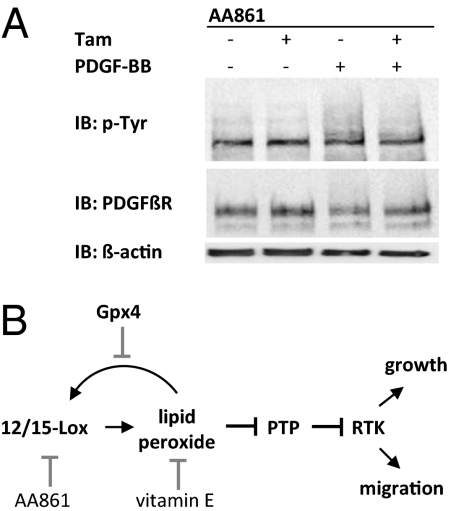
A final set of experiments was performed to describe in more detail the pathways and components that contributed to the increased lipid peroxidation after Gpx4 inactivation. These efforts focused on the regulators of arachidonic acid pathway, because Gpx4 has been identified as an antagonizer of arachidonic acid-oxygenating enzymes. Interestingly, addition of the 12/15-LOX inhibitor AA861 reduced the levels of lipid peroxidation in Tam-treated MERCreMER;Gpx4fl/fl cells to background levels (Fig. S7A). In agreement with a stimulatory effect of lipid peroxidation on PDGF β-receptor phosphorylation, AA861 also reverted the increased PDGF β-receptor phosphorylation in Gpx4-deleted cells (Fig. 5A). In contrast, the cyclooxygenase inhibitor indomethacin and the 5-LOX activating protein inhibitor MK886 had no major effects on PDGF β-receptor phosphorylation (Fig. S7B).
Fig. 5.
Accumulation of peroxidized lipids and increased PDGF receptor phosphorylation in Gpx4-deleted cells is dependent on 12/15-LOX–derived peroxides. (A) Addition of AA861 prevented PDGF β-receptor down-regulation and hyperphosphorylation of Gpx4−/− cells. (B) A schematic summary of a unique pathway for control of RTK signaling including peroxidized lipid-mediated oxidation of PTPs.
These experiments thus identify 12/15-LOX as the major oxygenase responsible for the accumulation of peroxidized lipids in cells lacking Gpx4. This was further supported by the finding that treatment with the NADPH oxidase inhibitor diphenyliodonium had no effect on PDGF receptor expression or phosphorylation levels (Fig. S5B).
Discussion
Together, the findings of the present study have uncovered a previously unrecognized pathway controlling RTK activity (Fig. 5B). The key components of this pathway include 12/15-LOX- and Gpx4-regulated formation of peroxidized lipids, which inhibit PTPs and thereby increase RTK signaling.
The results from analyses of effects of Gpx4 inactivation on the phosphorylation pattern of PDGF β-receptor and on the activation of different downstream signaling pathways are most consistent with inactivation of a subset of PDGF β-receptor–targeting PTPs. Analyses in cellular and in vitro models have provided indications that PTP domains indeed display intrinsic differences in oxidation susceptibility (13–15). Analyses of PTP-PEST oxidation in migrating endothelial cells have also implied that the restricted subcellular localization of different PTPs, together with a spatially restricted production of oxidizing agents, confers specificity to this regulatory mechanisms (39). These earlier findings should help in guiding future studies aiming at identification of the PTPs that are preferentially targeted by peroxidized lipids.
The ability of lipid peroxides to oxidize PTPs has not been appreciated previously. To date, NADPH oxidase-derived superoxide anion and H2O2 have been regarded as the major oxidizing mediators of PTPs (40, 41). However, here we found that 15-HPETE in the nanomolar range is much more effective in in vitro PTP oxidation than H2O2. The concentration of 15-HPETE required to achieve substantial PTP oxidation may be in the physiological range (42). It is noteworthy that 12/15-LOX does not only oxidize free fatty acids, such as arachidonic acid and linoleic acid, but that it is also capable of oxidizing esterified eicosanoids in membranes (43). Because Gpx4 also preferentially acts at the membrane, it is conceivable that the PTPs with a location suitable for PDGF receptor dephosphorylation are particularly affected by Gpx4. It should also be noted that NADPH oxidase releases superoxide anion extracellularly, whereas 12/15-LOX acts inside the cell. However, it should be emphasized that possible interactions between NADPH oxidase-derived superoxides and peroxidized lipids should be further studied.
Important topics for future studies will be analyses of mechanisms that control Gpx4 activity and possible interactions between RTK stimulation and Gpx4 and 12/15-Lox activities. It has been postulated that Gpx4, similar to other glutathione peroxidases, controls LOX and cyclooxygenase activities via the cellular peroxide tone (44, 45). Furthermore, future studies should also explore whether HPETEs other than 15-HPETE show similar capacity to oxidize and inactivate PTPs. In this context it should be noted that the oxidation of the lipid phosphatase PTEN is regulated by unidentified arachidonic acid metabolites (46). Other previous studies meriting attention include the findings that 13-OOH-octadecadienoic acid augments EGFR signaling and that 12-HPETE activates p38 MAPK in platelets (42, 47).
Increased levels of peroxidized lipids have been linked to many complex diseases with an inflammatory component like neurodegeneration, atherosclerosis, and type II diabetes (48). There is also an altered redox status in cancer cells, which may lead to increased levels of peroxidized lipids (49). The demonstration of the present study that peroxidized lipids are strong inducers of PTP oxidation should prompt further studies on how this effect of peroxidized lipids contributes to their physiological and pathophysiological effects.
Materials and Methods
Cell Lines, Cell Culture Conditions, and Chemicals.
The isolation of mouse embryonic fibroblasts from Gpx4+/fl and Gpx4fl/fl embryos, stable transfection with Tam-inducible MERCreMER (MER, mutated estrogen receptor), lentiviral add-back of wild-type Gpx4 in MERCreMER;Gpx4fl/fl cells, and cultivation of cells have been described recently (31). To induce the knockout, cells were treated for 48–60 h with 1 μM 4-OH-tamoxifen (# H7904; Sigma-Aldrich) dissolved in DMSO. Gpx4 expression levels were studied using a monoclonal peptide antibody raised against murine Gpx4 as previously described (31).
PTP Activity Assay.
A PTP activity assay of cell lysates, monitoring in vitro dephosphorylation of the Src optimal peptide (AEEEIYGEFEAKKK), was performed as previously described (14). A detailed description is provided in SI Materials and Methods.
In-Gel Assay of PTP Oxidation.
The assay was essentially performed as previously described (50). In brief, reverse in-gel assay cell lysates were prepared with or without iodoacetic acid in the dark. Immunoprecipitated TC-PTP and total cell lysates were separated on an acrylamide gel containing [γ-32P]-labeled Glu4Tyr peptide. The gel was subjected to several washing steps to renature and reduce the proteins before it was dried and exposed to film. A detailed description is provided in SI Materials and Methods.
oxPTP Antibody-Based Analyses of PTP Oxidation.
For analyses of oxidation of immunoprecipitated SHP-2, the oxPTP antibody was used essentially as described in ref. 14. The in vitro PTP oxidation experiment was carried out as previously described (13), with some minor modifications. For details see SI Materials and Methods.
Analyses of PDGF Receptor Phosphorylation and Signaling.
Cells were routinely stimulated with PDGF-BB (10 ng/mL final concentration) for 3 min at 37 °C in starve medium (DMEM supplemented with 100 μg/mL BSA). Immunoblotting analyses of PDGF receptor expression and phosphorylation followed established procedures (4). ERK and PLC-γ phosphorylation was also determined according to standard procedures. IP3 formation was monitored by labeling of cells with 3H-inositol and subsequent scintillation-based determination of IP3 on an eluate from an ion-exchange column (4). In vivo dephosphorylation was monitored in cells stimulated with PDGF-BB, blocked of further stimulation with the PDGF inhibitor AG1296, and harvested at different times after stimulation and analyzed for receptor phosphorylation. Further details on these analyses are provided in SI Materials and Methods.
PDGF-BB–Induced Cytoskeletal Changes.
Tam-treated cells were stimulated with PDGF-BB before staining with phalloidin-FITC for determination of lamellipodia formation, as outlined in more detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
LAR antibodies were kindly provided by Wiljan Hendriks (Radboud University, Nijmegen, The Netherlands). This work was supported by Deutsche Forschungsgemeinschaft Grant CO 291/2-2 (to M.C.), Deutsche Forschungsgemeinschaft Priority Programmes Grants SPP1087 (to G.W.B. and M.C.) and SPP1069 (to M.C.), and a travel fellowship from the European Molecular Biology Laboratory (to M.C.). A.Ö. was supported by the Swedish Research Council, the European Union-sponsored PTPNET, and the Swedish Cancer Society (Cancerfonden).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007909107/-/DCSupplemental.
References
- 1.Ostman A, Böhmer FD. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 2001;11:258–266. doi: 10.1016/s0962-8924(01)01990-0. [DOI] [PubMed] [Google Scholar]
- 2.Tonks NK. Protein tyrosine phosphatases: From genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Persson C, et al. Site-selective regulation of platelet-derived growth factor beta receptor tyrosine phosphorylation by T-cell protein tyrosine phosphatase. Mol Cell Biol. 2004;24:2190–2201. doi: 10.1128/MCB.24.5.2190-2201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kappert K, et al. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–2651. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- 6.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 7.Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc Natl Acad Sci USA. 2008;105:9959–9964. doi: 10.1073/pnas.0804336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinghoffer RA, Kazlauskas A. Identification of a putative Syp substrate, the PDGF beta receptor. J Biol Chem. 1995;270:22208–22217. doi: 10.1074/jbc.270.38.22208. [DOI] [PubMed] [Google Scholar]
- 9.Kovalenko M, et al. Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. J Biol Chem. 2000;275:16219–16226. doi: 10.1074/jbc.275.21.16219. [DOI] [PubMed] [Google Scholar]
- 10.Chiarugi P, et al. Insight into the role of low molecular weight phosphotyrosine phosphatase (LMW-PTP) on platelet-derived growth factor receptor (PDGF-r) signaling. LMW-PTP controls PDGF-r kinase activity through TYR-857 dephosphorylation. J Biol Chem. 2002;277:37331–37338. doi: 10.1074/jbc.M205203200. [DOI] [PubMed] [Google Scholar]
- 11.Rhee SG, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- 13.Groen A, et al. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2005;280:10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- 14.Persson C, et al. Preferential oxidation of the second phosphatase domain of receptor-like PTP-alpha revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2004;101:1886–1891. doi: 10.1073/pnas.0304403101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross SH, et al. Differential redox regulation within the PTP superfamily. Cell Signal. 2007;19:1521–1530. doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 17.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 18.Kwon J, et al. Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 2005;24:2331–2341. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh DK, et al. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Giannoni E, et al. Lymphocyte function-associated antigen-1-mediated T cell adhesion is impaired by low molecular weight phosphotyrosine phosphatase-dependent inhibition of FAK activity. J Biol Chem. 2003;278:36763–36776. doi: 10.1074/jbc.M302686200. [DOI] [PubMed] [Google Scholar]
- 21.Choi MH, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 22.Kanda M, et al. Glutaredoxin modulates platelet-derived growth factor-dependent cell signaling by regulating the redox status of low molecular weight protein-tyrosine phosphatase. J Biol Chem. 2006;281:28518–28528. doi: 10.1074/jbc.M604359200. [DOI] [PubMed] [Google Scholar]
- 23.Loh K, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo HA, et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Conrad M, Schneider M, Seiler A, Bornkamm GW. Physiological role of phospholipid hydroperoxide glutathione peroxidase in mammals. Biol Chem. 2007;388:1019–1025. doi: 10.1515/BC.2007.130. [DOI] [PubMed] [Google Scholar]
- 26.Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982;710:197–211. doi: 10.1016/0005-2760(82)90150-3. [DOI] [PubMed] [Google Scholar]
- 27.Mauri P, et al. Versatility of selenium catalysis in PHGPx unraveled by LC/ESI-MS/MS. Biol Chem. 2003;384:575–588. doi: 10.1515/BC.2003.065. [DOI] [PubMed] [Google Scholar]
- 28.Conrad M, et al. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol Cell Biol. 2005;25:7637–7644. doi: 10.1128/MCB.25.17.7637-7644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider M, et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23:3233–3242. doi: 10.1096/fj.09-132795. [DOI] [PubMed] [Google Scholar]
- 30.Yant LJ, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 31.Seiler A, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Loscalzo J. Membrane redox state and apoptosis: Death by peroxide. Cell Metab. 2008;8:182–183. doi: 10.1016/j.cmet.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kappert K, et al. Dynamic changes in the expression of DEP-1 and other PDGF receptor-antagonizing PTPs during onset and termination of neointima formation. FASEB J. 2007;21:523–534. doi: 10.1096/fj.06-6219com. [DOI] [PubMed] [Google Scholar]
- 35.Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278:739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- 36.Jandt E, Denner K, Kovalenko M, Ostman A, Böhmer FD. The protein-tyrosine phosphatase DEP-1 modulates growth factor-stimulated cell migration and cell-matrix adhesion. Oncogene. 2003;22:4175–4185. doi: 10.1038/sj.onc.1206652. [DOI] [PubMed] [Google Scholar]
- 37.Kovalenko M, et al. Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- 38.Mellstroöm K, et al. The effect of platelet-derived growth factor on morphology and motility of human glial cells. J Muscle Res Cell Motil. 1983;4:589–609. doi: 10.1007/BF00712117. [DOI] [PubMed] [Google Scholar]
- 39.Wu RF, et al. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonks NK. Redox redux: Revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 42.Coulon L, Calzada C, Moulin P, Véricel E, Lagarde M. Activation of p38 mitogen-activated protein kinase/cytosolic phospholipase A2 cascade in hydroperoxide-stressed platelets. Free Radic Biol Med. 2003;35:616–625. doi: 10.1016/s0891-5849(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 43.Funk CD, Cyrus T. 12/15-lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med. 2001;11:116–124. doi: 10.1016/s1050-1738(01)00096-2. [DOI] [PubMed] [Google Scholar]
- 44.Chen CJ, Huang HS, Chang WC. Depletion of phospholipid hydroperoxide glutathione peroxidase up-regulates arachidonate metabolism by 12S-lipoxygenase and cyclooxygenase 1 in human epidermoid carcinoma A431 cells. FASEB J. 2003;17:1694–1696. doi: 10.1096/fj.02-0847fje. [DOI] [PubMed] [Google Scholar]
- 45.Imai H, et al. Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase. J Biol Chem. 1998;273:1990–1997. doi: 10.1074/jbc.273.4.1990. [DOI] [PubMed] [Google Scholar]
- 46.Covey TM, Edes K, Fitzpatrick FA. Akt activation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene. 2007;26:5784–5792. doi: 10.1038/sj.onc.1210391. [DOI] [PubMed] [Google Scholar]
- 47.Glasgow WC, et al. The linoleic acid metabolite, (13S)-hydroperoxyoctadecadienoic acid, augments the epidermal growth factor receptor signaling pathway by attenuation of receptor dephosphorylation. Differential response in Syrian hamster embryo tumor suppressor phenotypes. J Biol Chem. 1997;272:19269–19276. doi: 10.1074/jbc.272.31.19269. [DOI] [PubMed] [Google Scholar]
- 48.Spiteller G. Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radic Biol Med. 2006;41:362–387. doi: 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 50.Markova B, Gulati P, Herrlich PA, Böhmer FD. Investigation of protein-tyrosine phosphatases by in-gel assays. Methods. 2005;35:22–27. doi: 10.1016/j.ymeth.2004.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.