Abstract
An important objective of pain research is to identify novel drug targets for the treatment of pathological persistent pain states, such as inflammatory and neuropathic pain. Mas-related G-protein–coupled receptors (Mrgprs) represent a large family of orphan receptors specifically expressed in small-diameter nociceptive primary sensory neurons. To determine the roles of Mrgprs in persistent pathological pain states, we exploited a mouse line in which a chromosomal locus spanning 12 Mrgpr genes was deleted (KO). Initial studies indicated that these KO mice show prolonged mechanical- and thermal-pain hypersensitivity after hind-paw inflammation compared with wild-type littermates. Here, we show that this mutation also enhances the windup response of dorsal-horn wide dynamic-range neurons, an electrophysiological model for the triggering of central pain sensitization. Deletion of the Mrgpr cluster also blocked the analgesic effect of intrathecally applied bovine adrenal medulla peptide 8–22 (BAM 8–22), an MrgprC11 agonist, on both inflammatory heat hyperalgesia and neuropathic mechanical allodynia. Spinal application of bovine adrenal medulla peptide 8–22 also significantly attenuated windup in wild-type mice, an effect eliminated in KO mice. These data suggest that members of the Mrgpr family, in particular MrgprC11, may constitute an endogenous inhibitory mechanism for regulating persistent pain in mice. Agonists for these receptors may, therefore, represent a class of antihyperalgesics for treating persistent pain with minimal side effects because of the highly specific expression of their targets.
Keywords: inflammation, neuropeptides, sensory neurons, Mrgpr
After tissue inflammation or nerve injury, increased afferent neuronal excitability (peripheral sensitization) and a state of dorsal-horn neuronal hyperexcitability (central sensitization) amplify ascending pain signals (1, 2), which, if uncontrolled, may lead to various unremitting pain symptoms (e.g., tactile allodynia, thermal hyperalgesia, or spontaneous pain) in a subset of patients (3, 4). Patients with such persistent pain states have few treatment options, in part because pain-specific drug targets are lacking (4, 5). One set of potential targets comprises a large family of orphan receptors known as Mas-related G-protein–coupled receptors (Mrgprs). Many Mrgprs (e.g., As, B4, B5, C11, and D) are expressed specifically on small-diameter, presumably nociceptive, nonpeptidergic sensory neurons in the dorsal root ganglia (DRG) (6, 7). Recent studies have begun to shed light on the physiological functions served by Mrgprs, including mediation of nonhistaminergic itch by MrgprA3 (8–11). Whether other Mrgprs mediate itch or regulate persistent pathological pain states is not clear (12–14).
Examining the function of Mrgprs in vivo has been challenging, because endogenous Mrgpr ligands have not been unequivocally identified and deletion of a single Mrgpr gene may not cause a detectable phenotype because of potential redundancy in the Mrgpr gene family (7, 15). To overcome these problems, we generated a mouse line in which 12 intact Mrgpr coding sequences (As, B4, B5, and C11) were simultaneously deleted; the resulting mice are referred to as Mrgpr-clusterΔ−/− (KO) mice (11). The deleted cluster contains most MrgprA and MrgprC genes and represents ∼50% of the potentially functional Mrgpr repertoire in mice (7). Importantly, the deleted Mrgprs are not required for neuronal survival or fate determination of small-diameter sensory neurons (11). Therefore, the KO mice may represent a useful tool for studying the functions and determining the roles of Mrgprs in pain in vivo. The KO mice respond normally to acute noxious thermal, mechanical, and chemical stimuli compared with wild-type (WT) littermates. However, they display prolonged mechanical- and thermal-pain hypersensitivity after intraplantar injection of complete Freund's Adjuvant (CFA) or carrageenen, whereas the development of neuropathic pain was similar between the two genotypes (11). Findings from our previous work motivated us to further examine the roles for Mrgprs in signaling and modulation of persistent pain states with different origins (i.e., inflammation and nerve injury) and investigate the underlying neurophysiological mechanisms in vivo. We also tested the effect of intrathecal administration of an MrgprC11 agonist, bovine adrenal medulla peptide 8–22 (BAM 8–22), on mouse pain behavior. Our results suggest that certain Mrgprs in mice may constitute endogenous inhibitors of pathological pain. These data also suggest that agonists, rather than antagonists, for MrgprC11 may represent a class of antihyperalgesics for persistent pain.
Results
Intense/Repetitive Noxious Input Activates an Endogenous Mrgpr Mechanism to Counteract the Sensitization of Pain Responses.
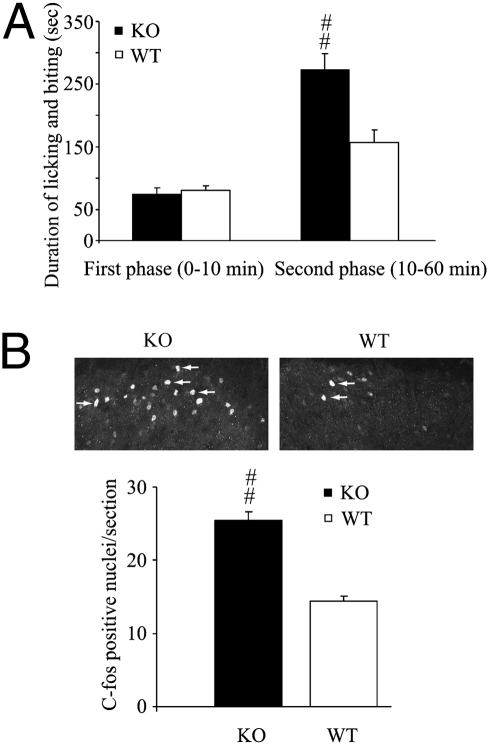
First, we examined whether formalin-induced tissue injury leads to an endogenous activation of Mrgprs to modulate spontaneous pain. The formalin test is a unique model of persistent pain that encompasses inflammatory, neurogenic, and central mechanisms of nociception (16–18). Importantly, spontaneous pain responses to two principally different stimuli, nociceptor activation (first phase) and tissue inflammation (second phase), can be readily revealed in the same test and separately analyzed. Formalin (2%; 5 μL) was injected into the plantar tissue of one hind paw. We observed that spontaneous pain behavior in the second phase (10–60 min post injection), which is driven largely by tissue inflammation and involves central sensitization of dorsal-horn neurons (16), was significantly potentiated in KO mice compared with WT littermates (Fig. 1A). Reflecting this behavioral phenotype, a greater increase of c-fos–expressing neurons in the ipsilateral laminae I and II of lumbar (L4–L6) spinal segments in KO mice than in WT mice was evident after an intraplantar injection of formalin (Fig. 1B). In contrast, the first (acute) phase of the formalin response (0–10 min post injection), which results predominantly from a direct chemical stimulation of the C fiber-afferent nociceptors, was not affected by the mutation (Fig. 1A).
Fig. 1.
KO mice display stronger dorsal-horn neuronal activation and enhanced pain responses after intraplantar formalin injection. (A) KO mice expressed enhanced spontaneous pain responses in the second phase of formalin-induced pain but responded normally in the first (acute) phase. (B) Transverse sections of L4–L5 spinal cord from WT and KO mice were stained with anti–c-fos antibody 3 h after intraplantar formalin injection (2%, 5 μL). Ten sections were chosen randomly from each mouse (three mice per genotype). c-fos–positive nuclei are indicated by arrows. KO mice had significantly more c-fos–positive cells than did WT mice, indicating that the KO mice had greater neuronal activation. Data are expressed as mean ± SEM.
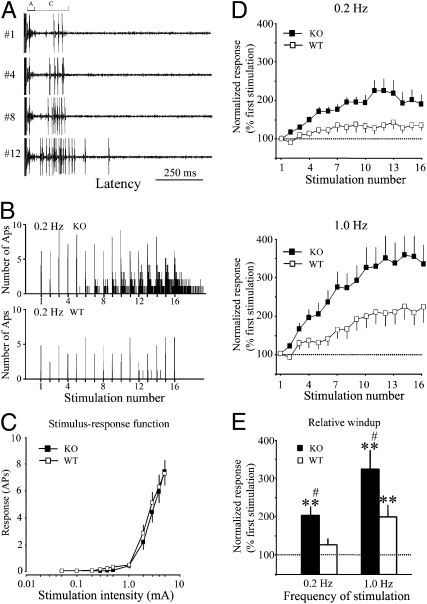
Next, we examined whether the enhanced inflammatory-pain response in KO mice is also manifested at the cellular level of spinal pain processing. Wide dynamic-range (WDR) neurons in the deep dorsal horn are important for spinal pain processing and are candidates for transmission cells in the gate theory of pain (2, 19, 20). They receive both innocuous and noxious sensory inputs from the periphery and display A fiber- and C fiber-mediated responses (A and C components, respectively) to a single intracutaneous electrical stimulus with an intensity above C fiber-activation threshold (Fig. 2A). Based on the axon-conduction velocities, WDR neuronal responses to electrical stimuli in mice were separated into a short latency A component (0–40 ms, excluding stimulus artifact) and a long latency C component (40–250 ms) (20). Typically, the excitability of some WDR neurons progressively increases in response to repetitive C fiber-afferent stimulation, a short-term activity-dependent neuronal sensitization called windup (21). Therefore, we recorded from WDR neurons and examined whether the deletion of the Mrgpr cluster would alter the excitability of WDR neurons. The effective frequency of the electrical stimulation for inducing windup is usually >0.3 Hz under physiological conditions, and a plateau level of windup is often reached at a higher-frequency stimulation of ∼1 Hz (20, 21). Accordingly, windup was examined by repetitive intracutaneous electrical stimuli (16 pulses, 3.0 mA/supra C fiber-activation threshold, 2.0 ms) applied at 0.2 and 1.0 Hz, with a minimum 10-min interval between each trial. We observed that WDR neurons in WT mice (n = 23) showed windup responses at the higher frequency of 1.0-Hz stimulation but rarely at 0.2-Hz stimulation (Fig. 2 B and D), consistent with previous studies (20, 21). To quantify the peak levels of windup, we measured the relative windup value, which is the averaged C component responses to the last 10 (7–16) stimuli of the trial normalized by the C component response to the first stimulus of each trial (input value). The relative windup values were significantly increased during 1.0- but not 0.2-Hz stimulation in WT mice compared with the respective baseline (Fig. 2E). Strikingly, unlike the case in WT mice, many WDR neurons in KO mice (n = 30) exhibited windup at the normally ineffective 0.2-Hz stimulation frequency (Fig. 2 B and D), and the relative windup value at 0.2-Hz stimulation in the KO group was significantly greater than the baseline (Fig. 2E). Importantly, the windup responses at both 0.2- and 1.0-Hz stimulation frequencies were significantly greater in KO than in WT mice (P < 0.05) (Fig. 2E). The mean recording depth of WDR neurons did not differ between WT (450 ± 23 μm) and KO mice (441 ± 22 μm, P > 0.05). In contrast with their differences in windup, the acute responses of WDR neurons to graded intracutaneous electrical stimulation (0.05–5.0 mA, 2.0 ms) were comparable between the WT and KO mice. The threshold and population stimulus-intensity response (S-R) functions of the C component were also similar in the two groups (Fig. 2C and Table 1). These data suggest that one or more Mrgprs within the KO cluster function to limit the extent of increased WDR neuronal excitability in response to repetitive C fiber stimulation.
Fig. 2.
WDR neurons in KO mice display enhanced windup to repetitive C fiber input. (A) A WDR neuron displayed typical A component (0–40 ms) and C component (40–250 ms) responses in response to suprathreshold intracutaneous electrical stimulation. This unit from a KO mouse showed progressive increases in C component (windup) in response to repetitive electrical stimulation of 0.2 Hz (16 pulses, 3.0 mA, 0.5 ms). (B) Histograms show responses of WDR neurons from KO and WT mice to 0.2-Hz stimulation. The KO neuron displayed windup, but the WT neuron did not. Bin size is 50 ms. APs, action potentials. (C) Stimulus-response functions of C components to graded electrical stimuli (0.1–5.0 mA, 2.0 ms) did not differ between WT (n = 23) and KO mice (n = 30). (D) C components of the responses to repetitive windup-inducing electrical stimulation applied at 0.2 Hz and 1.0 Hz in WT and KO mice were plotted against the stimulation number of each trial. (E) The averaged C components of the responses to the last 10 stimuli (7–16) of 0.2- and 1.0-Hz stimulation were significantly higher in KO than WT mice. Windup data are normalized to the response evoked by the first stimulation of each trial. Data are presented as mean ± SEM. **P < 0.01 vs. the input value; #P < 0.05 vs. WT group.
Table 1.
The thresholds and latency of the first A and C fiber-mediated action potentials in WDR neurons
| A latency (ms) | C latency (ms) | A threshold (mA) | C threshold (mA) | |
| WT | 14.0 ± 0.8 | 124 ± 5 | 0.23 ± 0.04 | 2.0 ± 0.3 |
| KO | 14.8 ± 0.6 | 108 ± 6 | 0.26 ± 0.03 | 2.3 ± 0.2 |
| WT pre-BAM | 14.2 ± 0.8 | 114 ± 5 | 0.16 ± 0.02 | 1.5 ± 0.3 |
| WT post-BAM | 12.1 ± 0.7 | 115 ± 4 | 0.17 ± 0.03 | 1.7 ± 0.4 |
| KO pre-BAM | 14.5 ± 0.6 | 126 ± 3 | 0.17 ± 0.02 | 1.7 ± 0.5 |
| KO post-BAM | 12.9 ± 0.6 | 118 ± 4 | 0.19 ± 0.03 | 1.9 ± 0.6 |
WT, wild type; KO, Mrgpr-cluster −/−; BAM, BAM8-22. Data are mean ± SEM.
Intrathecal Administration of BAM 8–22 Inhibits Persistent Inflammatory and Neuropathic Pain in WT but Not KO Mice.
One gene within the KO cluster that can potentially modulate pain responses is MrgprC11. An endogenous agonist of MrgprC11 is a 22-amino acid peptide called BAM 22 (6, 7); it belongs to the family of endogenous opioid peptides and is derived from the proenkephalin A gene. Interestingly, the N terminus of BAM 22 binds and activates opioid receptors, whereas the C terminus of the peptide activates mouse MrgprC11, rat MrgprC, and human MrgprX1. Previous studies have shown that a truncated BAM 22, which lacks its N terminus (BAM 8–22), specifically activates Mrgprs but not opioid receptors (6, 14). Importantly, among the 12 Mrgprs deleted in Mrgpr-cluster KO mice, only MrgprC11 is activated by BAM 8–22 in heterologous cells (9, 11, 14). In rats, several rodent MrgprC agonists, including BAM 8–22 and γ2-melanocyte-stimulating hormone (γ2-MSH), have been reported to have both pro- and antipain effects (12, 13, 22–25). Because these previous studies did not use transgenic animals that lack Mrgprs and the specificity of these agonists for Mrgprs has not been fully established, it has been difficult to determine whether the effects of these peptides in vivo are mediated by Mrgprs.
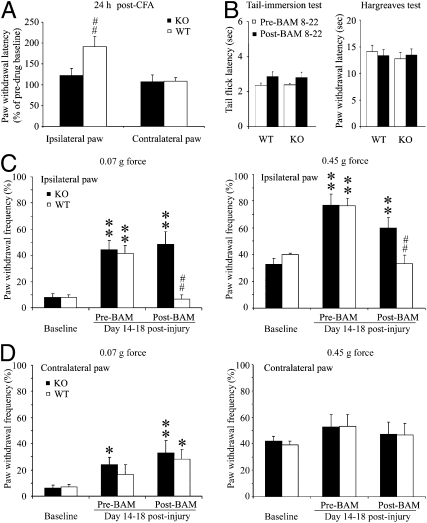
Because the spinal cord is an important site for pain modulation and Mrgprs are likely expressed on the central as well as the peripheral terminals of DRG neurons (12, 13), we examined the effects of intrathecal BAM 8–22 (1 mM, 5 μL) on pain behavior in WT and KO mice. This peptide on its own elicited mild, short-lived (less than 5 min) scratching and tail-biting behavior in some WT mice, but this response was similar in KO mice, suggesting that it is not mediated by Mrgprs (or at least not by those contained within the deleted cluster). We next examined the ability of BAM 8–22 to modulate persistent inflammatory pain by examining enhanced pain sensitivity to a noxious heat stimulus as monitored by the Hargreaves test 24 h after intraplantar injection of CFA (6 μL, 50%) into one hind paw. In the absence of BAM 8–22, thermal hyperalgesia was comparable between the two genotypes: the paw-withdrawal latencies (PWL) of the ipsilateral hind paw in KO and WT mice were 4.3 ± 0.7 s and 3.3 ± 0.3 s, respectively. In WT mice, a single intrathecal injection of BAM 8–22 (1 mM, 5 μL) was able to alleviate thermal hyperalgesia at 30 min postinjection, increasing the PWL by 1.9-fold compared with predrug baseline (Fig. 3A, WT, n = 13, ipsilateral paw). In contrast, this antihyperalgesic effect of BAM 8–22 was not observed in KO mice (Fig. 3A, KO, n = 10), indicating that it is Mrgpr-dependent. In addition, intrathecal BAM 8–22 did not significantly affect the PWL of the contralateral (control side) hind paw to acute radiant heat in either group (Fig. 3A, contralateral paw). These data suggest that Mrgprs (most likely, Mrgpr C11) play a role in mediating the effect of exogenously administered BAM 8–22 to attenuate inflammatory thermal hyperalgesia in WT mice.
Fig. 3.
Intrathecal injection of BAM 8–22 inhibits persistent inflammatory pain and neuropathic pain in WT but not KO mice. (A) Intrathecal (i.th.) injection of BAM 8–22 (1 mM, 5 μL) significantly alleviated thermal hyperalgesia in the ipsilateral hind paw 24 h after intraplantar injection of CFA (6 μL, 50%) in WT (n = 12) but not KO mice (n = 10). BAM 8–22 did not affect PWL of the contralateral hind paw in either group. (B) The same dose of BAM 8–22 did not significantly change the tail-flick latency in the tail-immersion test (50 °C) in WT (n = 10) or KO mice (n = 10). In addition, the tail-flick latencies were not significantly different between the two groups at pre- and postdrug conditions. PWL of the contralateral hind paw to radiant heat (Hargreaves test) in the CFA experiment was similar before and after intrathecal BAM 8–22 injection in both groups. (C) BAM 8–22 (0.5 mM, 5 μL, i.th.) also attenuated mechanical-pain hypersensitivity induced by CCI of the sciatic nerve in WT mice but not KO mice. The PWF of the ipsilateral hind paw to low-force (0.07 g) and high-force (0.45 g) punctuate stimulation was significantly increased from the preinjury levels in both KO and WT mice 14–18 d postinjury. BAM 8–22 significantly reduced the PWF of the ipsilateral hind paw in response to low- and high-force stimuli in WT mice (n = 7) but not KO mice (n = 8) after 30 min. (D) BAM 8–22 did not significantly reduce the PWF of the contralateral hind paw in either group. Data are expressed as mean ± SEM. *P < 0.05 and **P < 0.01 vs. preinjury value; ##P < 0.01 vs. predrug value.
To determine whether intrathecal BAM 8–22 also affects acute thermal nociception, we performed tail-immersion tests in naïve WT and KO mice. The tail-flick latencies after intrathecal injection of BAM 8–22 were not significantly different from the respective predrug values in either WT (n = 10) or KO mice (n = 10) (Fig. 3B). This finding is in line with the observation that the peptide did not significantly change the sensitivities to acute radiant heat (Hargreaves test) in WT (n = 15) and KO (n = 14) (Fig. 3B) mice. In addition, thermal sensitivities were comparable between the two groups before and after BAM 8–22 treatment (Fig. 3B).
There are substantial differences between neuropathic-pain and inflammatory-pain states (e.g., etiology, pathology, and treatment strategy) (4, 26–28). Because mechanical allodynia is the most common and disabling stimulus-evoked symptom of neuralgia and is often difficult to treat, we determined whether BAM 8–22 can also reduce neuropathic mechanical allodynia in mice. We subjected mice to the chronic constriction-injury (CCI) model, in which the sciatic nerve is ligated loosely with a suture. The effect of this manipulation on mechanical-pain sensitivity was tested at 14–18 d after injury by measuring paw-withdrawal frequency to punctuate mechanical stimuli of different strengths. Intrathecal injection of BAM 8–22 (0.5 mM, 5 μL) significantly attenuated CCI-induced mechanical-pain hypersensitivity to both low-force (0.07 g) and high-force (0.45 g) stimuli applied to the ipsilateral hind paw in WT mice (Fig. 3C) (n = 7). This antihyperalgesic effect of the peptide was eliminated in KO mice (Fig. 3C) (n = 8), although the development of mechanical allodynia itself was not affected by the mutation. BAM 8–22 did not significantly change paw-withdrawal responses on the uninjured side (Fig. 3D, contralateral paw). This result suggests that the antiallodynic effect of intrathecal BAM 8–22 under neuropathic conditions is also mediated by Mrgprs.
Spinal Application of BAM 8–22 Attenuates Windup in WT but Not KO Mice.
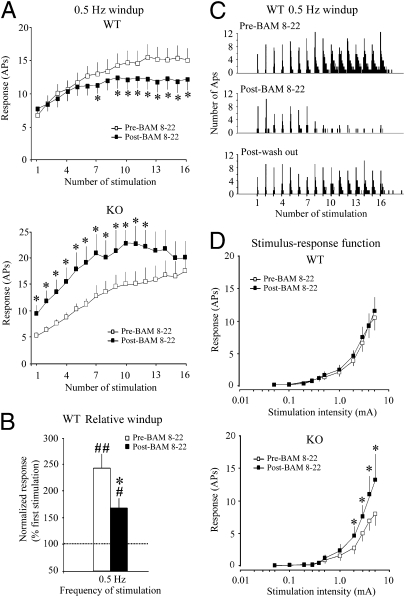
Next, we asked whether the antihyperalgesic effect of BAM 8–22 can be seen at the level of central nociceptive processing. Because intrathecal BAM 8–22 inhibited both inflammatory and neuropathic pain, we postulated that MrgprC11 agonists might attenuate spinal neuronal sensitization involved in persistent pain. We examined the effects of topical spinal application of BAM 8–22 (0.1 mM, 30 μL) on the windup of WDR neuronal responses to repetitive noxious inputs. We used 0.5-Hz stimulation frequency, because it can induce windup but does not saturate the response (21). Therefore, it can be used to examine the effect of either facilitatory or inhibitory drug action on windup. In WT mice (n = 25), windup to 0.5-Hz stimulation was significantly attenuated after spinal superfusion with BAM 8–22 (Fig. 4 A–C, WT), consistent with the antihyperalgesic effect of BAM 8–22 in our behavioral studies. In KO mice, by contrast, the effect of BAM 8–22 was not simply eliminated but rather, reversed: the peptide significantly increased the input value and C component responses of WDR neurons to 0.5-Hz stimulation (Fig. 4A, cf WT vs. KO, black squares). Population S-R functions of C fiber-mediated responses of WDR neurons to graded electrical stimulation were similar before and after drug administration in WT mice (n = 25) (Fig. 4D). However, in KO mice, BAM 8–22 significantly increased the number of C component responses to graded electrical stimulation at intensities >1.0 mA (n = 17) (Fig. 4D). Nevertheless, the threshold and latency of the first C fiber-mediated action potential was unaffected by administration of the peptide in either WT or KO mice (Table 1). These results suggest that the inhibitory effects of BAM 8–22 on windup, a measure of short-term neuronal hyperexcitability, are mediated by Mrgprs. Furthermore, deletion of Mrgprs unmasks a potentiating effect of the peptide on the same response mediated by another class of receptors.
Fig. 4.
BAM 8–22 inhibits windup in WT mice. (A) The C components of WDR neuronal response to 0.5-Hz stimulation were plotted as a function of stimulus number before and after BAM 8–22 administration. (B) The averaged C component responses for the last 10 stimuli during 0.5-Hz stimulation in WT mice were normalized by the respective response evoked by the first stimulation of each trial (input value). The relative windup in WT mice was significantly decreased by BAM 8–22 compared with the predrug level. Because of a significant increase of input in KO mice after BAM 8–22 treatment, windup data were not normalized. (C) The histograms show an example of the inhibitory effect of BAM 8–22 on the windup of a WDR neuron in WT mice at 0.5-Hz stimulation. The windup response was substantially attenuated 10–30 min after BAM 8–22 application and was partially recovered 10–30 min after saline washout. Bin size is 50 ms. (D) BAM 8–22 (0.1 mM, 30 μL) significantly increased the C component response to graded electrical stimulation at intensities of 2.0–5.0 mA in KO (n = 17) but not WT mice (n = 25) at 10–30 min after spinal topical application. Data are expressed as mean ± SEM. *P < 0.05 and **P < 0.01 vs. the predrug condition; #P < 0.05 vs. the input value.
Discussion
Receptors Within a Cluster of Mrgprs May Mediate an Endogenous Inhibitory Mechanism for Persistent Pain and Hyperalgesia.
Our previous behavioral studies indicated that deletion of the Mrgpr gene cluster exaggerates inflammatory-pain responses while leaving acute nociceptive-pain sensation intact (11), but it left open the question of the underlying neurobiological mechanism(s). Intense nociceptor activation through C fiber-evoked responses in dorsal-horn neurons results in a state of central sensitization manifested as an increased neuronal response to subsequent stimuli (2, 29). The windup phenomenon in WDR neurons reflects an activity-dependent short-term increase in neuronal excitability (21, 30, 31). Like many biological functions, the windup response to changes in stimulation frequency may be represented by a sigmoidal function. We postulate that a facilitation of windup by removal of endogenous inhibition is manifested as a reduction in the effective-frequency threshold for eliciting this response from 1.0- to 0.2-Hz stimulation. A similar phenotype was observed in mice lacking the μ-opioid–receptor gene (20). The facilitated windup in KO mice is consistent with the enhanced inflammatory- and formalin-pain behavioral phenotype. In contrast to windup, the acute responses of WDR neurons to graded electrical stimulation (e.g., S-R function and threshold) were not significantly different between the two genotypes. These electrophysiological data suggest that the encoding of acute noxious stimuli by WDR neurons is largely intact in KO mice, consistent with the observation that KO mice respond to nociceptive mechanical- and thermal-pain in a manner similar to their WT littermates (Fig. 3 B–D).
Together, our behavioral and electrophysiological experiments provide complementary lines of evidence that an endogenous mechanism mediated by a cluster of Mrgprs inhibits persistent pain behavior and a spinal electrophysiological correlate of hyperalgesia, presumably by acting on sensory afferent fibers in the dorsal horn. Apparently, this Mrgpr-mediated pain inhibition is not tonically active but is triggered only by intense noxious inputs (e.g., by inflammation or repetitive electrical activation of C fibers), wherein it functions to attenuate pathological pain severity and counteract dorsal-horn neuronal sensitization.
The identities of the specific Mrgpr receptor and its endogenous ligand that mediate this inhibitory influence in WT mice remain to be determined, although MrgprC11 and BAM 22 are potential candidates. A previous study has shown that BAM 22 expression is up-regulated in rat dorsal horn and small-sized DRG neurons after CFA-induced hind-paw inflammation (32). Future studies are needed to determine whether changes in the expression of Mrgprs also occur after tissue inflammation and nerve injury.
BAM 8–22 Inhibits Persistent Pain and Spinal Neuronal Sensitization Through Activation of Mrgprs.
In the current study, intrathecal administration of BAM 8–22 suppressed thermal hyperalgesia induced by hind paw inflammation in WT mice. This finding is in accordance with previous observations in rats that intrathecal administration of BAM 8–22 suppresses inflammatory pain and spinal c-fos expression induced by intraplantar formalin injection (22, 32, 33) and diminishes NMDA-evoked nocifensive behaviors (12). Importantly, the antihyperalgesic effect of intrathecal BAM 8–22 was not observed in KO mice, suggesting that the effect is Mrgpr-dependent. Peripheral-nerve injury-induced mechanical allodynia also was attenuated by intrathecal BAM 8–22 in WT mice but not in KO mice. These findings show that spinal administration of BAM 8–22 attenuates both thermal- and mechanical-pain hypersensitivity in mice under different pathological conditions (tissue inflammation and nerve injury), presumably through activation of Mrgprs at central terminals of DRG neurons. Among the 12 deleted Mrgprs in Mrgpr-clusterΔ−/− mice, MrgprC11 likely contributes most to the inhibitory effect, because BAM 8–22 is a specific agonist of MrgprC11 (11, 14). However, additional experiments that use mice with a single MrgprC11 deletion and MrgprC11 rescue in cluster KO mice will be needed to directly determine the role of MrgprC11 in the antihyperalgesic effect. Because MrgprC11 is an ortholog of human MrgprX1, which can also be activated by BAM 8–22 (6), BAM 8–22 delivered through a spinal route may represent a promising treatment for pathological pain states. Notably, BAM 8–22 is unlikely to compromise protective physiological pain, because it did not affect baseline nociception (e.g., tail immersion test and paw withdrawal threshold/latency on the uninjured side).
An important site for integration of nociceptive information and analgesic action is the dorsal horn (34–36). BAM 8–22 significantly inhibited windup in WT mice but not in KO mice. These results suggest that the inhibitory action of BAM 8–22 on windup in WT mice is Mrgpr-dependent. Major analgesics (e.g., opioids, NMDA-receptor antagonists, and adenosine) that alleviate chronic pain also exert a strong inhibition on windup (21, 37, 38). Therefore, the inhibition of windup by BAM 8–22 correlates well with its antihyperalgesic effect on behavior. Unlike windup, the threshold and S-R functions for C fiber-mediated responses in WDR neurons to graded electrical stimuli were not significantly changed by BAM 8–22 in WT mice, consistent with the fact that BAM 8–22 did not affect baseline nociceptive-pain sensitivity in WT mice.
In KO mice, BAM 8–22 increased, rather than decreased, the C fiber-mediated responses of WDR neurons to graded electrical stimulation at intensities >1 mA, indicating a facilitatory effect of BAM 8–22 on neuronal response to acute noxious input in the absence of the Mrgpr cluster. This excitatory action of BAM 8–22 on WDR neurons may be mediated through an Mrgpr-independent mechanism or by Mrgpr family members that were not part of the deleted cluster. However, we do not think that BAM 8–22 should increase acute peripheral-pain sensitivity in KO mice, because the threshold for activation of the C component in WDR neurons did not change after BAM 8–22 treatment in these mutants. Therefore, the facilitatory effect of BAM 8–22 seen in mutant WDR neurons is likely caused by activation of a receptor(s) expressed in the spinal cord (e.g., on WDR neurons themselves, interneurons, or descending fibers). The dual and opposing influences of BAM 8–22 on pain transmission uncovered by our experiments may help to reconcile some of the previous conflicting reports regarding the effect of this peptide on pain behavior (13, 39).
Potential Mechanisms for Mrgpr-Mediated Pain Inhibition.
Our study suggests that activation of certain Mrgprs normally suppresses the WDR windup response and also attenuates behavioral hyperalgesia. This might imply an inhibitory action of the relevant endogenous ligand on the primary sensory neurons that express these receptors. However, we previously observed an Mrgpr-dependent increase in intracellular calcium levels after BAM 8–22 treatment in DRG cells, which we proposed may underlie the itch response induced by intradermal injection of BAM 8–22 in WT mice (11). Although BAM 8–22 may activate different Mrgpr family members in the periphery versus at central terminals, it is also possible that this peptide might exert different effects on pain and other sensations through the same receptor when applied at different locations. For example, capsaicin, which excites DRG neurons and induces intense burning pain in the periphery through the receptor TrpV1, depresses presynaptic excitation and inhibits pain when applied centrally (40, 41). This effect could result from presynaptic terminal depolarization, which would decrease the amplitude of action potentials and induce presynaptic inhibition (42). Alternatively, BAM 8–22 might modulate cellular activities differently at the cell body (e.g., pattern of action-potential firing) than at central terminals (e.g., neurotransmitter release). Such differences could occur as a result of disparate distributions and compartmentalization of Mrgprs, intracellular signaling machinery (e.g., Gq and Gi), and receptors or channels (e.g., calcium channels) whose activity may be modulated by Mrgpr activation (43, 44). In light of the important roles of high voltage-activated (HVA) calcium channels in excitatory neurotransmitter release and pain inhibition (24, 44–47), it would be interesting to examine whether BAM 8–22 inhibits HVA calcium current in small DRG neurons through MrgprC11.
Alternatively, either of two circuit mechanisms could also explain the inhibitory role of Mrgprs in persistent pain. First, MrgprC11 ligands may activate DRG neurons that synapse onto inhibitory interneurons located in the substantia gelatinosa (lamina II) of the spinal cord. Such a mechanism would be consistent with the fact that these ligands increase intracellular calcium in cultured DRG neurons (11). This local inhibitory circuit has been identified by previous studies (48) and has been suggested to receive input from relatively large-diameter, more rapidly conducting C fibers. Because Mrgprs are expressed in large C fiber neurons (diameter = 20–25 μm) that centrally project to lamina II (9, 49), Mrgpr-expressing neurons may be engaged in this inhibitory circuitry. Alternatively, the activation of Mrgprs may lead to the release of inhibitory neurotransmitters or neuromodulators onto excitatory second-order neurons, thereby inhibiting the pain pathway. PKC-γ interneurons, which may contribute to pathological pain, might also play a role in BAM 8–22-induced pain inhibition and should be investigated in future studies (50–52).
In summary, the present results together with previous data (11) suggest that certain Mrgprs (mouse MrgprC11 and human MrgprX1) may constitute an inhibitory mechanism for pathological pain and spinal neuronal sensitization, although additional experiments are required to determine the underlying cellular and molecular mechanisms. Because human MrgprX1 expression is restricted to DRG neurons, specific agonists of this receptor might provide relief from chronic pain while producing few side effects. If so, then such agonists could represent a class of analgesic agents for the treatment of patients with chronic pain.
Experimental Procedures
Production of Mrgpr-ClusterΔ−/− Mice.
The deletion of a cluster of Mrgpr genes in the mouse germline was described in a previous study (11). Briefly, chimeric M Mrgpr-clusterΔ−/− (KO) mice were produced by blastocyst injection of positive embryonic stem cells. The KO mice were generated by mating chimeric mice to C57BL/6 mice. Mice were backcrossed to C57BL/6 mice for at least five generations. The Mrgpr-clusterΔ−/− mice were fertile, appeared healthy, and were indistinguishable by their behavior and appearance from the WT littermates.
CCI Model of Neuropathic Pain in Mice.
A CCI at the left sciatic nerve was induced in adult male mice. Inhalation anesthesia was induced with a constant level of isoflurane (2.0%) delivered through a nose cone. Under aseptic conditions, the left sciatic nerve at the middle thigh level was separated from the surrounding tissue and loosely tied with three nylon sutures (9-0 nonabsorbable monofilament; S&T AG). The distance between two adjacent ligatures was around 0.5 mm. None of the mice displayed autotomy or exhibited marked motor deficits.
Behavioral Studies.
All behavioral tests were performed by an experimenter blinded to the genotype. The mice used in the tests were 2- to 3-mo-old males (20–30 g). All experiments were performed under the protocol approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Formalin test.
Formalin (5 μL 2% formalin in PBS) was injected into the plantar region of one hind paw, and spontaneous pain behavior (licking and biting) was recorded for 60 min as previously described (23, 37).
CFA-induced heat hyperalgesia.
The intraplantar region of one hind paw of each mouse was injected with 6 μL 50% CFA solution in saline. Thermal-pain sensitivity was assessed by recording PWL on exposure to a defined radiant-heat stimulus (Hargreaves test) before CFA injection and 30 min after injection (41).
Tail-immersion test.
Mice were gently restrained in a 50-mL conical tube that the mice voluntarily entered. The protruding one-third of the tail was then dipped into a 50 °C water bath. Latency to respond to the heat stimulus with vigorous flexion of the tail was measured three times and averaged.
CCI-induced mechanical allodynia.
Mechanical sensitivity was assessed with the von Frey test by the frequency method (53). Two calibrated von Frey monofilaments (low force = 0.07 g; high force = 0.45 g) were used. Each von Frey filament was applied perpendicularly to the plantar side of each hind paw for ∼1 s; the stimulation was repeated 10 times to both hind paws. The occurrence of paw withdrawal in each of these 10 trials was expressed as a percent response frequency: PWF = (number of paw withdrawals/10 trials) × 100%.
Drug and Intrathecal Injection.
BAM 8–22 was purchased from Tocris and suspended in 0.9% saline. The drug was injected intrathecally under brief isoflurane (1.5%) anesthesia to reduce stress. A 30-gauge, 0.5-in needle connected to a 10-μL syringe was inserted into one side of the L5 or L6 spinous process at an angle of ∼20° above the vertebral column and slipped into the groove between the spinous and transverse processes. The needle was moved carefully forward to the intervertebral space. A tail flick indicated that the tip of the needle was inserted into the subarachnoid space.
Electrophysiological Recording of WDR Neurons
Electrophysiological recording of WDR neurons in the dorsal horn of the spinal cord was performed by an experimenter blinded to the genotype as previously described (20). Mice were paralyzed with pancuronium bromide (0.15 mg/kg i.p.) during neurophysiological recording. Throughout the experiment, anesthesia was maintained with a constant level of isoflurane (1.5%) carried in med-air. A spinal unit with a cutaneous receptive field located in the plantar area of the hind paw was located by applying mechanical stimuli. WDR neurons were defined as those that responded to both innocuous and noxious mechanical stimuli and that had increasing rates of response to increasing intensities of stimuli. Electrical stimuli were applied through a pair of fine needles inserted s.c. across the central plantar area of the hind paw 0.3–0.4 cm apart. Extracellular recordings of individual neurons were obtained by using fine-tip (<1.0 μm) paralyn-coated tungsten microelectrodes (8 mΩ at 1 kHz). BAM 8–22 or vehicle control was applied directly to the exposed surface of the spinal cord at the recording segment in a volume of 30–50 μL after predrug tests. The effects of BAM 8–22 on the spontaneous activity of WDR neurons were examined within 0–10 min after application. The evoked neuronal responses were recorded 10–30 min after drug application. Only one neuron in each animal was used to test the drug effects. The postdrug responses were compared with the predrug responses, allowing each neuron to act as its own control.
Data Analysis.
The number of action potentials evoked by graded electrical stimuli was compared between two genotypes by a two-way mixed-model ANOVA with Fisher's protected least significant difference (LSD) post hoc test. Student t test was used to compare the recording depth, activation threshold, latency of the first A fiber-mediated response, and latency of the first C fiber-mediated response between the two groups. For windup, the raw data were the number of action potentials in the C component evoked by each stimulus in a train of repetitive electrical stimuli. Because the number of action potentials in the C component varies among WDR neurons, the raw data for each neuron were normalized to the first response in each trial (input) and then averaged. A two-way mixed-model ANOVA was used to compare windup and averaged C component responses to the last 10 stimuli (7–16) of the trial between the two genotypes and between pre- and postdrug conditions. Data are presented as mean ± SEM. P < 0.05 was considered significant.
Acknowledgments
We thank Claire Levine, MS, for editing the manuscript and Yixun Geng for assistance with the mice. D.J.A and X.D. are investigators at the Howard Hughes Medical Institute. The work was supported by the Johns Hopkins Blaustein Pain Research Fund Award (to Y.G. and X.D.), an Alfred P. Sloan Neuroscience grant (to X.D.), and a Whitehall Foundation grant (to X.D.). This work was also supported by National Institutes of Health Grants NS26363 (to S.N.R.), NS048499 (to D.J.A.), and NS054791 and NS58481 (to X.D.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 3.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron R. Mechanisms of disease: Neuropathic pain—a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 5.MacPherson RD. New directions in pain management. Drugs Today (Barc) 2002;38:135–145. doi: 10.1358/dot.2002.38.2.668325. [DOI] [PubMed] [Google Scholar]
- 6.Lembo PM, et al. Proenkephalin A gene products activate a new family of sensory neuron—specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 7.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 8.Rau KK, et al. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, et al. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–948. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- 10.Cavanaugh DJ, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365.. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Hu Z, Quirion R, Hong Y. Modulation of NMDA receptors by intrathecal administration of the sensory neuron-specific receptor agonist BAM8-22. Neuropharmacology. 2008;54:796–803. doi: 10.1016/j.neuropharm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Grazzini E, et al. Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci USA. 2004;101:7175–7180. doi: 10.1073/pnas.0307185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SK, et al. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci USA. 2002;99:14740–14745. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci USA. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seal RP, et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 20.Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J Neurosci. 2006;26:4298–4307. doi: 10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrero JF, Laird JM, López-García JA. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 22.Hong Y, Dai P, Jiang J, Zeng X. Dual effects of intrathecal BAM22 on nociceptive responses in acute and persistent pain—potential function of a novel receptor. Br J Pharmacol. 2004;141:423–430. doi: 10.1038/sj.bjp.0705637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T, Cai Q, Hong Y. Intrathecal sensory neuron-specific receptor agonists bovine adrenal medulla 8-22 and (Tyr6)-gamma2-MSH-6-12 inhibit formalin-evoked nociception and neuronal Fos-like immunoreactivity in the spinal cord of the rat. Neuroscience. 2006;141:965–975. doi: 10.1016/j.neuroscience.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Ikeda SR. Modulation of ion channels and synaptic transmission by a human sensory neuron-specific G-protein-coupled receptor, SNSR4/mrgX1, heterologously expressed in cultured rat neurons. J Neurosci. 2004;24:5044–5053. doi: 10.1523/JNEUROSCI.0990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honan SA, McNaughton PA. Sensitisation of TRPV1 in rat sensory neurones by activation of SNSRs. Neurosci Lett. 2007;422:1–6. doi: 10.1016/j.neulet.2007.04.083. [DOI] [PubMed] [Google Scholar]
- 26.Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci USA. 1999;96:7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez Parkitna J, et al. Comparison of gene expression profiles in neuropathic and inflammatory pain. J Physiol Pharmacol. 2006;57:401–414. [PubMed] [Google Scholar]
- 29.Melzack R, Coderre TJ, Katz J, Vaccarino AL. Central neuroplasticity and pathological pain. Ann N Y Acad Sci. 2001;933:157–174. doi: 10.1111/j.1749-6632.2001.tb05822.x. [DOI] [PubMed] [Google Scholar]
- 30.Vikman KS, Kristensson K, Hill RH. Sensitization of dorsal horn neurons in a two-compartment cell culture model: Wind-up and long-term potentiation-like responses. J Neurosci. 2001;21:RC169. doi: 10.1523/JNEUROSCI.21-19-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 32.Cai M, Chen T, Quirion R, Hong Y. The involvement of spinal bovine adrenal medulla 22-like peptide, the proenkephalin derivative, in modulation of nociceptive processing. Eur J Neurosci. 2007;26:1128–1138. doi: 10.1111/j.1460-9568.2007.05755.x. [DOI] [PubMed] [Google Scholar]
- 33.Zeng X, Huang H, Hong Y. Effects of intrathecal BAM22 on noxious stimulus-evoked c-fos expression in the rat spinal dorsal horn. Brain Res. 2004;1028:170–179. doi: 10.1016/j.brainres.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda H, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 35.Costigan M, Woolf CJ. No DREAM, no pain. Closing the spinal gate. Cell. 2002;108:297–300. doi: 10.1016/s0092-8674(02)00640-2. [DOI] [PubMed] [Google Scholar]
- 36.De Felipe C, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 37.Knabl J, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 39.Ndong C, et al. Role of rat sensory neuron-specific receptor (rSNSR1) in inflammatory pain: Contribution of TRPV1 to SNSR signaling in the pain pathway. Pain. 2009;143:130–137. doi: 10.1016/j.pain.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Kusudo K, Ikeda H, Murase K. Depression of presynaptic excitation by the activation of vanilloid receptor 1 in the rat spinal dorsal horn revealed by optical imaging. Mol Pain. 2006;2:8. doi: 10.1186/1744-8069-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 42.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Thaler C, Brehm P. Calcium channels in Xenopus spinal neurons differ in somas and presynaptic terminals. J Neurophysiol. 2001;86:269–279. doi: 10.1152/jn.2001.86.1.269. [DOI] [PubMed] [Google Scholar]
- 44.Delmas P, Abogadie FC, Buckley NJ, Brown DA. Calcium channel gating and modulation by transmitters depend on cellular compartmentalization. Nat Neurosci. 2000;3:670–678. doi: 10.1038/76621. [DOI] [PubMed] [Google Scholar]
- 45.Callaghan B, et al. Analgesic alpha-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in rat sensory neurons via GABAB receptor activation. J Neurosci. 2008;28:10943–10951. doi: 10.1523/JNEUROSCI.3594-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerevich Z, et al. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altier C, Zamponi GW. Targeting Ca2+ channels to treat pain: T-type versus N-type. Trends Pharmacol Sci. 2004;25:465–470. doi: 10.1016/j.tips.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 50.Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 53.Guan Y, Yaster M, Raja SN, Tao YX. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]